Abstract
In the title centrosymmetric mononuclear nickel(II) complex, [Ni(C7H5ClNO)2], the NiII ion, lying on an inversion center, is four-coordinated by two O and two imine N atoms from two 4-chloro-2-iminomethylphenolate ligands, forming a distorted square-planar geometry. In the crystal structure, molecules are linked into a two-dimensional network parallel to the bc plane by C—H⋯O hydrogen bonds.
Related literature
For related structures, see: Hong (2007 ▶); Kamenar et al. (1990 ▶); Li et al. (2005 ▶, 2007 ▶); Zhou et al. (2004 ▶).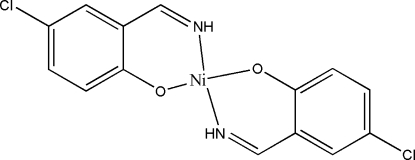
Experimental
Crystal data
[Ni(C7H5ClNO)2]
M r = 367.85
Monoclinic,

a = 15.775 (6) Å
b = 5.685 (2) Å
c = 7.894 (3) Å
β = 93.864 (18)°
V = 706.3 (5) Å3
Z = 2
Mo Kα radiation
μ = 1.76 mm−1
T = 298 (2) K
0.18 × 0.17 × 0.17 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.743, T max = 0.755
4102 measured reflections
1532 independent reflections
1296 reflections with I > 2σ(I)
R int = 0.029
Refinement
R[F 2 > 2σ(F 2)] = 0.030
wR(F 2) = 0.084
S = 1.04
1532 reflections
100 parameters
1 restraint
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.45 e Å−3
Δρmin = −0.30 e Å−3
Data collection: SMART (Bruker, 2002 ▶); cell refinement: SAINT (Bruker, 2002 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809004279/ci2768sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809004279/ci2768Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C7—H7⋯O1i | 0.93 | 2.54 | 3.318 (3) | 142 |
Symmetry code: (i)  .
.
Acknowledgments
The author acknowledges Liaodong University for funding this study.
supplementary crystallographic information
Comment
Recently, the author has reported the crystal structure of a Schiff base nickel(II) complex (Hong, 2007). As an extension of the work on the structural investigation of nickel(II) complexes, the title complex is reported here.
The title compound is a centrosymmetric mononuclear nickel(II) complex (Fig. 1), which is similar to those reported previously (Kamenar et al., 1990). The Ni atom, lying on the inversion center, is four-coordinated by two O and two imine N atoms from two 4-chloro-2-iminomethylphenol ligands, forming a square-planar geometry. The bond lengths (Table 1) related to the metal centre are comparable to the values in similar nickel(II) complexes (Zhou et al., 2004; Li et al., 2005; Li et al., 2007).
In the crystal structure, the molecules are linked into a two-dimensional network parallel to the bc plane by C—H···O hydrogen bonds (Table 1).
Experimental
5-Chlorosalicylaldehyde (1.0 mmol, 156.6 mg) and Ni(CH3COO)2.4H2O (0.5 mmol, 125.0 mg) were dissolved in methanol solution containing a small quantity of ammonia (30 ml). The mixture was stirred at room temperature for 30 min to give a clear brown solution. After keeping the solution in air for a few days, brown block-shaped crystals were formed.
Refinement
Atom H1 was located from a difference Fourier map and refined isotropically, with the N1–H1 distance restrained to 0.90 (1) Å. Other H atoms were placed in idealized positions and constrained to ride on their parent atoms with C–H distances of 0.93 Å, and with Uiso(H) set to 1.2Ueq(C).
Figures
Fig. 1.
The molecular structure of the title compound, showing 30% probability displacement ellipsoids and the atom-numbering scheme. Unlabelled atoms are related to labelled atoms by the symmetry operation (1 - x, 1 - y, - z).
Crystal data
| [Ni(C7H5ClNO)2] | F(000) = 372 |
| Mr = 367.85 | Dx = 1.730 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 1954 reflections |
| a = 15.775 (6) Å | θ = 2.8–27.6° |
| b = 5.685 (2) Å | µ = 1.76 mm−1 |
| c = 7.894 (3) Å | T = 298 K |
| β = 93.864 (18)° | Block, brown |
| V = 706.3 (5) Å3 | 0.18 × 0.17 × 0.17 mm |
| Z = 2 |
Data collection
| Bruker SMART CCD area-detector diffractometer | 1532 independent reflections |
| Radiation source: fine-focus sealed tube | 1296 reflections with I > 2σ(I) |
| graphite | Rint = 0.029 |
| ω scans | θmax = 27.0°, θmin = 2.6° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −20→18 |
| Tmin = 0.743, Tmax = 0.755 | k = −7→7 |
| 4102 measured reflections | l = −10→9 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.030 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.084 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.0495P)2 + 0.0905P] where P = (Fo2 + 2Fc2)/3 |
| 1532 reflections | (Δ/σ)max = 0.001 |
| 100 parameters | Δρmax = 0.45 e Å−3 |
| 1 restraint | Δρmin = −0.30 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Ni1 | 0.5000 | 0.5000 | 0.0000 | 0.03469 (15) | |
| Cl1 | 0.06466 (4) | 0.34882 (15) | 0.19374 (11) | 0.0778 (3) | |
| N1 | 0.45858 (11) | 0.2416 (3) | 0.1094 (2) | 0.0421 (4) | |
| O1 | 0.39683 (9) | 0.6527 (2) | −0.01584 (18) | 0.0411 (3) | |
| C1 | 0.31247 (13) | 0.3565 (3) | 0.1140 (3) | 0.0374 (4) | |
| C2 | 0.32363 (13) | 0.5745 (4) | 0.0315 (3) | 0.0369 (4) | |
| C3 | 0.25045 (14) | 0.7137 (4) | −0.0045 (3) | 0.0445 (5) | |
| H3 | 0.2554 | 0.8554 | −0.0619 | 0.053* | |
| C4 | 0.17234 (15) | 0.6450 (4) | 0.0433 (3) | 0.0501 (6) | |
| H4 | 0.1252 | 0.7403 | 0.0189 | 0.060* | |
| C5 | 0.16322 (14) | 0.4331 (5) | 0.1283 (3) | 0.0493 (6) | |
| C6 | 0.23191 (14) | 0.2906 (4) | 0.1628 (3) | 0.0439 (5) | |
| H6 | 0.2252 | 0.1487 | 0.2190 | 0.053* | |
| C7 | 0.38261 (14) | 0.1990 (4) | 0.1480 (3) | 0.0412 (5) | |
| H7 | 0.3722 | 0.0571 | 0.2015 | 0.049* | |
| H1 | 0.4993 (14) | 0.133 (4) | 0.131 (4) | 0.080* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ni1 | 0.0430 (2) | 0.0274 (2) | 0.0335 (2) | 0.00107 (14) | 0.00128 (15) | 0.00308 (13) |
| Cl1 | 0.0488 (4) | 0.0872 (6) | 0.0994 (6) | −0.0016 (3) | 0.0197 (3) | 0.0169 (4) |
| N1 | 0.0470 (10) | 0.0325 (9) | 0.0465 (10) | 0.0054 (7) | 0.0023 (8) | 0.0076 (7) |
| O1 | 0.0437 (8) | 0.0314 (7) | 0.0483 (9) | 0.0025 (6) | 0.0049 (6) | 0.0063 (6) |
| C1 | 0.0467 (11) | 0.0305 (10) | 0.0348 (10) | −0.0012 (8) | 0.0020 (8) | −0.0010 (8) |
| C2 | 0.0447 (11) | 0.0312 (9) | 0.0345 (10) | 0.0018 (8) | 0.0017 (8) | −0.0021 (8) |
| C3 | 0.0510 (12) | 0.0351 (11) | 0.0469 (13) | 0.0042 (9) | 0.0004 (10) | 0.0025 (9) |
| C4 | 0.0470 (12) | 0.0463 (14) | 0.0565 (14) | 0.0086 (10) | −0.0006 (10) | −0.0021 (10) |
| C5 | 0.0454 (13) | 0.0497 (13) | 0.0530 (14) | −0.0026 (10) | 0.0052 (10) | −0.0009 (11) |
| C6 | 0.0517 (12) | 0.0379 (11) | 0.0424 (12) | −0.0045 (10) | 0.0060 (9) | 0.0008 (9) |
| C7 | 0.0513 (12) | 0.0316 (10) | 0.0406 (11) | −0.0007 (9) | 0.0037 (9) | 0.0066 (8) |
Geometric parameters (Å, °)
| Ni1—O1i | 1.8414 (15) | C1—C7 | 1.434 (3) |
| Ni1—O1 | 1.8414 (15) | C2—C3 | 1.412 (3) |
| Ni1—N1i | 1.8455 (18) | C3—C4 | 1.370 (3) |
| Ni1—N1 | 1.8455 (18) | C3—H3 | 0.93 |
| Cl1—C5 | 1.738 (2) | C4—C5 | 1.391 (4) |
| N1—C7 | 1.280 (3) | C4—H4 | 0.93 |
| N1—H1 | 0.897 (10) | C5—C6 | 1.365 (3) |
| O1—C2 | 1.315 (2) | C6—H6 | 0.93 |
| C1—C6 | 1.403 (3) | C7—H7 | 0.93 |
| C1—C2 | 1.417 (3) | ||
| O1i—Ni1—O1 | 180.00 (4) | C4—C3—C2 | 121.5 (2) |
| O1i—Ni1—N1i | 93.89 (7) | C4—C3—H3 | 119.2 |
| O1—Ni1—N1i | 86.11 (7) | C2—C3—H3 | 119.2 |
| O1i—Ni1—N1 | 86.11 (7) | C3—C4—C5 | 120.3 (2) |
| O1—Ni1—N1 | 93.89 (7) | C3—C4—H4 | 119.9 |
| N1i—Ni1—N1 | 180.00 (10) | C5—C4—H4 | 119.9 |
| C7—N1—Ni1 | 128.97 (15) | C6—C5—C4 | 120.2 (2) |
| C7—N1—H1 | 120 (2) | C6—C5—Cl1 | 119.4 (2) |
| Ni1—N1—H1 | 111 (2) | C4—C5—Cl1 | 120.37 (19) |
| C2—O1—Ni1 | 127.63 (13) | C5—C6—C1 | 120.6 (2) |
| C6—C1—C2 | 120.15 (18) | C5—C6—H6 | 119.7 |
| C6—C1—C7 | 118.94 (19) | C1—C6—H6 | 119.7 |
| C2—C1—C7 | 120.91 (19) | N1—C7—C1 | 124.10 (19) |
| O1—C2—C3 | 118.37 (19) | N1—C7—H7 | 118.0 |
| O1—C2—C1 | 124.41 (18) | C1—C7—H7 | 118.0 |
| C3—C2—C1 | 117.21 (19) |
Symmetry codes: (i) −x+1, −y+1, −z.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C7—H7···O1ii | 0.93 | 2.54 | 3.318 (3) | 142 |
Symmetry codes: (ii) x, −y+1/2, z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: CI2768).
References
- Bruker (2002). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Hong, Z. (2007). Acta Cryst. E63, m2026.
- Kamenar, B., Kaitner, B., Ferguson, G. & Waters, T. N. (1990). Acta Cryst. C46, 1920–1923.
- Li, J.-M., Jiang, Y.-M., Li, C.-Z. & Zhang, S.-H. (2007). Acta Cryst. E63, m447–m449.
- Li, J.-M., Jiang, Y.-M., Wang, Y.-F. & Liang, D.-W. (2005). Acta Cryst. E61, m2160–m2162.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Zhou, J., Chen, Z.-F., Wang, X.-W., Tan, Y.-S., Liang, H. & Zhang, Y. (2004). Acta Cryst. E60, m568–m570.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809004279/ci2768sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809004279/ci2768Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



