Summary
Numerous autoimmune and inflammatory disorders stem from the dysregulation of hematopoietic cell activation. The activity of inositol lipid and protein tyrosine phosphatases, and the receptors that recruit them, is critical for prevention of these disorders. Balanced signaling by inhibitory and activating receptors is now recognized to be an important factor in tuning cell function and inflammatory potential. In this review, we provide an overview of current knowledge of membrane proximal events in signaling by inhibitory/regulatory receptors focusing on structural and functional characteristics of receptors and their effectors Src homology 2 (SH2) domain-containing tyrosine phosphatase 1 and SH2 domain-containing inositol 5-phosphatase-1. We review use of new strategies to identify novel regulatory receptors and effectors. Finally, we discuss complementary actions of paired inhibitory and activating receptors, using FcγRIIA and FcγRIIB regulation human basophil activation as a prototype.
Keywords: phosphatases, SHIP-1, SHP-1, Fc Receptors, paired receptors, signal transduction
Introduction
The balanced expression and function of activating and inhibitory receptors plays a key role in the regulation of immune cell activation. FcγRIIB, the inhibitory receptor for immunoglobulin G (IgG) constant regions, influences the development of immune responses, as well as allergic and autoimmune disease (1, 2). This receptor is expressed on B cells, dendritic cells, monocytes, mast cells, and basophils. Its action balances that of activating Fc and antigen receptors and other receptors whose signaling function requires phosphatidylinositol 3,4,5P3 (PtdIns3,4,5P3). From studies using knockout mice, we have learned that the absence of either FcγRIIB or its principal downstream effector, the Src homology 2 (SH2) domain-containing inositol 5-phosphatase-1 (SHIP-1), results in autoimmunity, dysregulation of B cells, enhanced mast cell degranulation, and increased IgG- and IgE-mediated systemic anaphylaxis (3–5). In humans, polymorphisms in FcγRIIB have been associated with systemic lupus erythematosus (6). In this review, we discuss recent advances in understanding the immunoregulatory functions of FcγRIIB and its effectors.
The FcγRIIB interface with cytoplasmic effectors
The basis of FcγRIIB propagation of inhibitory signals was unknown until the mid-1990s. Pivotal to understanding this process were the findings of Amigorena et al. (7) who demonstrated that a 13 amino acid sequence in the cytoplasmic tail was necessary for FcγRIIB inhibition of antigen receptor-mediated B-cell activation. Muta et al. (8) then demonstrated sufficiency, showing that this sequence could mediate inhibition when expressed in an inert molecular context. Finally, these authors showed that phosphorylation of a tyrosine within this sequence is required for its function. In 1995, the phosphorylated form of the 13 amino acid ‘inhibitor’ sequence [EAENTIT(p)YSLLKH] from the cytoplasmic tail of FcγRIIB was used to isolate likely effectors; three proteins of 150, 70, and 65 kDa were found (9, 10). The 65 and 70 kDa proteins were identified as the SH2-containing phosphotyrosine phosphatases (PTPs) SHP-1 (SH2 domain-containing phosphatase-1) and SHP-2, and the third more minor species was identified as SHIP-1 (Fig. 1). These experiments were the first to implicate phosphatases as the primary effectors of inhibitory receptor signaling. Subsequent studies using the full-length cytoplasmic tail of FcγRIIB demonstrated that SHIP-1 bound with greater affinity than either SHP-1 or SHP-2 (11–15).
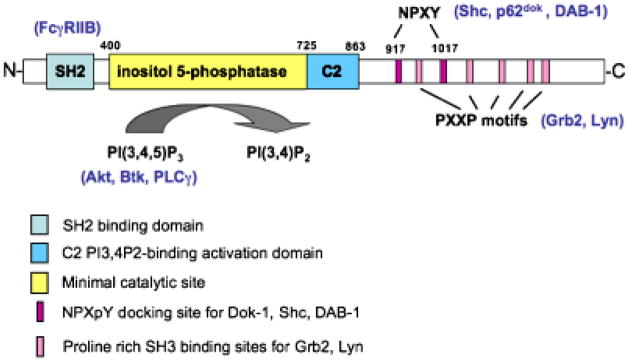
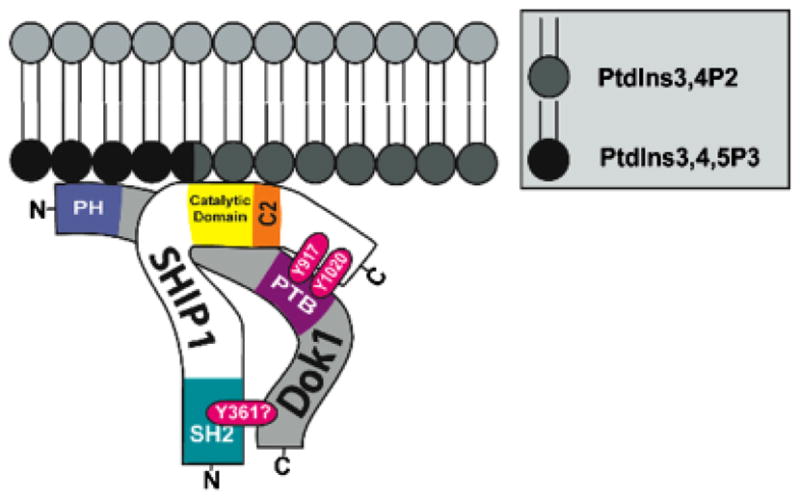
Fig. 1. Structure of SHIP-1.
The multiple functional domains of SHIP-1 include an N-terminal SH2 domain, which has been shown to interact with FcγRIIB. Immediately C-terminal of the catalytic domain is a newly recognized C2 allosteric activation domain. When bound by PtdIns3,4P2, this region was found to increase the enzyme activity of SHIP-1. The C-terminal domain NPXY motifs (917 and 1017) interact with phosphotyrosine binding domain (PTB) of Shc, p62dok, and DAB-1. The proline-rich PXXP motifs have been shown to interact with Grb2.
These findings triggered a lengthy debate as to the relative importance of tyrosine versus lipid phosphatases in mediating FcγRIIB function. In the 1995 study by D’Ambrosio et al. (9), SHP-1 hypomorphic motheaten mice (mev/mev) were employed to conclude that tyrosine phosphatase is required for inhibition of the proliferative response. In 1996, Ono et al. (15) used SHIP-1 knockout mice to implicate the lipid phosphatase in inhibition of calcium signaling. Some closure was achieved when a second phosphotyrosine in the cytoplasmic tail of FcγRIIB (Y327) was shown to be a docking site for the SH2 domain of adapter protein Grb2. Grb2 forms a complex with SHIP-1 via an SH3 domain interaction with a proline-rich sequence in SHIP-1 (16, 17). Upon FcγRIIB biphosphorylation, a tripartite structure is formed in which the SHIP-1 SH2 domain binds pY309, and SHIP-1-associated Grb2 binds pY327 (17) (Fig. 2). The earlier studies of D’Ambrosio (9) employed the isolated inhibitory 13 amino acid peptide and a C-terminally truncated FcγRIIB that, while containing Y309, was missing Y327. Thus, while the full-length receptor preferentially engages SHIP-1, the receptor used by D’Ambrosio preferentially engaged SHP-1.
Fig. 2. Proposed progressive activation of SHIP-1 resulting in formation of anMPSC.

(A) Lyn is associated with a portion of resting BCR. (B) Co-aggregation of the BCR with FcγRIIB by antigen/IgG complexes results in phosphorylation of the ITIM consensus sequence Y(319)SSL. (C) The pITIM binds the SHIP-1 SH2 domain, and the Y327 terminal phosphotyrosine recruits Grb2 to form a bidentate interaction between SHIP1/Grb-2/FcγRIIB. (D) Lyn phosphorylates the tyrosine in the NPXY motif, creating a docking site for the adapter molecule p62dok (E). (F) Lyn phosphorylates the ITIM on Dok-1 (Y361DEP), creating a ‘preferred’ SH2 binding site for the N-terminal SH2 domain of SHIP-1. (G) This interaction results in release of SHIP-1 from FcγRIIB, and results in (H), an mobile PtdIns3,4,5P3 scavenger complex (MPSC), which is now free to migrate to remote membrane sites where it inhibits lipid hydrolysis.
Differential actions of SHIP-1 versus SHP-1
The inositol (SHIP-1 and SHIP-2) and tyrosine phosphatases (SHP-1 and SHP-2) bind phosphorylated FcγRIIB immunoreceptor tyrosine-based inhibitory motif (ITIM) consensus sequences via their SH2 domains. SHIP-1 dephosphorylates the 5′-phosphate in phosphatidylinositides (18, 19), hydrolyzing PtdIns3,4,5P3 that is produced upon antigen receptor stimulation. PtdIns3,4,5P3 is required for membrane translocation by a number of critical effectors, e.g. Bruton’s tyrosine kinase (Btk), and perhaps phospholipase C-γ, Vav, and SOS. SHIP-1 also mediates inhibitory signaling via its adapter molecule Dok-1, which binds p21 Ras guanosine trisphosphatase activating protein (RasGAP), a negative regulator of Ras signaling (20).
SHP-1 functions as a negative regulator of signaling events by dephosphorylating a broad range of tyrosine-phosphorylated signaling effectors. The lack of functional SHP-1 in the autosomal recessive motheaten mouse strain results in chronic inflammation and systemic autoimmunity (21). SHP-1 mediates signaling through a wide variety of receptors including C-Kit, granulocyte-macrophage colony-stimulating factor, killer immunoglobulin receptor (KIR), B-cell and T-cell antigen receptor, CD5, CD72, and CD22 (reviewed in 22). In B cells, this cytoplasmic tyrosine phosphatase targets substrates including the B-cell receptor (BCR) Igα–Igβ subunits, Syk, and SH2 domain-containing lymphocyte phosphoprotein of 65 kDa (SLP-65) (also known as B-cell linker protein) (23–26). As will be discussed in more detail below, SHP-1 recruitment to inhibitory receptors leads to only local inhibition of signaling, i.e. it dephosphorylates tyrosines only on proteins brought together by co-aggregation of receptors.
Structure and functions of SHIP-1
The SHIP family of inositol phosphatases includes SHIP-1, expressed primarily in hematopoietic cells, and SHIP-2 that is found ubiquitously (27). In most hematopoietic cells, SHIP-1 is expressed in much higher levels than SHIP-2, but levels of these enzymes are dynamically regulated during cell activation (28–31). SHIP-1, the focus here, contains multiple functional domains, conferring diverse actions (Fig. 1). The first 100 residues of the N-terminal domain contain an SH2 domain that mediates pITIM binding. The following 300 amino acid segment, while predicted to be highly structured, has no known function. The minimal catalytic site of the inositol 5′-phosphatase domain falls between amino acids 400 and 866. This region confers phosphatase activity against PtdIns3,4,5P3 and inositol 1,3,4,5-tetrakisphosphate (Ins1,3,4,5P4). It appears that the C-terminal region of this previously defined ‘minimal’ catalytic phosphatase domain is not required for hydrolysis of PtdIns3,4,5P3 but rather is a lipid-binding C2 domain, which may serve as a target site for allosteric enhancement of SHIP-1 activity (32). Using a SHIP-1 construct lacking this C2 domain, which spans amino acids 725 to 863, Ong et al. (32) determined that this region serves as a site for allosteric activation of SHIP-1. Addition of PtdIns3,4P2, the product of SHIP-1’s hydrolysis of PtdIns3,4,5P3, or a small-molecule agonist enhanced SHIP-1’s enzymatic activity, but only if the C2 domain was present (32). Removal of the C2 domain has no qualitative effect on the ability of the construct to hydrolyze PtdIns3,4,5P3 into PtdIns3,4P2. The authors suggested that binding of PtdIns3,4P2 leads to a conformational change, enhancing SHIP-1 activity. C-terminal from the catalytic domain of SHIP-1 lies an extended sequence containing multiple motifs involved in protein–protein interaction. These include two NPXY motifs (N914 and N1017), which, when phosphorylated, serve as docking sites for phosphotyrosine-binding domains (PTB) in Dok-1, Shc, and DAB-1 (18, 20, 33, authors’ unpublished data). This segment also contains proline-rich regions that bind SH3 domain-containing proteins such as Grb-2 (Fig. 1).
We hypothesize that FcγRIIB signaling is mediated by the processive activation of SHIP-1 via a mechanism that involves the multiple protein–protein interactions noted above (Fig. 2). In mice, co-aggregation of the BCR or FcεR1 with FcγRIIB results in Lyn-mediated phosphorylation of Y309, the tyrosine within the ITIM of FcγRIIB, as well as the more C-terminal Y327. The pITIM binds SHIP-1, and the C-terminal phosphotyrosine (Y327) recruits Grb-2 to form a tripartate complex (17). Lyn then phosphorylates the NPXY tyrosine(s) of the sequestered SHIP-1, generating a binding site for the Dok-1 PTB domain. Sequestration of Dok-1 at the FcγRIIB/SHIP-1 complex facilitates phosphorylation of its C-terminal tyrosines by Lyn. Among these tyrosines is a consensus-binding site (YXLP) for the SHIP-1 SH2 domain. This phosphorylation therefore creates a circumstance in which FcγRIIB pY309 and the Dok-1 pY(XLP) compete for the SHIP-1 SH2 domain. It seems likely that formation of a bidentate SHIP-1/Dok-1 complex would be favored energetically and would result in the displacement of SHIP-1 from the ITIM of FcγRIIB. This would be consistent with the relative ease of co-immunoprecipitating SHIP-1/Dok-1 complexes compared with SHIP-1/FcγRIIB complexes following BCR/FcγRIIB co-aggregation (34). The now mobile heterodimer of SHIP-1 and Dok-1, which we refer to as the ‘mobile PtdIns3,4,5P3 scavenger complex’ (MPSC), is free to engage in other interactions and to hydrolyze its substrates. We speculate that this complex may be localized to membrane regions enriched in its substrate (PtdIns3,4,5P3) by virtue of the demonstrated binding specificity of the Dok-1 pleckstrin homology (PH) domain for PtdIns3,4,5P3 (35) (Fig. 3). However, the C2 domain of SHIP-1, described above as an allosteric enhancement site for SHIP-1 activity, binds PtdIns3,4P2 (32) and may also function in a focusing capacity (36).
Fig. 3. MPSC interaction with the plasma membrane.
The Dok-1 PH domain docks to PtdIns3,4,5P3 stabilizing SHIP-1/Dok-1 interaction with the plasma membrane. Recent work has shown the C2 domain of SHIP-1 interacts with PtdIns3,4P2, resulting in allosteric activation of SHIP-1. The conversion of PtdIns3,4,5P3 to PtdIns3,4P2 prevents further activation of Tec family kinases. In addition, PtdIns3,4P2 is now available to interact with TAPP1, the outcome of which remains uncertain.
Hydrolysis of PtdIns3,4,5P3 may affect downstream responses to antigen by at least two mechanisms. PtdIns3,4,5P3 is required for translocation of Tec kinases (Itk, Btk) and Akt to the plasma membrane, leading to their participation in receptor signaling (37–42). PtdIns3,4,5P3 accumulation at the cell membrane during BCR signaling is immediate and transient (43, 44). A second effect of SHIP-1 is mediated by generation of PtdIns3,4P2. This lipid may enhance SHIP-1 activity but also presumably ‘activates’ its binding partners TAPP1 and TAPP2 (45, 46). An additional function of the SHIP-1/Dok-1 complex involves phosphorylated Dok-1 interaction with RasGAP. RasGAP binds to Dok-1 via phosphotyrosines Y(295)AEP and Y(362)DEP (47, 48). Dok-1 activation of RasGAP, an inhibitor of Ras, may underlie the reported FcγRIIB inhibition of Ras activation (49).
SHIP-1 has been implicated as the mediator of many inhibitory functions within FcγRIIB-containing signaling complexes. It is required for inhibition of antigen receptor-mediated calcium signaling (15), CD86 upregulation (50), and proliferation in B cells (51–53). It is likely involved in FcγRIIB inhibition of dendritic cell maturation (54). In mast cells, co-aggregation with FcεRI leads to inhibition of degranulation and cytokine production (55).
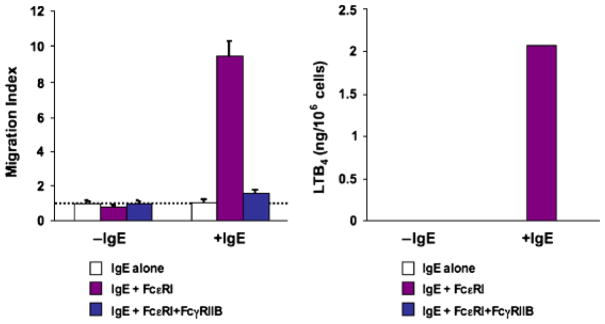
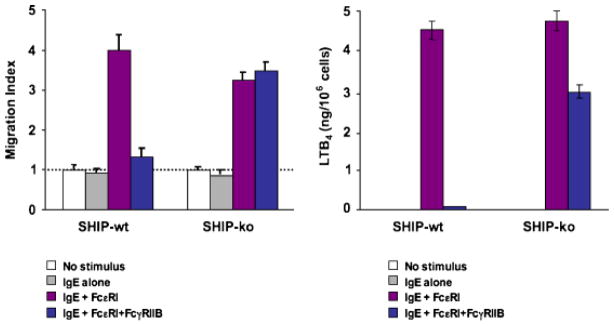
We recently extended these observations by exploring FcγRIIB inhibition of FcεRI-mediated leukotriene biosynthesis. Murine bone marrow-derived mast cells from wildtype and SHIP-1 knockout mice were sensitized with IgE, washed, and stimulated with either rabbit F(ab′)2 anti-mouse Ig antibodies resulting in cross-linking of FcεR1, or with intact antibodies, resulting in co-aggregation of FcεR1 with FcγRIIB. Cells were placed in the lower chamber of a transwell, and the effect of the stimuli on T-cell chemotaxis from the upper chamber was assessed. Using this system, we had previously shown that FcεRI signaling induces T-effector cell chemotaxis via production of LTB4 (56). Here we showed that co-aggregation of FcγRIIB with FcεR1 inhibits LTB4 production and T0 migration (Fig. 4). Finally, this inhibition is dependent on SHIP-1 (Fig. 5).
Fig. 4. Co-aggregation of FcγRIIB with FcεR1 inhibits LTB4 production and T-cell migration.
Murine bone marrow-derived mast cells from wildtype mice were sensitized with IgE, washed and stimulated with either rabbit F(ab′)2 anti-mouse immunoglobulin antibodies, resulting in aggregation of FcεR1 alone, or with intact antibodies, resulting in co-aggregation of FcεR1 with FcγRIIB. Co-aggregation of IgE with FcγRIIB resulted in inhibition of migration (A) LTB4 production (B).
Fig. 5. SHIP-1 is required for FcγRIIB-mediated inhibition of FcεRI-induced LTB4 production.
Bone marrow-derived mast cells from wildtype and SHIP-1 knockout mice were sensitized with IgE, washed, and stimulated with either rabbit F(ab′)2 anti-mouse immunoglobulin antibodies resulting in cross-linking of FcεR1, or with intact antibodies, resulting in co-aggregation of FcεR1 with FcγRIIB. In the absence of SHIP-1, co-aggregation of IgE with FcγRIIB did not inhibit migration (A) or LTB4 production (B).
The formation and function of the MPSC may explain the unique ability of SHIP-1 to inhibit signaling by remotely stimulated receptors whose function requires PtdIns3,4,5P3. Studies conducted by Vely et al. (57) nearly a decade ago demonstrated that while inhibitory receptors that utilize SHP-1 as their primary effector (e.g. KIRs) are only able to inhibit signaling by co-aggregated receptors, those that utilize SHIP-1 are able to inhibit signaling by remotely stimulated receptors. Extending these observations to a physiologic setting, we showed the activation of FcγRIIB signaling in immature B cells led to inhibition of CXCL12-induced calcium mobilization and migration (58). We further showed that this trans inhibition was dependent upon SHIP-1, as it was not seen in B cells from SHIP-1−/− animals. In an extension of these studies, we showed that chronic BCR signaling that also activates the SHIP-1/Dok-1 circuit has the same effect. Consistent with a role for SHIP-1 in inhibition, signaling through CXCR4 by CXCL12 is dependent on PtdIns3,4,5P3 (58). Thus, we hypothesize that the MPSC is able to translocate to distal regions of the plasma membrane and be retained at sites rich in PtdIns3,4,5P3 and PtdInsP3,4P2. The consequence of this translocation is inhibition of signaling by remotely stimulated receptors. This is a distinguishing factor between SHIP-1 and SHP-1: SHIP-1 can function in trans, whereas SHP-1 acts only locally, i.e. on receptors within complexes with which it is directly associated (59).
Structure and function of SHP-1
Four isoforms of SHP-1 have been identified (60). For the purpose of this review, we focus on the hematopoietic cell-predominant, full-length isoform, which contains two SH2 domains, each of which may recognize distinct ITIM sequences, a tyrosine phosphatase catalytic domain, and a C-terminal tail (Fig. 6). SHP-2, which is similar in structure to SHP-1, appears to function primarily as a positive mediator of signaling (61–63). Because of the sequence homology between SHP-1 and SHP-2, resolution of the crystal structure of SHP-2 was informative regarding how the N-terminal SH2 domain regulates activity of the catalytic PTP domain (64, 65). In the cytosol, when SHP-2 is in its inactive state, the N-terminal SH2 domain interacts extensively with the residues of the catalytic domain, directly blocking the phosphatase catalytic site. Regulation of SHP-1 was shown to be similarly influenced by the N-terminal SH2 domain (66, 67). The intramolecular association of the NXGDY/F motif of the SH2 domain with the catalytic cleft of the protein tyrosine phosphatase domain is both necessary and sufficient for auto-inhibition (60, 67). Regulation of SHP-1 by this steric mechanism could explain why it only functions locally; it only acts when tethered to pITIMs. Whereas the N-terminal SH2 domain serves in a capacity to regulate enzymatic activity, biochemical studies by Bruhns et al. indicated that both N- and C-terminal SH2 domains of SHP-1 must bind phosphorylated ITIMs for the catalytic domain to achieve maximal activation (60, 68, 69).
Fig. 6. SHP-1 structure.
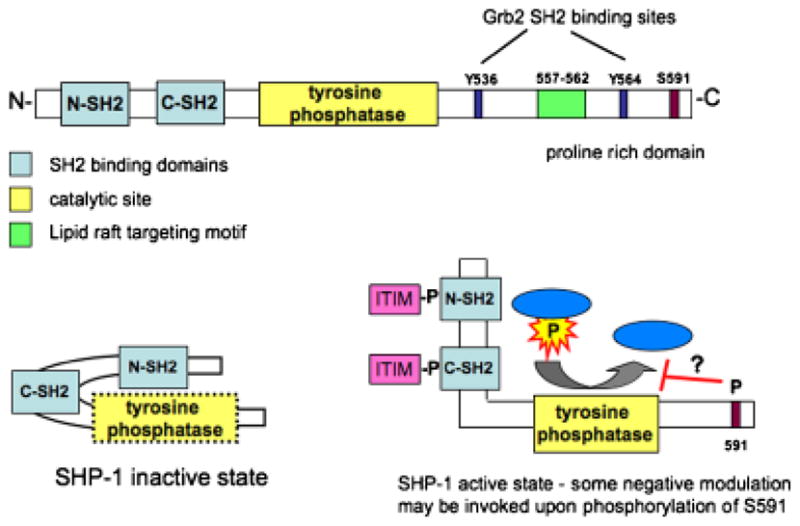
(A) SHP-1 is composed of two SH2 domains, which are proposed to have distinct specificities, followed by the catalytic site. The C-terminal tail contains Grb2 SH2 binding sites (Y536 and Y564), a newly identified lipid raft targeting motif, and a C-terminal serine, which when phosphorylated is thought to inhibit SHP-1 function. (B) In a resting state, the N-terminal SH2 domain interacts with the tyrosine phosphatase domain, preventing enzyme activity. Interaction of the two SH2 domains with pITIMs on adjacent proteins results in exposure and resulting activation of the catalytic domain.
The C-terminal region of SHP-1 is highly divergent from that of SHP-2 and likely confers the specificity of its actions. The C-terminal portion of SHP-1 contains functional domains/motifs that include two tyrosines at 536 and 534 that have been proposed to serve an adapter function by recruitment of Grb-2 (70, 71). The catalytic activity of SHP-1 is inhibited to some extent by phosphorylation of its serine (S591) (60, 72, 73). Recently, Sankarshanan et al. (74) identified a 6 amino acid sequence (557–562) in the C-terminal tail as a lipid raft targeting motif. Mutational analysis revealed that this sequence is sufficient to target lipid rafts and that its absence leads to loss of SHP-1 inhibition of TCR-mediated signaling (74).
What determines alternate pITIM binding to SH2 domains by SHIP-1 and SHP-1?
Inhibitory receptors fall into categories with respect to the effectors they utilize to mediate their function. Alternate use of inositol and tyrosine phosphatases is dictated by pITIM preference of the SH2 domains of these effectors. The basis of this specificity has been addressed using three approaches: correlation of sequence and SH2 binding of different receptors, ITIM mutational analysis, and binding studies of SH2 using peptide libraries. Early mutational studies demonstrated the hydrophobic residue at the Y-2 position of the ITIM motif is important for SHP-1 binding but not SHIP-1 binding (57, 75). These findings were supported by analyses of binding specificity of known inhibitory receptors (76, 77).
In order to identify a unique ‘motif zip code’ for each SH2 domain, Cantley and colleagues (76, 78) used pY peptide libraries to determine sequences surrounding the phosphotyrosines preferred by specific SH2 domains. Using this work as a basis, Yaffe and Cantley (79, 80) developed a web-based peptide library-based algorithm to identify sequence motifs likely to bind specific protein domains (http://scansite.mit.edu). Using a modified technique, Sweeney and colleagues (81–83) identified sequence specificity for SHP-1 and SHIP-1 SH2 domains. They identified the consensus binding sequences for the SHP-1 N-SH2 to be LXpY(M/F)X(F/M) and for C-SH2 to be (T/v/i)XpY(A/t)X(L/m/v) where X is any amino acid, and lower case letters represent less frequently selected residues. SHIP-1, which contains a single SH2 domain, bound peptides with the consensus sequence of pY(Y/S/T/v)(L/y/f)(L/i/v). When dissociation constants of the selected peptide sequences were determined, several peptides associated with the SH2 domains of SHIP-1, SHP-1, and SHP-2 with a similar affinity (81). The peptide NNITpYSLLMHP had dissociation constants of 2.1 ± 0.1 and 2.2 ± 0.6 μM from SHP-1 N-SH2 and C-SH2 domains, respectively (82). This sequence resembles the SHIP-1-preferring pITIM of FcγRIIB in humans (NTITpYSLLMPH) and mice (NTITpYSLLKHP) and further supports SHP-1 binding to the ITIM of FcγRIIB. Overlapping specificities of the SH2 domains of various effector molecules allows for the contribution of other factors in determining in vivo binding specificities. These peptide library-binding studies have corroborated past mutational and correlative studies and have provided information suggestive of additional binding constraints. Although these resources may allow identification of new candidate ITIMs and thus new inhibitory receptors, it is important to note that other properties of proteins, such as their interaction to form a complex, contribute to binding with effectors. Table 1 lists ITIM sequences of various inhibitory receptors and effectors that have been identified in vivo and in vitro. ITIM-containing receptors that have been shown to interact with SHIP-1 include FcγRIIB, myeloid-associated immunoglobulin-like receptor-1 (MAIR-1), also known as leukocyte mono-Ig-like receptor 1 (LMIR1) or CD300A, and MAFA, whose functional interaction with ITIM was demonstrated in rat RBL cells but whose significance in other species is uncertain due to lack of conservation across species. Much of the work done in determining consensus sequences has utilized murine SH2 domains. In most cases, ITIM-binding motifs have been conserved through evolution. However, occasionally, as in the case of PD-1, one or more amino acid residues in the motif are significantly different (VAYEEL, mouse; VDYGEL, human). The functional significance of these changes is unclear.
Table 1.
SHIP and SHP Binding
| Name |
Cell expression |
Ligand |
ITIM sequence (I/V/L/S-x-Y-xx-L/V) |
Effector* |
|---|---|---|---|---|
| FcγRIIB (CD32) | B, MC, Baso | Fc region of Ig | ITYSLL (hu, m) | SHIP-1 in vivo |
| SHP-1, SHP-2 in vitro | ||||
| MAFA | MC, NK | Cadherins, mannose binding | SIYSTL (rat only) | SHIP-1 in vivo |
| CD22 | B | CD45 | VSYAIL (m) | SHIP-1, SHP-1 |
| ISYTTL (hu) | ||||
| PECAM (CD31) | MC, Baso, PMN | αvβ3 | VEYTEV (m) | SHP-2 in vivo |
| Endothelial cell | PECAM | VQYTEV (hu) | SHP-1 in vitro | |
| Platelet, NK, T&B subsets | CD38 | TVYSEV (hu) | ||
| LIR-1/ILT2 | B, MC, Baso, Eos, PMN, NK | Classical and non-classical | NLYAAV (hu) | SHP-1 in vivo |
| MHC class I | ||||
| VTYAEV (hu) | ||||
| VTYAQL (hu) | ||||
| SIYATL (hu) | ||||
| LMIR-1/CD300A | MC, B, Eos, myeloid cells | unknown | VEYSTL (m) | SHIP-1, SHP-1, SHP-2 in vivo |
| MAIR-1 | VEYSTV (hu) | |||
| LHYSSV (m) | ||||
| LHYASV (hu) | ||||
| NKG2A | NK | Qa-1 | ITYAEL (hu) | SHP-1, SHP-2 |
| VTYAEL (m) | ||||
| PD-1 | T cells | PD-1L, PD-2L | VAYEEL (m) | SHP-2 |
| VDYGEL (hu) | ||||
| TEYATI (hu, m) | ||||
| Ly49/KIR | NK, T cell subsets | Class I | VTYSMV (m) | SHP-1 |
| LAIR-1 | hu mononuclear leukocytes | EpCAM | VTYIQL (m) | SHP-1 |
| VTYAQL (hu) | ||||
| Gp49B | MC, Mono, NK | αvβ3 | IVYAQV (m) | SHP-1, SHP-2 in vivo |
| VTYAQL (m) | SHIP-1 in vitro | |||
| PIR-B | B, MC, Mono, DC, granulocytes | HLA class I | SLYASV (m) | SHP-1, SHP-2 in vivo |
| VTYAQL (m) | ||||
| SVYATL (m) | ||||
| SIRP-α | Broad tissue expression | CD47/IAP | ITYADL (hu, m) | SHP-1, SHP-2 in vivo |
| LTYADL (hu, m) | ||||
| TEYASI (hu, m) | ||||
| SEYASV (hu, m) | ||||
| Siglec-3 (CD33) | Sialic acid residues | Sialic acid | LHYASL (hu) | SHP-1 in vivo |
| TEYSEV (hu) | ||||
| FcγRIIA | PMN, Mono, Baso, platelets | Fc region of Ig | IGYTLF (hu) | SHIP, SHP-1 in vivo |
| GGYMTL (hu) | ||||
| NIYLTL (hu) | ||||
| FcRH3 | B | unknown | VLYSEL (hu) | SHP-1, SHP-2 |
| VIYTEV (m) | ||||
| FcRL5 | B | unknown | VVYSEV (hu) | SHP-1 |
| IIYSEV (hu) | ||||
| VIYTEV (m) | ||||
| CD72 | B | CD5 | ITYADL (hu, m) | SHP-1 |
| LTYENV (m) | ||||
| ITYENV (hu) |
SHP-1, SHP-2, SHIP binding may not be confirmed for all sequences listed.
B, B cell; MC, mast cell; Baso, basophil; NK, NK cell; PMN, neutrophil; Mono, monocyte/macrophage; DC, dendritic cell; m, murine sequence; hu, human sequence.
Adding to this complexity, SHIP-1 has been shown to interact with both ITIMs and immunoreceptor tyrosine-based activation motifs (ITAMs). When this occurs, two opposing actions may result. First, the circumstances of SHIP-1 recruitment and activation may redirect signal transduction to alternate but not necessarily inhibitory pathways. As mentioned earlier, hydrolysis of PtdIns3,4,5P3 results in accumulation of PtdIns3,4P2. When PH domain binding of various downstream effectors was assessed by Manna et al. (42), TAPP1 showed clear binding preference to PtdIns3,4P2 over PtdIns3,4,5P3. Thus, recruitment and activation of TAPP1 to the plasma membrane following BCR ligation (84) would be altered considerably in the presence of SHIP-1, which increases PtdInsP3,4P2. Subsequent downstream events following TAPP1 activation have yet to be characterized.
Alternatively, SHIP-1 may be recruited directly to downregulate activation. Kimura et al. (85) demonstrated that following IgE receptor aggregation in RBL-2H3 cells, phosphorylation of SHIP-1 is an early event resulting from interaction with the ITAM of the β subunit of FcεR1. Here activation of SHIP-1 serves to inhibit signaling through FcεR1. A number of studies have demonstrated that SHIP-1 is phosphorylated efficiently after clustering of ITAM containing Fcγ receptors (86–88). Using a chimeric receptor containing the extracellular domain of CD8 and the ITAM-containing tail of the cytoplasmic tail of human restricted FcγRIIA, Nakamura et al. (88) were able to demonstrate that the ITAM of FcγRIIA is capable of binding SHIP-1; this binding requires tyrosine phosphorylation of at least one of the tyrosine residues in the ITAM (Y288 or Y304) of FcγRIIA. They proposed that two distinct outcomes result when SHIP-1 is activated. Paired ITIM/ITAM co-clustering, as occurs with BCR/FcγRIIB aggregation, results in potent inhibition that is able to completely block cell activation. Recruitment of SHIP-1 exclusively by ITAM-containing receptors induces inhibitory signaling to a lesser degree and is incapable of completely blocking activation.
Our own studies suggest that either aggregation of BCR or FcγRIIB/BCR co-aggregation leads to the formation of inhibitory complexes that contain SHIP-1. These complexes are capable of translocating to remote sites and thereby mediate global suppression of signaling through PtdIns3,4,5-P3-dependent receptors. In contrast, regulator tyrosine phosphatases, recruited by ITAM-containing receptors, act locally and mediate feedback regulation of the activating receptor.
What factors determine FcγRIIB usage of SHIP-1 versus SHP-1?
Multiple studies have shown that both SHIP-1 and SHP-1 bind the pITIM of murine FcγRIIB (89), but SHIP-1 has been established as the primary effector of FcγRIIB (9, 11–15). In the mouse, secondary receptor interactions with the SHIP-1-associated Grb-2 facilitate engagement of this effector pathway (Fig. 2C). Human FcγRIIB lacks the tyrosine that mediates Grb-2 binding. Therefore, activation of the SHP-1 pathway may have a greater role in human FcγRIIB signaling. Nonetheless, SHIP-1–Grb2–Dok-1 complexes have been co-immunoprecipitated following co-aggregation of FcγRIIB with FcεRI on human mast cells (90).
In vitro studies using beads coated with a low versus a high density of pITIMs indicate that higher order aggregation of FcγRIIB leads to biased activation of SHP-1 over SHIP-1 (69). Beginning from the premise that SHP-1 activation requires engagement of both of its SH2 domains, one might imagine that this requirement is satisfied when pITIMs from two FcγRIIB molecules are brought into close proximity by a highly polyvalent immune complex (Fig. 7B). Alternatively SHP-1 could interact with a single FcγRIIB pITIM and concurrently with a pITIM from a distinct juxtaposed receptor, such as FcγRIIA (Fig. 8). Finally, some studies indicate that it is only the N-terminal SHP-1 SH2 that is autoregulatory (67). Binding of the N-terminal SHP-1 SH2 domain to a single pITIM is sufficient for minimal phosphatase activity, but to achieve maximal activation, both SH2 domains must be engaged. This may explain how higher order FcγRIIB cross-linking favors SHP-1 activation relative to SHIP-1.
Fig. 7. Proposed SHIP-1 versus SHP-1 binding.
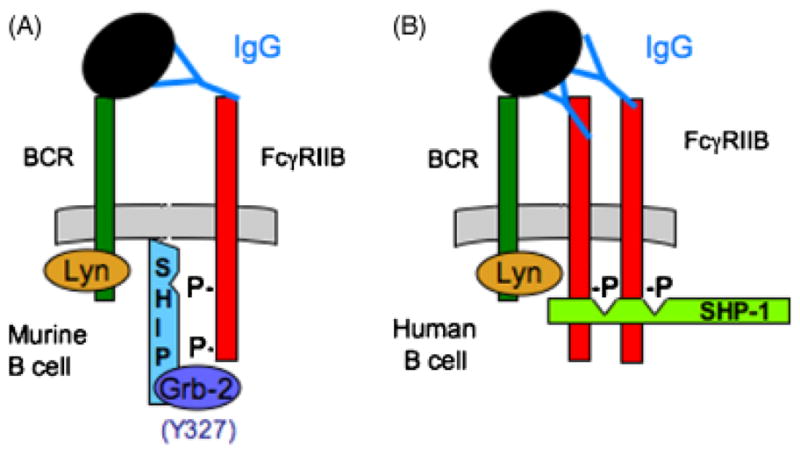
(A) In a murine B cell, tripartate interaction of FcγRIIB/Grb-2/SHIP-1 follows FcR and BCR co-aggregation. (B) In the human B cell, Y327 in the C-terminal tail of FcγRIIB is absent, possibly allowing for SHP-1 interaction. Because SHP-1 has two SH2 domains, higher order cross-linking of FcγRIIB may be required for this interaction to occur.
Fig. 8. Proposed SHP-1 binding in cell expressing both FcγRIIA and FcγRIIB.
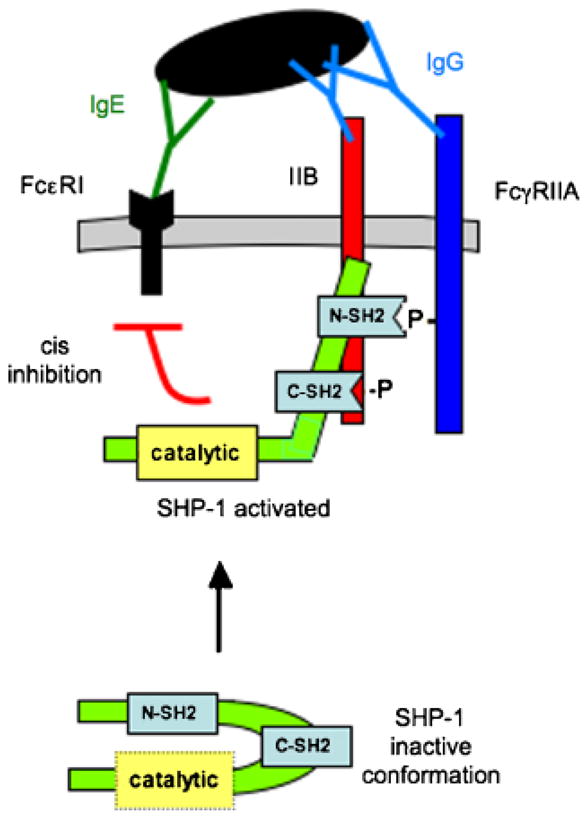
SHP-1 has two SH2 domains with distinct binding capabilities: one may bind the pITIM on FcγRIIB, the other the N-terminal pITAM of FcγRIIA (106). This interaction results in exposure and activation of the catalytic domain, which dephosphorylate tyrosines in cis.
The enigma of paired inhibitory and activating receptor expression: insight from the FcγRIIA/B paradigm
As work over the past decade has revealed increasing numbers of activating and inhibitory immunoreceptors, one of the striking findings has been that many of these receptors exist as closely homologous pairs. Most surprising is that these pairs are often co-expressed and seem to have the same specificity but have opposing activities. This relationship is typified by Fc receptors but is also an important feature in the function of KIRs, paired Ig-like receptors, signal-regulatory proteins, Ig-like transcripts, MAIRs, and leukocyte mono-Ig-like receptors (LMIRs) (91). While the purpose and thus evolutionary advantage of co-expressing receptors with equivalent specificity but opposing signaling function is unclear, some insight is provided by studies of FcγRIIA and B.
Archetypal of paired receptors are the human stimulatory and inhibitory receptors for IgG constant regions, FcγRIIA and FcγRIIB. Evidence at the clinical level demonstrates that the receptors have distinct and important functions. Polymorphisms in both FcγRIIA and FcγRIIB have been associated with lupus (1, 92). Further, their expression appears to be subject to independent acute regulation. For example, interferon-γ and C5a increase expression of activating FcγR and decrease expression of inhibitory FcγRs (93–95). On innate effector cells, transforming growth factor-β, interleukin-4 (IL-4), IL-10, and IL-13 increase FcγRIIB expression, and decrease activating FcγR (96–100). In contrast, IL-4 stimulation decreases FcγRIIB on activated B cells (101). Thus regulation is cell and stimulus specific. These studies and a recent review (102) provide supporting evidence that relative expression levels of activating and inhibitory FcγR tune cells for differential responses to immune complexes. Because these receptors share their ligand, it seems likely that they function concurrently to modulate cell activation.
Our own recent studies of ex vivo activation of human basophils support the concept of competing activating and inhibitory pathways. Both FcγRIIA and FcγRIIB are expressed by human basophils (Fig. 9). We found that these receptors must be co-engaged in order to achieve inhibition of FcεRI signaling in these cells (Fig. 10). Blocking antibodies against either FcγRIIA or FcγRIIB reversed the inhibitory effects of serum containing anti-allergen antibodies (P < 0.01). The mechanisms underlying the effect are not altogether clear. However, it would appear that IgG anti-allergen antibodies form immune complexes upon addition of antigen. These complexes or allergen alone (in the case of non-immune serum) bind to the basophils via anti-allergen IgE loaded on FcεRI. If IgG in the complex co-engages only the inhibitory FcγRIIB, inhibition is inefficient or absent. However, if the immune-complexed IgG binds both FcγRIIB and the ‘activating’ FcγRIIA, inhibition is robust. We hypothesize the following: because the number of FcεRI bound by IgE specific for the allergen is likely very small (due to competing IgE of other specificities) relative to the number of FcγRIIB available to bind IgG, FcγRIIB phosphorylation by FcεRI-activated SRC family kinases is likely to be inefficient. However, if FcγRIIA is also recruited into this aggregate, much more SRC family kinase activation will occur, leading to enhanced inhibition.
Fig. 9. Analysis of FcR expression by human peripheral blood basophils.
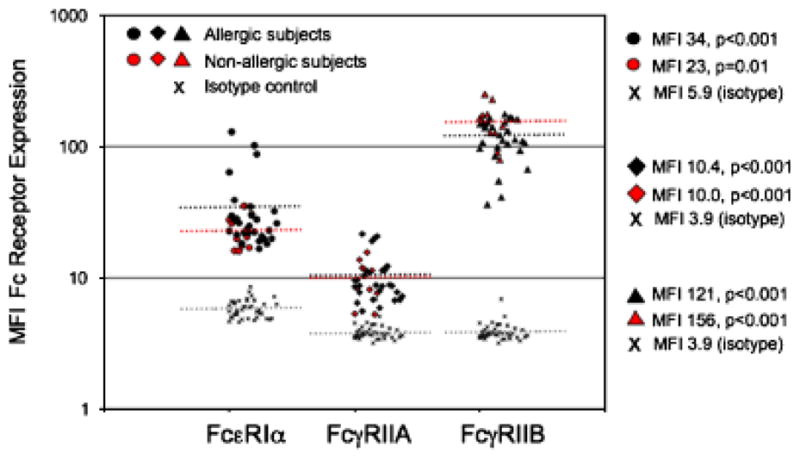
Human peripheral blood was collected in sodium heparin tubes. Cells were fixed, and then stained with the basophil-specific marker, CD203c antibodies against FcεR1α (polyclonal rabbit from Serotec), FcγRIIA (IV.3), or FcγRIIB (2B6) were used to determine surface expression of the respective Fc receptors (108). Both antibodies have been altered so that their Fc regions no longer bind FcγR (both were kind gifts from Macrogenics). Subjects were divided into those denying any allergy symptoms (open symbols), or those who reported suffering from allergies (closed symbols). FcεR1 expression was significantly correlated with serum IgE levels, P < 0.01 (data not shown).
Fig. 10. FcγRIIA and FcγRIIB are both required for immune complex-mediated inhibition of FcεRI signaling.
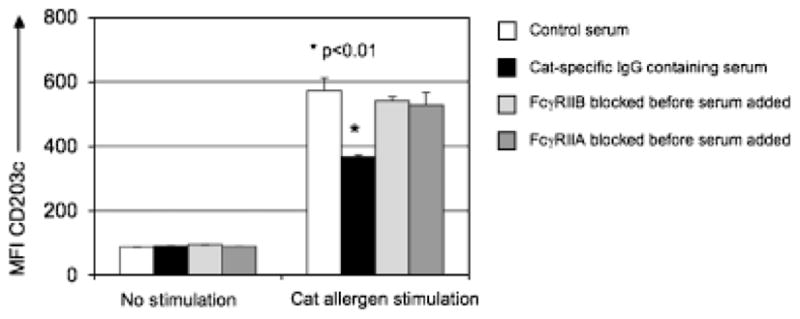
Human peripheral blood was collected from a cat allergen-sensitized individual in a sodium heparin tube. Next, the cells were incubated with blocking antibodies against either FcγRIIA or FcγRIIB (figure legend). Serum from a subject on immunotherapy containing high levels of cat allergen-specific IgG was added (20% w/v) to the whole blood samples for 3 h before a 10 min stimulation with cat hair extract (normalized to a Fel d1 concentration of 0.01 μg/ml). Activation was stopped by placing the cells on ice. After stimulation, red blood cells were lysed, and cells were stained with pan-leukocyte marker anti-CD45, basophil-specific marker anti-CD203c, and anti-IgE. CD203c was also used as a marker of basophil activation (109). CD203c expression was lower when serum containing cat allergen-specific IgG was added before stimulation with cat allergen, when compared with control serum (no cat allergen-specific IgG), P < 0.01. When FcγRIIA or FcγRIIB was blocked before addition of the serum, the inhibitory effect was reversed.
It is possible that this mechanism is involved in the activation of other paired stimulatory and inhibitory receptors. For example, it could be important in reinforcing inhibitory signaling by KIRs in cell synapses where ligands occur on opposing cell membranes and synapse architecture may be unfavorable for ITIM phosphorylation.
SHIP-1 and feedback regulation of immunoreceptor signaling
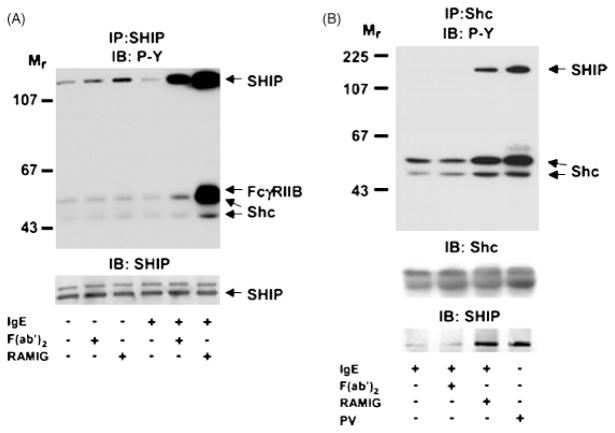
SHIP-1 also plays a critical role in feedback inhibition following signaling through the BCR and FcεR1. In B cells and mast cells (rat basophilic leukemia), SHIP-1 phosphorylation is enhanced by aggregation of BCR and FcεR1 alone using F(ab′)2 cross-linking antibodies or antigen (10, 58, 103, 104) (Fig. 11). Likewise, when IgE-sensitized bone marrow mast cells were stimulated with supraoptimal antigen levels, phosphorylation of SHIP-1 was observed. This correlated with decreased mast cell degranulation. When cells lacking SHIP-1 were used, degranulation was not decreased under the same conditions (105). It has been proposed that SHIP-1 binds and is activated by the ITAM of the FcεRI β chain, but attempts to co-immunoprecipitate SHIP-1 with the β chain have been unsuccessful. Using a CD8/FcγRIIA fusion protein, Nakamura et al. (88) were able to demonstrate functional interaction of SHIP-1 with the ITAM-containing cytoplasmic tail of FcγRIIA. Further, by comparing the phagocytic index of bone marrow-derived macrophages, the authors determined that the actions of FcγRIIB and SHIP-1 were independent, but both maintained a negative regulatory role.
Fig. 11. Tyrosine phosphorylation and association of SHIP-1 and Shc following FcγRIIB co-aggregation with FcεRI.
RBL-mFcγRIIB cells were cultured with or without sensitizing IgE and stimulated with rabbit anti-mouse IgG F(ab′)2, rabbit anti-mouse intact IgG, or pervanadate (PV) for 4 min as indicated. Lysates of total cellular proteins were prepared and proteins were immunoprecipitated with anti-SHIP-1 (A) or anti-Shc (B) antibodies. Immune complexes were resolved by SDS-PAGE, transferred to membranes, and analyzed by immunoblotting with anti-phosphotyrosine antibodies. Membranes were stripped and reprobed with anti-SHIP-1 or anti-Shc antibodies as indicated. Published with permission from Ott et al. (104).
Advancing the idea that SHIP-1 SH2 binding motifs can be somewhat promiscuous, Huang and colleagues used transfectants of FcγRIIA, FcγRIIB, SHP-1, and SHIP-1 to demonstrate that FcγRIIA-mediated phagocytosis could be inhibited by the presence of excess SHP-1, SHIP-1, or FcγRIIB (106, 107). In sum, our knowledge of how feedback regulatory pathways involving SHIP-1 are recruited to ITAM receptors is evolving but remains somewhat limited.
Conclusions
As we learn more about pathways involved in regulation of immune function, additional targets will be identified that hold promise for clinical treatments. A recently described pharmacologic activator of SHIP-1 could be of clinical utility in damping inflammation (32). However, new biologic agents capable of interacting with activating and/or inhibitory receptors or their effectors could have deleterious effects by upsetting the dynamics of receptor regulation. Of additional consideration are genetic variations that affect receptor signaling, as these may direct the course of treatment, e.g. FcR variants and lupus. The ongoing pursuit of improved understanding of the mechanisms involved in regulation of cellular inflammation will help identify areas of highest risk and benefit.
References
- 1.Takai T. Fc receptors and their role in immune regulation and autoimmunity. J Clin Immunol. 2005;25:1–18. doi: 10.1007/s10875-005-0353-8. [DOI] [PubMed] [Google Scholar]
- 2.Malbec O, Daeron M. The mast cell IgG receptors and their roles in tissue inflammation. Immunol Rev. 2007;217:206–221. doi: 10.1111/j.1600-065X.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 3.Ujike A, et al. Modulation of immunoglobulin (Ig)E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG. J Exp Med. 1999;189:1573–1579. doi: 10.1084/jem.189.10.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huber M, Helgason CD, Damen JE, Liu L, Humphries RK, Krystal G. The Src homology 2-containing inositol phosphatase (SHIP) is the gatekeeper of mast cell degranulation. Proc Natl Acad Sci USA. 1998;95:11330–11335. doi: 10.1073/pnas.95.19.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helgason CD, et al. A dual role for Src homology 2 domain-containing inositol-5-phosphatase (SHIP) in immunity: aberrant development and enhanced function of b lymphocytes in ship −/− mice. J Exp Med. 2000;191:781–794. doi: 10.1084/jem.191.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown EE, Edberg JC, Kimberly RP. Fc receptor genes and the systemic lupus erythematosus diathesis. Autoimmunity. 2007;40:567–581. doi: 10.1080/08916930701763710. [DOI] [PubMed] [Google Scholar]
- 7.Amigorena S, et al. Cytoplasmic domain heterogeneity and functions of IgG Fc receptors in B lymphocytes. Science. 1992;256:1808–1812. doi: 10.1126/science.1535455. [DOI] [PubMed] [Google Scholar]
- 8.Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzweig MC, Ravetch JV. A 13-aminoacid motif in the cytoplasmic domain of Fc gamma RIIB modulates B-cell receptor signalling. Nature. 1994;368:70–73. doi: 10.1038/368070a0. [DOI] [PubMed] [Google Scholar]
- 9.D’Ambrosio D, et al. Recruitment and activation of PTP1C in negative regulation of antigen receptor signaling by Fc gamma RIIB1. Science. 1995;268:293–297. doi: 10.1126/science.7716523. [DOI] [PubMed] [Google Scholar]
- 10.D’Ambrosio D, Fong DC, Cambier JC. The SHIP phosphatase becomes associated with Fc gammaRIIB1 and is tyrosine phosphorylated during ‘negative’ signaling. Immunol Lett. 1996;54:77–82. doi: 10.1016/s0165-2478(96)02653-3. [DOI] [PubMed] [Google Scholar]
- 11.Chacko GW, Tridandapani S, Damen JE, Liu L, Krystal G, Coggeshall KM. Negative signaling in B lymphocytes induces tyrosine phosphorylation of the 145-kDa inositol polyphosphate 5-phosphatase, SHIP. J Immunol. 1996;157:2234–2238. [PubMed] [Google Scholar]
- 12.Tridandapani S, Kelley T, Pradhan M, Cooney D, Justement LB, Coggeshall KM. Recruitment and phosphorylation of SH2-containing inositol phosphatase and Shc to the B-cell Fc gamma immunoreceptor tyrosine-based inhibition motif peptide motif. Mol Cell Biol. 1997;17:4305–4311. doi: 10.1128/mcb.17.8.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Famiglietti SJ, Nakamura K, Cambier JC. Unique features of SHIP, SHP-1 and SHP-2 binding to FcgammaRIIb revealed by surface plasmon resonance analysis. Immunol Lett. 1999;68:35–40. doi: 10.1016/s0165-2478(99)00027-9. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura K, Brauweiler A, Cambier JC. Effects of Src homology domain 2 (SH2)-containing inositol phosphatase (SHIP), SH2-containing phosphotyrosine phosphatase (SHP)-1, and SHP-2 SH2 decoy proteins on Fc gamma RIIB1–effector interactions and inhibitory functions. J Immunol. 2000;164:631–638. doi: 10.4049/jimmunol.164.2.631. [DOI] [PubMed] [Google Scholar]
- 15.Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(gamma)RIIB. Nature. 1996;383:263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- 16.Fong DC, et al. Mutational analysis reveals multiple distinct sites within Fc gamma receptor IIB that function in inhibitory signaling. J Immunol. 2000;165:4453–4462. doi: 10.4049/jimmunol.165.8.4453. [DOI] [PubMed] [Google Scholar]
- 17.Isnardi I, Lesourne R, Bruhns P, Fridman WH, Cambier JC, Daeron M. Two distinct tyrosine-based motifs enable the inhibitory receptor FcgammaRIIB to cooperatively recruit the inositol phosphatases SHIP1/2 and the adapters Grb2/Grap. J Biol Chem. 2004;279:51931–51938. doi: 10.1074/jbc.M410261200. [DOI] [PubMed] [Google Scholar]
- 18.Damen JE, et al. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc Natl Acad Sci USA. 1996;93:1689–1693. doi: 10.1073/pnas.93.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lioubin MN, Algate PA, Tsai S, Carlberg K, Aebersold A, Rohrschneider LR. p150Ship, a signal transduction molecule with inositol polyphosphate-5-phosphatase activity. Genes Dev. 1996;10:1084–1095. doi: 10.1101/gad.10.9.1084. [DOI] [PubMed] [Google Scholar]
- 20.Tamir I, et al. The RasGAP-binding protein p62dok is a mediator of inhibitory FcgammaRIIB signals in B cells. Immunity. 2000;12:347–358. doi: 10.1016/s1074-7613(00)80187-9. [DOI] [PubMed] [Google Scholar]
- 21.Tsui HW, Siminovitch KA, de Souza L, Tsui FW. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat Genet. 1993;4:124–129. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Somani AK, Siminovitch KA. Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling. Semin Immunol. 2000;12:361–378. doi: 10.1006/smim.2000.0223. [DOI] [PubMed] [Google Scholar]
- 23.Adachi T, Wienands J, Wakabayashi C, Yakura H, Reth M, Tsubata T. SHP-1 requires inhibitory co-receptors to down-modulate B cell antigen receptor-mediated phosphorylation of cellular substrates. J Biol Chem. 2001;276:26648–26655. doi: 10.1074/jbc.M100997200. [DOI] [PubMed] [Google Scholar]
- 24.Cyster JG, Goodnow CC. Protein tyrosine phosphatase 1C negatively regulates antigen receptor signaling in B lymphocytes and determines thresholds for negative selection. Immunity. 1995;2:13–24. doi: 10.1016/1074-7613(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 25.Pani G, Kozlowski M, Cambier JC, Mills GB, Siminovitch KA. Identification of the tyrosine phosphatase PTP1C as a B cell antigen receptor-associated protein involved in the regulation of B cell signaling. J Exp Med. 1995;181:2077–2084. doi: 10.1084/jem.181.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dustin LB, et al. Expression of dominant-negative src-homology domain 2-containing protein tyrosine phosphatase-1 results in increased Syk tyrosine kinase activity and B cell activation. J Immunol. 1999;162:2717–2724. [PubMed] [Google Scholar]
- 27.Krystal G. Lipid phosphatases in the immune system. Semin Immunol. 2000;12:397–403. doi: 10.1006/smim.2000.0222. [DOI] [PubMed] [Google Scholar]
- 28.Pesesse X, Deleu S, De Smedt F, Drayer L, Erneux C. Identification of a second SH2-domain-containing protein closely related to the phosphatidylinositol polyphosphate 5-phosphatase SHIP. Biochem Biophys Res Commun. 1997;239:697–700. doi: 10.1006/bbrc.1997.7538. [DOI] [PubMed] [Google Scholar]
- 29.Muraille E, Pesesse X, Kuntz C, Erneux C. Distribution of the src-homology-2-domain-containing inositol 5-phosphatase SHIP-2 in both non-haemopoietic and haemopoietic cells and possible involvement of SHIP-2 in negative signalling of B-cells. Biochem J. 1999;342:697–705. [PMC free article] [PubMed] [Google Scholar]
- 30.Brauweiler A, Tamir I, Marschner S, Helgason CD, Cambier JC. Partially distinct molecular mechanisms mediate inhibitory FcgammaRIIB signaling in resting and activated B cells. J Immunol. 2001;167:204–211. doi: 10.4049/jimmunol.167.1.204. [DOI] [PubMed] [Google Scholar]
- 31.Sly LM, Rauh MJ, Kalesnikoff J, Song CH, Krystal G. LPS-induced upregulation of SHIP is essential for endotoxin tolerance. Immunity. 2004;21:227–239. doi: 10.1016/j.immuni.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Ong CJ, et al. Small-molecule agonists of SHIP1 inhibit the phosphoinositide 3-kinase pathway in hematopoietic cells. Blood. 2007;110:1942–1949. doi: 10.1182/blood-2007-03-079699. [DOI] [PubMed] [Google Scholar]
- 33.Siegel J, Li Y, Whyte P. SHIP-mediated inhibition of K562 erythroid differentiation requires an intact catalytic domain and Shc binding site. Oncogene. 1999;18:7135–7148. doi: 10.1038/sj.onc.1203212. [DOI] [PubMed] [Google Scholar]
- 34.Brauweiler AM, Tamir I, Cambier JC. Bilevel control of B-cell activation by the inositol 5-phosphatase SHIP. Immunol Rev. 2000;176:69–74. doi: 10.1034/j.1600-065x.2000.00612.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhao M, Schmitz AA, Qin Y, Di Cristofano A, Pandolfi PP, Van Aelst L. Phosphoinositide 3-kinase-dependent membrane recruitment of p62(dok) is essential for its negative effect on mitogen-activated protein (MAP) kinase activation. J Exp Med. 2001;194:265–274. doi: 10.1084/jem.194.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 37.Buhl AM, Cambier JC. Phosphorylation of CD19 Y484 and Y515, and linked activation of phosphatidylinositol 3-kinase, are required for B cell antigen receptor-mediated activation of Bruton’s tyrosine kinase. J Immunol. 1999;162:4438–4446. [PubMed] [Google Scholar]
- 38.Bolland S, Pearse RN, Kurosaki T, Ravetch JV. SHIP modulates immune receptor responses by regulating membrane association of Btk. Immunity. 1998;8:509–516. doi: 10.1016/s1074-7613(00)80555-5. [DOI] [PubMed] [Google Scholar]
- 39.Falasca M, Logan SK, Lehto VP, Baccante G, Lemmon MA, Schlessinger J. Activation of phospholipase C gamma by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomlinson MG, Heath VL, Turck CW, Watson SP, Weiss A. SHIP family inositol phosphatases interact with and negatively regulate the Tec tyrosine kinase. J Biol Chem. 2004;279:55089–55096. doi: 10.1074/jbc.M408141200. [DOI] [PubMed] [Google Scholar]
- 41.Aman MJ, Lamkin TD, Okada H, Kurosaki T, Ravichandran KS. The inositol phosphatase SHIP inhibits Akt/PKB activation in B cells. J Biol Chem. 1998;273:33922–33928. doi: 10.1074/jbc.273.51.33922. [DOI] [PubMed] [Google Scholar]
- 42.Manna D, Albanese A, Park WS, Cho W. Mechanistic basis of differential cellular responses of phosphatidylinositol 3,4-bisphosphate- and phosphatidylinositol 3,4,5-trisphosphate-binding pleckstrin homology domains. J Biol Chem. 2007;282:32093–32105. doi: 10.1074/jbc.M703517200. [DOI] [PubMed] [Google Scholar]
- 43.Astoul E, Watton S, Cantrell D. The dynamics of protein kinase B regulation during B cell antigen receptor engagement. J Cell Biol. 1999;145:1511–1520. doi: 10.1083/jcb.145.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cantrell DA. Regulation and function of serine kinase networks in lymphocytes. Curr Opin Immunol. 2003;15:294–298. doi: 10.1016/s0952-7915(03)00052-9. [DOI] [PubMed] [Google Scholar]
- 45.Dowler S, et al. Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem J. 2000;351:19–31. doi: 10.1042/0264-6021:3510019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allam A, Marshall AJ. Role of the adaptor proteins Bam32, TAPP1 and TAPP2 in lymphocyte activation. Immunol Lett. 2005;97:7–17. doi: 10.1016/j.imlet.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 47.Wick MJ, Dong LQ, Hu D, Langlais P, Liu F. Insulin receptor-mediated p62dok tyrosine phosphorylation at residues 362 and 398 plays distinct roles for binding GTPase-activating protein and Nck and is essential for inhibiting insulin-stimulated activation of Ras and Akt. J Biol Chem. 2001;276:42843–42850. doi: 10.1074/jbc.M102116200. [DOI] [PubMed] [Google Scholar]
- 48.Shah K, Shokat KM. A chemical genetic screen for direct v-Src substrates reveals ordered assembly of a retrograde signaling pathway. Chem Biol. 2002;9:35–47. doi: 10.1016/s1074-5521(02)00086-8. [DOI] [PubMed] [Google Scholar]
- 49.Tridandapani S, Phee H, Shivakumar L, Kelley TW, Coggeshall KM. Role of SHIP in FcgammaRIIb-mediated inhibition of Ras activation in B cells. Mol Immunol. 1998;35:1135–1146. doi: 10.1016/s0161-5890(98)00097-2. [DOI] [PubMed] [Google Scholar]
- 50.Brauweiler A, et al. Differential regulation of B cell development, activation, and death by the Src homology 2 domain-containing 5′ inositol phosphatase (SHIP) J Exp Med. 2000;191:1545–1554. doi: 10.1084/jem.191.9.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desponts C, Hazen AL, Paraiso KH, Kerr WG. SHIP deficiency enhances HSC proliferation and survival but compromises homing and repopulation. Blood. 2006;107:4338–4345. doi: 10.1182/blood-2005-12-5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Helgason CD, et al. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev. 1998;12:1610–1620. doi: 10.1101/gad.12.11.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Q, et al. SHIP is a negative regulator of growth factor receptor-mediated PKB/Akt activation and myeloid cell survival. Genes Dev. 1999;13:786–791. doi: 10.1101/gad.13.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalergis AM, Ravetch JV. Inducing tumor immunity through the selective engagement of activating Fcgamma receptors on dendritic cells. J Exp Med. 2002;195:1653–1659. doi: 10.1084/jem.20020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daeron M, Malbec O, Latour S, Arock M, Fridman WH. Regulation of high-affinity IgE receptor-mediated mast cell activation by murine low-affinity IgG receptors. J Clin Invest. 1995;95:577–585. doi: 10.1172/JCI117701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ott VL, Cambier JC, Kappler J, Marrack P, Swanson BJ. Mast cell-dependent migration of effector CD8+ T cells through production of leukotriene B4. Nat Immunol. 2003;4:974–981. doi: 10.1038/ni971. [DOI] [PubMed] [Google Scholar]
- 57.Vely F, et al. Differential association of phosphatases with hematopoietic co-receptors bearing immunoreceptor tyrosine-based inhibition motifs. Eur J Immunol. 1997;27:1994–2000. doi: 10.1002/eji.1830270825. [DOI] [PubMed] [Google Scholar]
- 58.Brauweiler A, Merrell K, Gauld SB, Cambier JC. Cutting Edge: acute and chronic exposure of immature B cells to antigen leads to impaired homing and SHIP1-dependent reduction in stromal cell-derived factor-1 responsiveness. J Immunol. 2007;178:3353–3357. doi: 10.4049/jimmunol.178.6.3353. [DOI] [PubMed] [Google Scholar]
- 59.Blery M, et al. Reconstituted killer cell inhibitory receptors for major histocompatibility complex class I molecules control mast cell activation induced via immunoreceptor tyrosine-based activation motifs. J Biol Chem. 1997;272:8989–8996. doi: 10.1074/jbc.272.14.8989. [DOI] [PubMed] [Google Scholar]
- 60.Poole AW, Jones ML. A SHPing tale: perspectives on the regulation of SHP-1 and SHP-2 tyrosine phosphatases by the C-terminal tail. Cell Signal. 2005;17:1323–1332. doi: 10.1016/j.cellsig.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 61.Siminovitch KA, Neel BG. Regulation of B cell signal transduction by SH2-containing protein-tyrosine phosphatases. Semin Immunol. 1998;10:329–347. doi: 10.1006/smim.1998.0125. [DOI] [PubMed] [Google Scholar]
- 62.Lechleider RJ, et al. Activation of the SH2-containing phosphotyrosine phosphatase SH-PTP2 by its binding site, phosphotyrosine 1009, on the human platelet-derived growth factor receptor. J Biol Chem. 1993;268:21478–21481. [PubMed] [Google Scholar]
- 63.Sly LM, Rauh MJ, Kalesnikoff J, Buchse T, Krystal G. SHIP, SHIP2, and PTEN activities are regulated in vivo by modulation of their protein levels: SHIP is up-regulated in macrophages and mast cells by lipopolysaccharide. Exp Hematol. 2003;31:1170–1181. doi: 10.1016/j.exphem.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 64.Eck MJ, Pluskey S, Trub T, Harrison SC, Shoelson SE. Spatial constraints on the recognition of phosphoproteins by the tandem SH2 domains of the phosphatase SH-PTP2. Nature. 1996;379:277–280. doi: 10.1038/379277a0. [DOI] [PubMed] [Google Scholar]
- 65.Barford D, Neel BG. Revealing mechanisms for SH2 domain mediated regulation of the protein tyrosine phosphatase SHP-2. Structure. 1998;6:249–254. doi: 10.1016/s0969-2126(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 66.Pei D, Lorenz U, Klingmuller U, Neel BG, Walsh CT. Intramolecular regulation of protein tyrosine phosphatase SH-PTP1: a new function for Src homology 2 domains. Biochemistry. 1994;33:15483–15493. doi: 10.1021/bi00255a030. [DOI] [PubMed] [Google Scholar]
- 67.Pei D, Wang J, Walsh CT. Differential functions of the two Src homology 2 domains in protein tyrosine phosphatase SH-PTP1. Proc Natl Acad Sci USA. 1996;93:1141–1145. doi: 10.1073/pnas.93.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bruhns P, Marchetti P, Fridman WH, Vivier E, Daeron M. Differential roles of N- and C-terminal immunoreceptor tyrosine-based inhibition motifs during inhibition of cell activation by killer cell inhibitory receptors. J Immunol. 1999;162:3168–3175. [PubMed] [Google Scholar]
- 69.Lesourne R, Bruhns P, Fridman WH, Daeron M. Insufficient phosphorylation prevents fc gamma RIIB from recruiting the SH2 domain-containing protein-tyrosine phosphatase SHP-1. J Biol Chem. 2001;276:6327–6336. doi: 10.1074/jbc.M006537200. [DOI] [PubMed] [Google Scholar]
- 70.Kon-Kozlowski M, Pani G, Pawson T, Siminovitch KA. The tyrosine phosphatase PTP1C associates with Vav, Grb2, and mSos1 in hematopoietic cells. J Biol Chem. 1996;271:3856–3862. doi: 10.1074/jbc.271.7.3856. [DOI] [PubMed] [Google Scholar]
- 71.Minoo P, Zadeh MM, Rottapel R, Lebrun JJ, Ali S. A novel SHP-1/Grb2-dependent mechanism of negative regulation of cytokine-receptor signaling: contribution of SHP-1 C-terminal tyrosines in cytokine signaling. Blood. 2004;103:1398–1407. doi: 10.1182/blood-2003-07-2617. [DOI] [PubMed] [Google Scholar]
- 72.Brumell JH, et al. Regulation of Src homology 2-containing tyrosine phosphatase 1 during activation of human neutrophils. Role of protein kinase C. J Biol Chem. 1997;272:875–882. doi: 10.1074/jbc.272.2.875. [DOI] [PubMed] [Google Scholar]
- 73.Yang J, et al. Crystal structure of human protein-tyrosine phosphatase SHP-1. J Biol Chem. 2003;278:6516–6520. doi: 10.1074/jbc.M210430200. [DOI] [PubMed] [Google Scholar]
- 74.Sankarshanan M, Ma Z, Iype T, Lorenz U. Identification of a novel lipid raft-targeting motif in Src homology 2-containing phosphatase 1. J Immunol. 2007;179:483–490. doi: 10.4049/jimmunol.179.1.483. [DOI] [PubMed] [Google Scholar]
- 75.Burshtyn DN, Yang W, Yi T, Long EO. A novel phosphotyrosine motif with a critical amino acid at position – 2 for the SH2 domain-mediated activation of the tyrosine phosphatase SHP-1. J Biol Chem. 1997;272:13066–13072. doi: 10.1074/jbc.272.20.13066. [DOI] [PubMed] [Google Scholar]
- 76.Songyang Z, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 77.Huang H, et al. Defining the specificity space of the human src-homology 2 domain. Mol Cell Proteomics. 2008;7:768–784. doi: 10.1074/mcp.M700312-MCP200. [DOI] [PubMed] [Google Scholar]
- 78.Songyang Z, Cantley LC. ZIP codes for delivering SH2 domains. Cell. 2004;116:S41–S43. doi: 10.1016/s0092-8674(04)00041-8. [DOI] [PubMed] [Google Scholar]
- 79.Yaffe MB, Leparc GG, Lai J, Obata T, Volinia S, Cantley LC. A motif-based profile scanning approach for genome-wide prediction of signaling pathways. Nat Biotechnol. 2001;19:348–353. doi: 10.1038/86737. [DOI] [PubMed] [Google Scholar]
- 80.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sweeney MC, Wavreille AS, Park J, Butchar JP, Tridandapani S, Pei D. Decoding protein–protein interactions through combinatorial chemistry: sequence specificity of SHP-1, SHP-2, and SHIP SH2 domains. Biochemistry. 2005;44:14932–14947. doi: 10.1021/bi051408h. [DOI] [PubMed] [Google Scholar]
- 82.Imhof D, Wavreille AS, May A, Zacharias M, Tridandapani S, Pei D. Sequence specificity of SHP-1 and SHP-2 Src homology 2 domains. Critical roles of residues beyond the pY+3 position. J Biol Chem. 2006;281:20271–20282. doi: 10.1074/jbc.M601047200. [DOI] [PubMed] [Google Scholar]
- 83.Wavreille AS, Garaud M, Zhang Y, Pei D. Defining SH2 domain and PTP specificity by screening combinatorial peptide libraries. Methods. 2007;42:207–219. doi: 10.1016/j.ymeth.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marshall AJ, Krahn AK, Ma K, Duronio V, Hou S. TAPP1 and TAPP2 are targets of phosphatidylinositol 3-kinase signaling in B cells: sustained plasma membrane recruitment triggered by the B-cell antigen receptor. Mol Cell Biol. 2002;22:5479–5491. doi: 10.1128/MCB.22.15.5479-5491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kimura T, Sakamoto H, Appella E, Siraganian RP. The negative signaling molecule SH2 domain-containing inositol-polyphosphate 5-phosphatase (SHIP) binds to the tyrosine-phosphorylated beta subunit of the high affinity IgE receptor. J Biol Chem. 1997;272:13991–13996. doi: 10.1074/jbc.272.21.13991. [DOI] [PubMed] [Google Scholar]
- 86.Cameron AJ, Allen JM. The human high-affinity immunoglobulin G receptor activates SH2-containing inositol phosphatase (SHIP) Immunology. 1999;97:641–647. doi: 10.1046/j.1365-2567.1999.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maresco DL, Osborne JM, Cooney D, Coggeshall KM, Anderson CL. The SH2-containing 5′-inositol phosphatase (SHIP) is tyrosine phosphorylated after Fc gamma receptor clustering in monocytes. J Immunol. 1999;162:6458–6465. [PubMed] [Google Scholar]
- 88.Nakamura K, Malykhin A, Coggeshall KM. The Src homology 2 domain-containing inositol 5-phosphatase negatively regulates Fcgamma receptor-mediated phagocytosis through immunoreceptor tyrosine-based activation motif-bearing phagocytic receptors. Blood. 2002;100:3374–3382. doi: 10.1182/blood-2002-03-0787. [DOI] [PubMed] [Google Scholar]
- 89.Mertsching E, et al. A mouse Fcgamma-Fcepsilon protein that inhibits mast cells through activation of FcgammaRIIB, SH2 domain-containing inositol phosphatase 1, and SH2 domain-containing protein tyrosine phosphatases. J Allergy Clin Immunol. 2008;121:441–447. e445. doi: 10.1016/j.jaci.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 90.Kepley CL, et al. Co-aggregation of FcgammaRII with FcepsilonRI on human mast cells inhibits antigen-induced secretion and involves SHIP-Grb2-Dok complexes. J Biol Chem. 2004;279:35139–35149. doi: 10.1074/jbc.M404318200. [DOI] [PubMed] [Google Scholar]
- 91.Izawa K, et al. Functional analysis of activating receptor LMIR4 as a counterpart of inhibitory receptor LMIR3. J Biol Chem. 2007;282:17997–18008. doi: 10.1074/jbc.M701100200. [DOI] [PubMed] [Google Scholar]
- 92.Kono H, et al. FcgammaRIIB Ile232Thr transmembrane polymorphism associated with human systemic lupus erythematosus decreases affinity to lipid rafts and attenuates inhibitory effects on B cell receptor signaling. Hum Mol Genet. 2005;14:2881–2892. doi: 10.1093/hmg/ddi320. [DOI] [PubMed] [Google Scholar]
- 93.Guyre PM, Morganelli PM, Miller R. Recombinant immune interferon increases immunoglobulin G Fc receptors on cultured human mononuclear phagocytes. J Clin Invest. 1983;72:393–397. doi: 10.1172/JCI110980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Woolhiser MR, Okayama Y, Gilfillan AM, Metcalfe DD. IgG-dependent activation of human mast cells following up-regulation of FcgammaRI by IFN-gamma. Eur J Immunol. 2001;31:3298–3307. doi: 10.1002/1521-4141(200111)31:11<3298::aid-immu3298>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 95.Shushakova N, et al. C5a anaphylatoxin is a major regulator of activating versus inhibitory FcgammaRs in immune complex-induced lung disease. J Clin Invest. 2002;110:1823–1830. doi: 10.1172/JCI200216577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pricop L, et al. Differential modulation of stimulatory and inhibitory Fc gamma receptors on human monocytes by Th1 and Th2 cytokines. J Immunol. 2001;166:531–537. doi: 10.4049/jimmunol.166.1.531. [DOI] [PubMed] [Google Scholar]
- 97.Tridandapani S, Siefker K, Teillaud JL, Carter JE, Wewers MD, Anderson CL. Regulated expression and inhibitory function of Fcgamma RIIb in human monocytic cells. J Biol Chem. 2002;277:5082–5089. doi: 10.1074/jbc.M110277200. [DOI] [PubMed] [Google Scholar]
- 98.Tridandapani S, et al. TGF-beta 1 suppresses [correction of supresses] myeloid Fc gamma receptor function by regulating the expression and function of the common gamma-subunit. J Immunol. 2003;170:4572–4577. doi: 10.4049/jimmunol.170.9.4572. [DOI] [PubMed] [Google Scholar]
- 99.Guriec N, Daniel C, Le Ster K, Hardy E, Berthou C. Cytokine-regulated expression and inhibitory function of FcgammaRIIB1 and -B2 receptors in human dendritic cells. J Leukoc Biol. 2006;79:59–70. doi: 10.1189/jlb.0305155. [DOI] [PubMed] [Google Scholar]
- 100.Liu Y, Gao X, Masuda E, Redecha PB, Blank MC, Pricop L. Regulated expression of FcgammaR in human dendritic cells controls cross-presentation of antigen–antibody complexes. J Immunol. 2006;177:8440–8447. doi: 10.4049/jimmunol.177.12.8440. [DOI] [PubMed] [Google Scholar]
- 101.Rudge EU, Cutler AJ, Pritchard NR, Smith KG. Interleukin 4 reduces expression of inhibitory receptors on B cells and abolishes CD22 and Fc gamma RII-mediated B cell suppression. J Exp Med. 2002;195:1079–1085. doi: 10.1084/jem.20011435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 103.Fong DC, Malbec O, Arock M, Cambier JC, Fridman WH, Daeron M. Selective in vivo recruitment of the phosphatidylinositol phosphatase SHIP by phosphorylated Fc gammaRIIB during negative regulation of IgE-dependent mouse mast cell activation. Immunol Lett. 1996;54:83–91. doi: 10.1016/s0165-2478(96)02654-5. [DOI] [PubMed] [Google Scholar]
- 104.Ott VL, Tamir I, Niki M, Pandolfi PP, Cambier JC. Downstream of kinase, p62(dok), is a mediator of Fc gamma IIB inhibition of Fc epsilon RI signaling. J Immunol. 2002;168:4430–4439. doi: 10.4049/jimmunol.168.9.4430. [DOI] [PubMed] [Google Scholar]
- 105.Gimborn K, Lessmann E, Kuppig S, Krystal G, Huber M. SHIP down-regulates FcepsilonR1-induced degranulation at supraoptimal IgE or antigen levels. J Immunol. 2005;174:507–516. doi: 10.4049/jimmunol.174.1.507. [DOI] [PubMed] [Google Scholar]
- 106.Ganesan LP, Fang H, Marsh CB, Tridandapani S. The protein-tyrosine phosphatase SHP-1 associates with the phosphorylated immunoreceptor tyrosine-based activation motif of Fc gamma RIIa to modulate signaling events in myeloid cells. J Biol Chem. 2003;278:35710–35717. doi: 10.1074/jbc.M305078200. [DOI] [PubMed] [Google Scholar]
- 107.Huang ZY, Hunter S, Kim MK, Indik ZK, Schreiber AD. The effect of phosphatases SHP-1 and SHIP-1 on signaling by the ITIM-and ITAM-containing Fcgamma receptors FcgammaRIIB and FcgammaRIIA. J Leukoc Biol. 2003;73:823–829. doi: 10.1189/jlb.0902454. [DOI] [PubMed] [Google Scholar]
- 108.Veri MC, et al. Monoclonal antibodies capable of discriminating the human inhibitory Fcgamma-receptor IIB (CD32B) from the activating Fcgamma-receptor IIA (CD32A): biochemical, biological and functional characterization. Immunology. 2007;121:392–404. doi: 10.1111/j.1365-2567.2007.02588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kleine-Tebbe J, Erdmann S, Knol EF, Mac-Glashan DW, Jr, Poulsen LK, Gibbs BF. Diagnostic tests based on human basophils: potentials, pitfalls and perspectives. Int Arch Allergy Immunol. 2006;141:79–90. doi: 10.1159/000094495. [DOI] [PubMed] [Google Scholar]