Abstract
The title molecule, C14H18Cl4, possesses a crystallographically imposed inversion centre, which coincides with the centre of benzene ring. In the absence of classical intermolecular interactions, van der Waals forces help the molecules to pack in the crystal.
Related literature
For related crystal structures, see: Chen et al. (2005 ▶); Gao et al. (2009 ▶). For general background, see Amabilino & Stoddart (1995 ▶).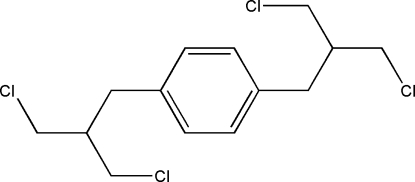
Experimental
Crystal data
C14H18Cl4
M r = 328.08
Monoclinic,

a = 6.518 (3) Å
b = 14.680 (6) Å
c = 8.433 (4) Å
β = 106.335 (5)°
V = 774.3 (6) Å3
Z = 2
Mo Kα radiation
μ = 0.75 mm−1
T = 291 (2) K
0.30 × 0.26 × 0.24 mm
Data collection
Bruker SMART APEX CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2000 ▶) T min = 0.807, T max = 0.842
4423 measured reflections
1679 independent reflections
1275 reflections with I > 2σ(I)
R int = 0.066
Refinement
R[F 2 > 2σ(F 2)] = 0.040
wR(F 2) = 0.113
S = 1.09
1679 reflections
82 parameters
H-atom parameters constrained
Δρmax = 0.32 e Å−3
Δρmin = −0.32 e Å−3
Data collection: SMART (Bruker, 2000 ▶); cell refinement: SAINT (Bruker, 2000 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809000609/cv2502sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809000609/cv2502Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors acknowledge the financial support of Jiangsu Polytechnic University, the Natural Science Foundation of China (grant No. 20872051) and the Key Laboratory of Fine Petrochemical Engineering of Jiangsu Province (grant No. KF0503).
supplementary crystallographic information
Comment
The molecular recognition between a π-electron-rich hydroquinone ring and π-electron-deficient cyclophane has provided the inspiration for the self-assembly of a large number of catenanes (Amabilino & Stoddart, 1995). In our study of the applications of fused bipyridine cyclophane compounds in the self-assembly of supramolecular systems, we obtained tetraethyl 2,2'-(p-phenylenedimethylene)dimalonate (Chen et al., 2005), which was used in the synthesis of 2,2'-(p-phenylenedimethylene)bis(propane-1,3-diol) (Gao et al., 2009). The title compound, (I), was obtained by the chlorination of the diol. Herewith we present the crystal structure of (I) (Fig. 1).
Experimental
The 2,2'-(p-phenylenedimethylene)bis(propane-1,3-diol), used in this study, was obtained in accordance with the Gao et al. (2005). In a flame-dryed, round-bottomed flask was placed SOCl2(5 mL) and p-C6H4[CH2CH(CH2OH)2]2(0.508 g,2 mmol) was slowly added under stirring. The mixture was heated up to 333 K. The solvent was evaporated and the resulting oil was chromatographed on a silica-gel column, yielding the title compound (0.51 g, 77%). M.p. 353–354 K.
Refinement
All H atoms were geometrically positioned (C–H 0.93-0.98%A) and treated as riding, with Uiso(H) = 1.2Ueq(C).
Figures
Fig. 1.
The molecular structure of (I) showing the atom-labelling scheme and 30% probability displacement ellipsoids [symmetry code: (A)-x, -y + 2, -z + 1].
Crystal data
| C14H18Cl4 | F(000) = 340 |
| Mr = 328.08 | Dx = 1.407 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 1607 reflections |
| a = 6.518 (3) Å | θ = 2.8–26.1° |
| b = 14.680 (6) Å | µ = 0.75 mm−1 |
| c = 8.433 (4) Å | T = 291 K |
| β = 106.335 (5)° | Block, colourless |
| V = 774.3 (6) Å3 | 0.30 × 0.26 × 0.24 mm |
| Z = 2 |
Data collection
| Bruker SMART APEX CCD diffractometer | 1679 independent reflections |
| Radiation source: sealed tube | 1275 reflections with I > 2σ(I) |
| graphite | Rint = 0.066 |
| φ and ω scans | θmax = 27.0°, θmin = 2.8° |
| Absorption correction: multi-scan (SADABS; Bruker, 2000) | h = −8→7 |
| Tmin = 0.807, Tmax = 0.842 | k = −18→14 |
| 4423 measured reflections | l = −10→10 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.040 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.113 | H-atom parameters constrained |
| S = 1.09 | w = 1/[σ2(Fo2) + (0.0483P)2 + 0.0345P] where P = (Fo2 + 2Fc2)/3 |
| 1679 reflections | (Δ/σ)max < 0.001 |
| 82 parameters | Δρmax = 0.32 e Å−3 |
| 0 restraints | Δρmin = −0.32 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.The structures were solved with direct methods and refined with full-matrix least-squares techniques using the SHELXTL. The coordinates of the non-hydrogen atoms were refined anisotropically, and the positions of the H atoms were positioned geometrically and refined using a riding model with C—H = 0.93–0.97 Å, and with Uiso(H) = 1.2 timesUeq(C). |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.1873 (3) | 0.97482 (12) | 0.6208 (2) | 0.0415 (4) | |
| C2 | 0.0205 (4) | 1.01687 (13) | 0.6634 (2) | 0.0482 (5) | |
| H2 | 0.0327 | 1.0284 | 0.7741 | 0.058* | |
| C3 | −0.1637 (3) | 1.04196 (13) | 0.5447 (3) | 0.0477 (5) | |
| H3 | −0.2733 | 1.0705 | 0.5764 | 0.057* | |
| C4 | 0.3901 (3) | 0.94730 (13) | 0.7505 (3) | 0.0489 (5) | |
| H7A | 0.3999 | 0.9812 | 0.8511 | 0.059* | |
| H7B | 0.5123 | 0.9638 | 0.7122 | 0.059* | |
| C5 | 0.4008 (3) | 0.84467 (13) | 0.7898 (2) | 0.0410 (4) | |
| H8 | 0.3984 | 0.8123 | 0.6877 | 0.049* | |
| C6 | 0.6155 (3) | 0.82501 (15) | 0.9136 (3) | 0.0543 (5) | |
| H9A | 0.6174 | 0.8515 | 1.0194 | 0.065* | |
| H9B | 0.7275 | 0.8539 | 0.8764 | 0.065* | |
| C7 | 0.2135 (3) | 0.81085 (13) | 0.8444 (2) | 0.0444 (5) | |
| H10A | 0.2273 | 0.7456 | 0.8629 | 0.053* | |
| H10B | 0.0828 | 0.8218 | 0.7571 | 0.053* | |
| Cl1 | 0.67066 (10) | 0.70596 (4) | 0.94037 (8) | 0.0736 (3) | |
| Cl2 | 0.19547 (9) | 0.86582 (4) | 1.03025 (7) | 0.0635 (2) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0465 (11) | 0.0298 (9) | 0.0488 (11) | −0.0015 (8) | 0.0141 (9) | 0.0050 (8) |
| C2 | 0.0586 (13) | 0.0437 (11) | 0.0440 (11) | 0.0046 (9) | 0.0172 (10) | −0.0002 (8) |
| C3 | 0.0535 (12) | 0.0389 (11) | 0.0560 (12) | 0.0063 (9) | 0.0241 (10) | 0.0036 (9) |
| C4 | 0.0424 (11) | 0.0438 (11) | 0.0570 (12) | −0.0062 (8) | 0.0082 (9) | 0.0026 (9) |
| C5 | 0.0389 (10) | 0.0396 (10) | 0.0443 (10) | 0.0017 (8) | 0.0114 (8) | −0.0017 (8) |
| C6 | 0.0368 (11) | 0.0513 (12) | 0.0718 (14) | 0.0052 (9) | 0.0101 (10) | 0.0022 (10) |
| C7 | 0.0397 (11) | 0.0414 (11) | 0.0480 (11) | −0.0012 (8) | 0.0058 (9) | 0.0009 (8) |
| Cl1 | 0.0629 (4) | 0.0603 (4) | 0.0915 (5) | 0.0227 (3) | 0.0119 (3) | 0.0086 (3) |
| Cl2 | 0.0614 (4) | 0.0795 (4) | 0.0538 (4) | 0.0101 (3) | 0.0228 (3) | 0.0004 (3) |
Geometric parameters (Å, °)
| C1—C3i | 1.382 (3) | C5—C7 | 1.504 (3) |
| C1—C2 | 1.383 (3) | C5—C6 | 1.520 (3) |
| C1—C4 | 1.515 (3) | C5—H8 | 0.9800 |
| C2—C3 | 1.380 (3) | C6—Cl1 | 1.786 (2) |
| C2—H2 | 0.9300 | C6—H9A | 0.9700 |
| C3—C1i | 1.382 (3) | C6—H9B | 0.9700 |
| C3—H3 | 0.9300 | C7—Cl2 | 1.796 (2) |
| C4—C5 | 1.540 (3) | C7—H10A | 0.9700 |
| C4—H7A | 0.9700 | C7—H10B | 0.9700 |
| C4—H7B | 0.9700 | ||
| C3i—C1—C2 | 118.01 (19) | C6—C5—C4 | 108.10 (16) |
| C3i—C1—C4 | 120.55 (19) | C7—C5—H8 | 107.2 |
| C2—C1—C4 | 121.44 (18) | C6—C5—H8 | 107.2 |
| C3—C2—C1 | 121.17 (19) | C4—C5—H8 | 107.2 |
| C3—C2—H2 | 119.4 | C5—C6—Cl1 | 112.74 (15) |
| C1—C2—H2 | 119.4 | C5—C6—H9A | 109.0 |
| C2—C3—C1i | 120.82 (19) | Cl1—C6—H9A | 109.0 |
| C2—C3—H3 | 119.6 | C5—C6—H9B | 109.0 |
| C1i—C3—H3 | 119.6 | Cl1—C6—H9B | 109.0 |
| C1—C4—C5 | 113.20 (15) | H9A—C6—H9B | 107.8 |
| C1—C4—H7A | 108.9 | C5—C7—Cl2 | 112.11 (13) |
| C5—C4—H7A | 108.9 | C5—C7—H10A | 109.2 |
| C1—C4—H7B | 108.9 | Cl2—C7—H10A | 109.2 |
| C5—C4—H7B | 108.9 | C5—C7—H10B | 109.2 |
| H7A—C4—H7B | 107.8 | Cl2—C7—H10B | 109.2 |
| C7—C5—C6 | 113.40 (16) | H10A—C7—H10B | 107.9 |
| C7—C5—C4 | 113.42 (15) | ||
| C3i—C1—C2—C3 | −0.4 (3) | C1—C4—C5—C6 | 176.91 (17) |
| C4—C1—C2—C3 | 179.85 (17) | C7—C5—C6—Cl1 | 64.6 (2) |
| C1—C2—C3—C1i | 0.4 (3) | C4—C5—C6—Cl1 | −168.74 (14) |
| C3i—C1—C4—C5 | −77.5 (2) | C6—C5—C7—Cl2 | 62.71 (19) |
| C2—C1—C4—C5 | 102.2 (2) | C4—C5—C7—Cl2 | −61.10 (19) |
| C1—C4—C5—C7 | −56.4 (2) |
Symmetry codes: (i) −x, −y+2, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: CV2502).
References
- Amabilino, D. B. & Stoddart, J. F. (1995). Chem. Rev.95, 2725–2737.
- Bruker (2000). SMART, SAINT and SADABS Bruker AXS Inc. Madison, Wisconsin, USA.
- Chen, A.-H., Wang, Z.-G., Yin, G.-D. & Wu, A.-X. (2005). Acta Cryst. E61, o3240–o3241.
- Gao, Y., Xi, H., Sun, X., Fu, Y. & Liu, L. (2009). Acta Cryst. E65, o170. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112-122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809000609/cv2502sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809000609/cv2502Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



