Abstract
In the title compound, C23H24N4OS, the piperidine and cyclohexane rings adopt twin chair conformations and the phenyl groups occupy equatorial positions. The dihedral angle between the two benzene rings is 10.25 (12)°. The crystal structure is stabilized by intermolecular N—H⋯O hydrogen bonds with the formation of centrosymmetric dimers.
Related literature
For background on the thiazolidinone system, see: Laurent et al. (2004 ▶). For the biological activities of thiazolidinones, see: Shih & Ke (2004 ▶), For bicyclic compounds, see: Jeyaraman & Avila, (1981 ▶). For ring conformational analysis, see: Cremer & Pople, (1975 ▶).
Experimental
Crystal data
C23H24N4OS
M r = 404.53
Monoclinic,

a = 8.3183 (3) Å
b = 10.8435 (4) Å
c = 22.7417 (8) Å
β = 92.483 (2)°
V = 2049.36 (13) Å3
Z = 4
Mo Kα radiation
μ = 0.18 mm−1
T = 293 K
0.25 × 0.20 × 0.15 mm
Data collection
Bruker APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker 1999 ▶) T min = 0.956, T max = 0.974
31153 measured reflections
7820 independent reflections
4068 reflections with I > 2σ(I)
R int = 0.053
Refinement
R[F 2 > 2σ(F 2)] = 0.062
wR(F 2) = 0.203
S = 1.04
7820 reflections
270 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.42 e Å−3
Δρmin = −0.33 e Å−3
Data collection: APEX2 (Bruker, 2004 ▶); cell refinement: SAINT (Bruker, 2004 ▶); data reduction: SAINT; program(s) used to solve structure: SIR92 (Altomare et al., 1993 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶) and Mercury (Macrae et al., 2006 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809005339/rk2127sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809005339/rk2127Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N4—H4A⋯O1i | 0.83 (2) | 2.03 (2) | 2.847 (2) | 169 (2) |
Symmetry code: (i)  .
.
Acknowledgments
The authors are grateful to Dr Babu Varghese, Senior Scientist, Indian Institute of Technology Madras, for his valuable suggestions and for the data collection.
supplementary crystallographic information
Comment
Thiazolidinones are an interesting backbone unit in medicinal chemistry and is responsible for numerous pharmacological and biological properties (Shih & Ke, 2004; Laurent et al., 2004), which inspires our research interest in this area towards the synthesis of thiazolidinone unit. The importance of bicyclic compounds as intermediates in the synthesis of a several physiologically active compounds have reviewed by Jeyaraman & Avila, (1981). Moreover, these bridged bicyclic compounds exhibit twin chair, chair–boat or twin boat conformations to be elucidated possessing interesting stereochemistry. In order to investigate the change in molecular conformation of piperidine and cyclohexane ring, the present investigation was made and confirmed by X–ray diffraction study.
We found, that six–membered heterocyclic piperidine ring (Fig. 1) adopt normal chair conformation with the puckering parameters (Cremer & Pople, 1975) being q1 and q2 are 0.0714 (19) Å and -0.567 (19) Å, respectively. The total puckering amplitude, QT=0.572 (19) Å; θ=173.03 (19)°. Similarly, the cyclohexane ring is also adopt normal chair conformation with the puckering parameters being q1 and q2 are 0.121 (2)Å and 0.552 (2) Å, respectively. The puckering amplitude, QT=0.562 (2) Å, θ=12.5 (2)°. The planar phenyl rings occupy equatorial orientation of the piperidine ring and its subtending angle between the phenyl ring and the best plane of the piperidine ring is 10.25 (12)°. The crystal structure is stabilized by intermolecular N4—H4A···O1i hydrogen bonds (Fig. 2) with formation of centrosymmetrical dimers. Symmetry code: (i) -x+1, -y+1, -z+1.
Experimental
To the boiling solution of the bicyclic thiosemicarbazone (0.01 mol) in ethanolic–chloroform (1:1 / v:v), ethylbromoacetate (0.01 mol), sodium acetate trihydrate (0.02 mol) and acetic acid few drops were added and refluxed for about 5–6 h. After the completion of reaction, excess of solvent was removed under reduced pressure and poured into water. After usual work–up, the solid was separated and purified by column chromatography using benzene–ethyl acetate (9:1 / v:v) as eluent on neutral alumina. Colourless crystals were grown by slow evaporation method using ethanol as solvent. 1H NMR (δ p.p.m.), DMSO–d6: 4.39 (s, 1H, H2a); 4.26 (s, 1H, H4a); 3.56 (s, 1H, H5e); 2.57 (s, 1H, H1e); 3.74 (s, 2H, S—CH2); 2.82 (m, 1H, H7a); 1.44 (m, 5H, H6e, H8e, H7e, H6a and H8a); 2.09 (s, 1H, NH at 3); 11.60 (bs, 1H, NH exchangeable); 7.25–7.60 & 7.80 (m, 10H aryl protons): 13C NMR (δ p.p.m.) DMSO–d6: 64.94 (C2); 63.57 (C4); 45.91 (C1); 39.88 (C5); 28.65 (C8); 27.28 (C6); 21.37 (C7); 32.92 (S—CH2); 173.98 (Cδb O); 163.00 (Cδb N) 142.54 (C2' & C4'); 128.16, 127.02, 126.95, 126.83, 126.77 (other aryl carbons).
Refinement
The H–atoms were bonded with C atoms were placed in calculated positions and were refined using a riding model, with aromatic C—H = 0.93 Å, methine C—H = 0.98 Å, methylene C—H = 0.97 Å. The displacement parameters were set for these H atoms as Uiso(H) = 1.2Ueq(C). The other H atoms were found from difference Fourier map and were refined isopropically.
Figures
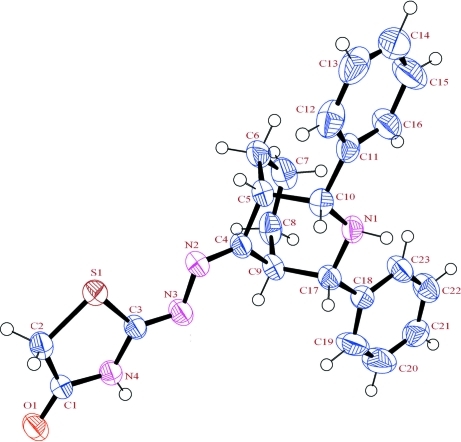
Fig. 1.
The asymmetric unit of title compound with the atom numbering scheme. Displacement ellipsoids drawn at 50% probability level. H atoms are presented as a small spheres of arbitrary radius.
Fig. 2.
Packing of molecules in the unit cell. Hydrogen bonds are shown by dotted lines.
Crystal data
| C23H24N4OS | F(000) = 856 |
| Mr = 404.53 | Dx = 1.311 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2yn | Cell parameters from 4403 reflections |
| a = 8.3183 (3) Å | θ = 3.9–24.7° |
| b = 10.8435 (4) Å | µ = 0.18 mm−1 |
| c = 22.7417 (8) Å | T = 293 K |
| β = 92.483 (2)° | Block, colourless |
| V = 2049.36 (13) Å3 | 0.25 × 0.20 × 0.15 mm |
| Z = 4 |
Data collection
| Bruker APEXII CCD diffractometer | 7820 independent reflections |
| Radiation source: Fine-focus sealed tube | 4068 reflections with I > 2σ(I) |
| Graphite | Rint = 0.053 |
| φ and ω scans | θmax = 33.2°, θmin = 1.8° |
| Absorption correction: multi-scan (SADABS; Bruker 1999) | h = −12→12 |
| Tmin = 0.956, Tmax = 0.974 | k = −16→16 |
| 31153 measured reflections | l = −34→34 |
Refinement
| Refinement on F2 | Primary atom site location: Direct |
| Least-squares matrix: Full | Secondary atom site location: Difmap |
| R[F2 > 2σ(F2)] = 0.062 | Hydrogen site location: Geom |
| wR(F2) = 0.203 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.1023P)2] where P = (Fo2 + 2Fc2)/3 |
| 7820 reflections | (Δ/σ)max = 0.001 |
| 270 parameters | Δρmax = 0.42 e Å−3 |
| 0 restraints | Δρmin = −0.33 e Å−3 |
Special details
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R–factor wR and goodness of fit S are based on F2, conventional R–factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > \ s(F2) is used only for calculating R–factors(gt) etc. and is not relevant to the choice of reflections for refinement. R–factors based on F2 are statistically about twice as large as those based on F, and R–factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.6862 (2) | 0.50259 (17) | 0.44666 (8) | 0.0387 (4) | |
| C2 | 0.8215 (2) | 0.52229 (19) | 0.40590 (9) | 0.0463 (5) | |
| H2A | 0.7801 | 0.5554 | 0.3686 | 0.056* | |
| H2B | 0.8749 | 0.4447 | 0.3985 | 0.056* | |
| C3 | 0.8430 (2) | 0.64074 (17) | 0.50239 (8) | 0.0377 (4) | |
| C4 | 1.0586 (2) | 0.83875 (17) | 0.58582 (8) | 0.0400 (4) | |
| C5 | 1.2108 (2) | 0.91183 (18) | 0.58360 (9) | 0.0439 (4) | |
| H5 | 1.2548 | 0.9015 | 0.5446 | 0.053* | |
| C6 | 1.3309 (3) | 0.8579 (2) | 0.62995 (11) | 0.0559 (6) | |
| H6A | 1.3594 | 0.7749 | 0.6182 | 0.067* | |
| H6B | 1.4283 | 0.9072 | 0.6310 | 0.067* | |
| C7 | 1.2677 (3) | 0.8535 (2) | 0.69020 (11) | 0.0627 (7) | |
| H7A | 1.2672 | 0.9363 | 0.7063 | 0.075* | |
| H7B | 1.3395 | 0.8038 | 0.7152 | 0.075* | |
| C8 | 1.0981 (3) | 0.8002 (2) | 0.69143 (10) | 0.0556 (5) | |
| H8A | 1.0554 | 0.8166 | 0.7297 | 0.067* | |
| H8B | 1.1041 | 0.7114 | 0.6867 | 0.067* | |
| C9 | 0.9813 (2) | 0.85244 (18) | 0.64362 (8) | 0.0430 (4) | |
| H9 | 0.8818 | 0.8039 | 0.6428 | 0.052* | |
| C10 | 1.1700 (2) | 1.04873 (17) | 0.59233 (8) | 0.0421 (4) | |
| H10 | 1.1015 | 1.0760 | 0.5587 | 0.050* | |
| C11 | 1.3226 (2) | 1.12617 (17) | 0.59453 (9) | 0.0442 (4) | |
| C12 | 1.4047 (3) | 1.1425 (2) | 0.54321 (11) | 0.0620 (6) | |
| H12 | 1.3648 | 1.1083 | 0.5080 | 0.074* | |
| C13 | 1.5469 (3) | 1.2102 (2) | 0.54460 (14) | 0.0733 (8) | |
| H13 | 1.6015 | 1.2207 | 0.5101 | 0.088* | |
| C14 | 1.6069 (3) | 1.2609 (3) | 0.59510 (15) | 0.0796 (9) | |
| H14 | 1.7020 | 1.3061 | 0.5954 | 0.095* | |
| C15 | 1.5281 (3) | 1.2454 (3) | 0.64502 (14) | 0.0804 (8) | |
| H15 | 1.5691 | 1.2807 | 0.6798 | 0.096* | |
| C16 | 1.3867 (3) | 1.1777 (2) | 0.64548 (11) | 0.0612 (6) | |
| H16 | 1.3349 | 1.1671 | 0.6806 | 0.073* | |
| C17 | 0.9389 (2) | 0.98974 (18) | 0.65116 (8) | 0.0420 (4) | |
| H17 | 0.8608 | 1.0127 | 0.6196 | 0.050* | |
| C18 | 0.8625 (2) | 1.01140 (19) | 0.70932 (9) | 0.0433 (4) | |
| C19 | 0.7131 (3) | 0.9629 (3) | 0.71957 (12) | 0.0766 (9) | |
| H19 | 0.6586 | 0.9191 | 0.6897 | 0.092* | |
| C20 | 0.6425 (3) | 0.9775 (3) | 0.77257 (13) | 0.0835 (9) | |
| H20 | 0.5424 | 0.9423 | 0.7783 | 0.100* | |
| C21 | 0.7169 (3) | 1.0423 (2) | 0.81652 (11) | 0.0594 (6) | |
| H21 | 0.6675 | 1.0544 | 0.8520 | 0.071* | |
| C22 | 0.8654 (3) | 1.0897 (2) | 0.80792 (10) | 0.0584 (6) | |
| H22 | 0.9184 | 1.1337 | 0.8381 | 0.070* | |
| C23 | 0.9384 (3) | 1.0737 (2) | 0.75539 (9) | 0.0534 (5) | |
| H23 | 1.0410 | 1.1055 | 0.7509 | 0.064* | |
| N1 | 1.08146 (19) | 1.06644 (15) | 0.64602 (7) | 0.0421 (4) | |
| N2 | 1.01593 (19) | 0.77332 (15) | 0.54109 (7) | 0.0433 (4) | |
| N3 | 0.87175 (19) | 0.70686 (16) | 0.54817 (7) | 0.0456 (4) | |
| N4 | 0.70888 (19) | 0.56801 (14) | 0.49729 (7) | 0.0403 (4) | |
| O1 | 0.57154 (16) | 0.43634 (14) | 0.43526 (6) | 0.0525 (4) | |
| S1 | 0.96100 (5) | 0.62925 (5) | 0.44083 (2) | 0.04262 (15) | |
| H1A | 1.049 (3) | 1.149 (2) | 0.6472 (10) | 0.060 (7)* | |
| H4A | 0.635 (3) | 0.569 (2) | 0.5206 (11) | 0.062 (7)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0368 (8) | 0.0358 (9) | 0.0432 (10) | −0.0020 (7) | −0.0019 (7) | −0.0086 (8) |
| C2 | 0.0459 (10) | 0.0462 (11) | 0.0470 (11) | −0.0060 (9) | 0.0046 (8) | −0.0151 (9) |
| C3 | 0.0384 (8) | 0.0344 (9) | 0.0400 (10) | −0.0012 (7) | −0.0040 (7) | −0.0051 (7) |
| C4 | 0.0427 (9) | 0.0348 (9) | 0.0418 (10) | −0.0007 (7) | −0.0065 (8) | −0.0070 (8) |
| C5 | 0.0460 (10) | 0.0370 (10) | 0.0485 (11) | −0.0033 (8) | −0.0007 (8) | −0.0112 (8) |
| C6 | 0.0490 (11) | 0.0369 (11) | 0.0804 (16) | 0.0033 (9) | −0.0145 (11) | −0.0086 (10) |
| C7 | 0.0733 (15) | 0.0435 (12) | 0.0682 (15) | 0.0093 (11) | −0.0331 (13) | −0.0022 (10) |
| C8 | 0.0817 (15) | 0.0359 (11) | 0.0484 (12) | 0.0033 (10) | −0.0067 (11) | −0.0013 (9) |
| C9 | 0.0486 (10) | 0.0375 (10) | 0.0425 (10) | −0.0076 (8) | −0.0023 (8) | −0.0073 (8) |
| C10 | 0.0488 (10) | 0.0380 (10) | 0.0392 (10) | 0.0028 (8) | 0.0005 (8) | −0.0013 (8) |
| C11 | 0.0522 (10) | 0.0300 (9) | 0.0508 (11) | 0.0024 (8) | 0.0074 (9) | 0.0009 (8) |
| C12 | 0.0833 (16) | 0.0441 (12) | 0.0604 (14) | 0.0053 (11) | 0.0242 (12) | 0.0023 (10) |
| C13 | 0.0814 (17) | 0.0487 (14) | 0.093 (2) | 0.0055 (13) | 0.0471 (16) | 0.0057 (14) |
| C14 | 0.0651 (15) | 0.0590 (16) | 0.117 (3) | −0.0164 (13) | 0.0349 (16) | −0.0108 (16) |
| C15 | 0.0707 (15) | 0.0826 (19) | 0.089 (2) | −0.0358 (14) | 0.0130 (14) | −0.0197 (16) |
| C16 | 0.0637 (13) | 0.0619 (14) | 0.0589 (14) | −0.0231 (11) | 0.0139 (11) | −0.0126 (12) |
| C17 | 0.0425 (9) | 0.0408 (10) | 0.0420 (10) | −0.0008 (8) | −0.0049 (8) | −0.0053 (8) |
| C18 | 0.0391 (9) | 0.0439 (11) | 0.0464 (11) | 0.0021 (8) | −0.0045 (8) | −0.0072 (8) |
| C19 | 0.0392 (11) | 0.120 (2) | 0.0705 (16) | −0.0151 (13) | 0.0006 (11) | −0.0420 (16) |
| C20 | 0.0465 (12) | 0.121 (3) | 0.0839 (19) | −0.0169 (15) | 0.0155 (12) | −0.0345 (18) |
| C21 | 0.0557 (12) | 0.0656 (15) | 0.0574 (13) | 0.0076 (11) | 0.0095 (10) | −0.0089 (12) |
| C22 | 0.0664 (14) | 0.0641 (14) | 0.0443 (12) | −0.0100 (11) | −0.0024 (10) | −0.0130 (10) |
| C23 | 0.0533 (11) | 0.0563 (13) | 0.0504 (12) | −0.0155 (10) | 0.0000 (10) | −0.0109 (10) |
| N1 | 0.0483 (8) | 0.0304 (8) | 0.0479 (9) | −0.0011 (7) | 0.0056 (7) | −0.0043 (7) |
| N2 | 0.0424 (8) | 0.0402 (9) | 0.0468 (9) | −0.0052 (7) | −0.0035 (7) | −0.0089 (7) |
| N3 | 0.0466 (8) | 0.0466 (9) | 0.0435 (9) | −0.0090 (7) | 0.0003 (7) | −0.0097 (7) |
| N4 | 0.0378 (8) | 0.0414 (9) | 0.0421 (9) | −0.0047 (7) | 0.0053 (7) | −0.0088 (7) |
| O1 | 0.0470 (7) | 0.0553 (9) | 0.0553 (9) | −0.0150 (6) | 0.0028 (6) | −0.0171 (7) |
| S1 | 0.0391 (2) | 0.0403 (3) | 0.0487 (3) | −0.00407 (19) | 0.00437 (19) | −0.0065 (2) |
Geometric parameters (Å, °)
| C1—O1 | 1.213 (2) | C11—C16 | 1.373 (3) |
| C1—N4 | 1.359 (2) | C11—C12 | 1.389 (3) |
| C1—C2 | 1.504 (3) | C12—C13 | 1.391 (4) |
| C2—S1 | 1.8009 (19) | C12—H12 | 0.9300 |
| C2—H2A | 0.9700 | C13—C14 | 1.349 (4) |
| C2—H2B | 0.9700 | C13—H13 | 0.9300 |
| C3—N3 | 1.278 (2) | C14—C15 | 1.346 (4) |
| C3—N4 | 1.367 (2) | C14—H14 | 0.9300 |
| C3—S1 | 1.7487 (18) | C15—C16 | 1.387 (3) |
| C4—N2 | 1.278 (2) | C15—H15 | 0.9300 |
| C4—C9 | 1.496 (3) | C16—H16 | 0.9300 |
| C4—C5 | 1.496 (3) | C17—N1 | 1.458 (2) |
| C5—C6 | 1.537 (3) | C17—C18 | 1.510 (3) |
| C5—C10 | 1.538 (3) | C17—H17 | 0.9800 |
| C5—H5 | 0.9800 | C18—C23 | 1.377 (3) |
| C6—C7 | 1.489 (3) | C18—C19 | 1.379 (3) |
| C6—H6A | 0.9700 | C19—C20 | 1.372 (3) |
| C6—H6B | 0.9700 | C19—H19 | 0.9300 |
| C7—C8 | 1.526 (3) | C20—C21 | 1.350 (4) |
| C7—H7A | 0.9700 | C20—H20 | 0.9300 |
| C7—H7B | 0.9700 | C21—C22 | 1.360 (3) |
| C8—C9 | 1.535 (3) | C21—H21 | 0.9300 |
| C8—H8A | 0.9700 | C22—C23 | 1.375 (3) |
| C8—H8B | 0.9700 | C22—H22 | 0.9300 |
| C9—C17 | 1.541 (3) | C23—H23 | 0.9300 |
| C9—H9 | 0.9800 | N1—H1A | 0.94 (3) |
| C10—N1 | 1.466 (2) | N2—N3 | 1.414 (2) |
| C10—C11 | 1.521 (3) | N4—H4A | 0.83 (2) |
| C10—H10 | 0.9800 | ||
| O1—C1—N4 | 124.69 (17) | C16—C11—C12 | 118.0 (2) |
| O1—C1—C2 | 123.70 (17) | C16—C11—C10 | 123.06 (18) |
| N4—C1—C2 | 111.60 (15) | C12—C11—C10 | 118.9 (2) |
| C1—C2—S1 | 107.74 (13) | C11—C12—C13 | 119.8 (3) |
| C1—C2—H2A | 110.2 | C11—C12—H12 | 120.1 |
| S1—C2—H2A | 110.2 | C13—C12—H12 | 120.1 |
| C1—C2—H2B | 110.2 | C14—C13—C12 | 121.2 (2) |
| S1—C2—H2B | 110.2 | C14—C13—H13 | 119.4 |
| H2A—C2—H2B | 108.5 | C12—C13—H13 | 119.4 |
| N3—C3—N4 | 121.05 (17) | C15—C14—C13 | 119.4 (2) |
| N3—C3—S1 | 126.91 (14) | C15—C14—H14 | 120.3 |
| N4—C3—S1 | 112.04 (13) | C13—C14—H14 | 120.3 |
| N2—C4—C9 | 129.74 (17) | C14—C15—C16 | 121.1 (3) |
| N2—C4—C5 | 118.26 (17) | C14—C15—H15 | 119.4 |
| C9—C4—C5 | 111.96 (15) | C16—C15—H15 | 119.4 |
| C4—C5—C6 | 107.51 (17) | C11—C16—C15 | 120.5 (2) |
| C4—C5—C10 | 108.35 (16) | C11—C16—H16 | 119.8 |
| C6—C5—C10 | 114.83 (16) | C15—C16—H16 | 119.8 |
| C4—C5—H5 | 108.7 | N1—C17—C18 | 110.86 (15) |
| C6—C5—H5 | 108.7 | N1—C17—C9 | 110.57 (15) |
| C10—C5—H5 | 108.7 | C18—C17—C9 | 110.78 (16) |
| C7—C6—C5 | 113.48 (18) | N1—C17—H17 | 108.2 |
| C7—C6—H6A | 108.9 | C18—C17—H17 | 108.2 |
| C5—C6—H6A | 108.9 | C9—C17—H17 | 108.2 |
| C7—C6—H6B | 108.9 | C23—C18—C19 | 116.5 (2) |
| C5—C6—H6B | 108.9 | C23—C18—C17 | 123.12 (18) |
| H6A—C6—H6B | 107.7 | C19—C18—C17 | 120.33 (18) |
| C6—C7—C8 | 113.11 (19) | C20—C19—C18 | 121.8 (2) |
| C6—C7—H7A | 109.0 | C20—C19—H19 | 119.1 |
| C8—C7—H7A | 109.0 | C18—C19—H19 | 119.1 |
| C6—C7—H7B | 109.0 | C21—C20—C19 | 120.7 (2) |
| C8—C7—H7B | 109.0 | C21—C20—H20 | 119.6 |
| H7A—C7—H7B | 107.8 | C19—C20—H20 | 119.6 |
| C7—C8—C9 | 113.88 (18) | C20—C21—C22 | 118.7 (2) |
| C7—C8—H8A | 108.8 | C20—C21—H21 | 120.7 |
| C9—C8—H8A | 108.8 | C22—C21—H21 | 120.7 |
| C7—C8—H8B | 108.8 | C21—C22—C23 | 121.1 (2) |
| C9—C8—H8B | 108.8 | C21—C22—H22 | 119.5 |
| H8A—C8—H8B | 107.7 | C23—C22—H22 | 119.5 |
| C4—C9—C8 | 107.61 (17) | C22—C23—C18 | 121.2 (2) |
| C4—C9—C17 | 107.63 (16) | C22—C23—H23 | 119.4 |
| C8—C9—C17 | 114.75 (16) | C18—C23—H23 | 119.4 |
| C4—C9—H9 | 108.9 | C17—N1—C10 | 115.60 (15) |
| C8—C9—H9 | 108.9 | C17—N1—H1A | 108.1 (14) |
| C17—C9—H9 | 108.9 | C10—N1—H1A | 107.7 (14) |
| N1—C10—C11 | 110.42 (15) | C4—N2—N3 | 113.58 (17) |
| N1—C10—C5 | 110.85 (16) | C3—N3—N2 | 108.82 (16) |
| C11—C10—C5 | 110.40 (16) | C1—N4—C3 | 117.12 (16) |
| N1—C10—H10 | 108.4 | C1—N4—H4A | 118.2 (17) |
| C11—C10—H10 | 108.4 | C3—N4—H4A | 124.0 (17) |
| C5—C10—H10 | 108.4 | C3—S1—C2 | 91.47 (8) |
| O1—C1—C2—S1 | 178.98 (16) | C4—C9—C17—N1 | −55.7 (2) |
| N4—C1—C2—S1 | −1.0 (2) | C8—C9—C17—N1 | 64.0 (2) |
| N2—C4—C5—C6 | −114.0 (2) | C4—C9—C17—C18 | −179.00 (15) |
| C9—C4—C5—C6 | 64.1 (2) | C8—C9—C17—C18 | −59.3 (2) |
| N2—C4—C5—C10 | 121.3 (2) | N1—C17—C18—C23 | −13.3 (3) |
| C9—C4—C5—C10 | −60.5 (2) | C9—C17—C18—C23 | 109.8 (2) |
| C4—C5—C6—C7 | −55.1 (2) | N1—C17—C18—C19 | 169.9 (2) |
| C10—C5—C6—C7 | 65.6 (2) | C9—C17—C18—C19 | −66.9 (3) |
| C5—C6—C7—C8 | 47.2 (2) | C23—C18—C19—C20 | 1.0 (4) |
| C6—C7—C8—C9 | −46.0 (3) | C17—C18—C19—C20 | 177.9 (3) |
| N2—C4—C9—C8 | 115.1 (2) | C18—C19—C20—C21 | 1.2 (5) |
| C5—C4—C9—C8 | −62.8 (2) | C19—C20—C21—C22 | −2.0 (5) |
| N2—C4—C9—C17 | −120.7 (2) | C20—C21—C22—C23 | 0.8 (4) |
| C5—C4—C9—C17 | 61.4 (2) | C21—C22—C23—C18 | 1.4 (4) |
| C7—C8—C9—C4 | 52.2 (2) | C19—C18—C23—C22 | −2.2 (3) |
| C7—C8—C9—C17 | −67.6 (2) | C17—C18—C23—C22 | −179.0 (2) |
| C4—C5—C10—N1 | 53.5 (2) | C18—C17—N1—C10 | 177.21 (15) |
| C6—C5—C10—N1 | −66.7 (2) | C9—C17—N1—C10 | 54.0 (2) |
| C4—C5—C10—C11 | 176.21 (16) | C11—C10—N1—C17 | −175.50 (16) |
| C6—C5—C10—C11 | 56.0 (2) | C5—C10—N1—C17 | −52.8 (2) |
| N1—C10—C11—C16 | 15.8 (3) | C9—C4—N2—N3 | 1.4 (3) |
| C5—C10—C11—C16 | −107.2 (2) | C5—C4—N2—N3 | 179.20 (16) |
| N1—C10—C11—C12 | −166.51 (18) | N4—C3—N3—N2 | −179.13 (16) |
| C5—C10—C11—C12 | 70.6 (2) | S1—C3—N3—N2 | 1.1 (2) |
| C16—C11—C12—C13 | −0.4 (3) | C4—N2—N3—C3 | −177.32 (17) |
| C10—C11—C12—C13 | −178.2 (2) | O1—C1—N4—C3 | −178.36 (18) |
| C11—C12—C13—C14 | −0.1 (4) | C2—C1—N4—C3 | 1.6 (2) |
| C12—C13—C14—C15 | 0.2 (4) | N3—C3—N4—C1 | 178.78 (18) |
| C13—C14—C15—C16 | 0.3 (5) | S1—C3—N4—C1 | −1.5 (2) |
| C12—C11—C16—C15 | 0.9 (4) | N3—C3—S1—C2 | −179.58 (19) |
| C10—C11—C16—C15 | 178.7 (2) | N4—C3—S1—C2 | 0.67 (15) |
| C14—C15—C16—C11 | −0.9 (5) | C1—C2—S1—C3 | 0.16 (15) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N4—H4A···O1i | 0.83 (2) | 2.03 (2) | 2.847 (2) | 169 (2) |
Symmetry codes: (i) −x+1, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: RK2127).
References
- Altomare, A., Cascarano, G., Giacovazzo, C. & Guagliardi, A. (1993). J. Appl. Cryst. 26, 343–350.
- Bruker (1999). SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2004). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc.97, 1354–1358.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Jeyaraman, R. & Avila, S. (1981). Chem. Rev.81, 149–174.
- Laurent, D. R. S., Gao, Q., Wu, D. & Serrano-Wu, M. H. (2004). Tetrahedron Lett.45, 1907–1910.
- Macrae, C. F., Edgington, P. R., McCabe, P., Pidcock, E., Shields, G. P., Taylor, R., Towler, M. & van de Streek, J. (2006). J. Appl. Cryst.39, 453–457.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Shih, M. H. & Ke, F. Y. (2004). Bioorg. Med. Chem.12, 4633–4643. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809005339/rk2127sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809005339/rk2127Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report