Summary
A byproduct of the largely stochastic generation of a diverse B-cell specificity repertoire is production of cells that recognize autoantigens. Indeed, recent studies indicate that more than half of the primary repertoire consists of autoreactive B cells that must be silenced to prevent autoimmunity. While this silencing can occur by multiple mechanisms, it appears that most autoreactive B cells are silenced by anergy, wherein they populate peripheral lymphoid organs and continue to express unoccupied antigen receptors yet are unresponsive to antigen stimulation. Here we review molecular mechanisms that appear operative in maintaining the antigen unresponsiveness of anergic B cells. In addition, we present new data indicating that the failure of anergic B cells to mobilize calcium in response to antigen stimulation is not mediated by inactivation of stromal interacting molecule 1, a critical intermediary in intracellular store depletion-induced calcium influx.
Keywords: B cell, tolerance, anergy, PI3K
Modes of B-cell tolerance
The ability of the adaptive immune system to provide protection against pathogens depends upon a diverse repertoire of antigen receptors that enable recognition of a seemingly infinite range of foreign protein and carbohydrate antigens. Diversity is generated early in lymphocyte development by random rearrangement of immunoglobulin (Ig) V, D, and J region gene segments. The random nature of this process leads inevitably to generation of receptors that recognize self-antigen. It is estimated that as much as 70% of early immature human B cells are self-reactive (1). It appears that in about a third of these self-reactive immature B cells autoreactivity is eliminated by receptor editing, wherein new Ig gene rearrangement generates an alternate light chain that pairs with the existing Ig heavy chain, altering specificity (2, 3). If editing fails to eliminate reactivity, the B cell is deleted by apoptosis (4). Experimental evidence shows that only a few percent of B cells meets this fate (5).
Although these modes of ‘central’ tolerance silence B cells in the bone marrow (BM), many self-reactive B cells escape to the periphery where they must be silenced by alternate mechanisms. Studies in the 1970s demonstrated that B cells from peripheral lymphoid organs can be rendered tolerant by exposure to antigen in vitro. In these early studies, the mechanism by which B cells were rendered tolerant was often referred to as clonal inactivation/abortion based on the fact that the frequency of antigen-specific, antibody-secreting cells fell drastically following tolerizing treatments. However, these experiments did not distinguish between the possibilities that this was due to death of the antibody-forming cell precursor or continued survival of these cells in a state unresponsive to antigen.
This question was first addressed by Pike and Nossal (6), who used a fluorescein (Flu) conjugated human γ globulin (HGG) to induce tolerance and subsequently analyzed the relationship between capacity to generate antibody responses and frequency of the Flu-binding B cells. They found that in mice treated as neonates or in utero with high doses of Flu-HGG, there was a significant reduction in the number of Flu-binding cells, suggesting editing or deletion. However, when they used lower antigen doses they found no reduction in the number of Flu-binding cells despite effective induction of tolerance. The authors coined the term ‘anergy’ to describe this mechanism of silencing in which autoreactive B cells persist yet are unresponsive to antigen.
Although the conclusions of Pike and Nossal would prove correct, there were caveats in the interpretation of these original experiments. For example, the antibody-forming cell precursor frequency they observed was much lower than would have been predicted based on the antigen-binding cell frequency. Therefore, many of the antigen-binding cells enumerated in the naive mouse may not have been responsive to the antigen, and these ‘irrelevant’ cells would have been retained after tolerance induction, leading to the false conclusion that antibody-secreting cell precursors were not deleted. Moreover, like all previous B-cell tolerance studies, the approach involved induction of tolerance using exogenous foreign antigen in contrast to the physiological situation in which the self-antigen would be present throughout the ontogeny of autoreactive B cells. Finally, the tolerogen used in the studies, Flu-HGG, may have bound the inhibitory IgG receptors (FcγRIIB) expressed by B cells, and this binding could have altered the subsequent immune response. Thus, although Pike and Nossal coined the concept of anergy, only later work proved that anergy is operative in the silencing of autoreactive B cells in vivo (7).
The first clear evidence that autoreactive B cells can inhabit peripheral lymphoid organs in an antigen unresponsive or anergic state came from studies using an Ig transgenic (tg) mouse in which B-cell receptor (BCR) specificity was fixed (7, 8). Goodnow and colleagues compared the effect on B cells of conditions in which cognate antigen is expressed in the animal from embryogenesis, to conditions where the antigen is absent and B cells remain naive. In this model, mice (MD4) co-expressed heavy chain (both μ and δ) and light chain transgenes to produce a BCR with high affinity (2 × 10−9 M) for hen egg lysozyme (HEL). These mice were bred with transgenic mice that express soluble HEL (ML5 mice). In an F1 hybrid of MD4 and ML5 mice that express a BCR recognizing ‘self’ HEL, B cells develop relatively normally as indicated by appearance in the periphery of transitional 1 (T1) and T2 cells. However, the number of mature follicular B cells is greatly reduced compared with MD4 mice. Interestingly, in MD4 × ML5 mice, most splenic B cells reside in a phenotypic stage reminiscent of late transitional cells. Chronic exposure of peripheral B cells to HEL (serum levels greater than 10–20 ng/ml) results in anergy, as defined by unresponsiveness to antigen stimulation. This unresponsiveness is not due to inaccessibility of antigen receptors as a consequence of bound self-antigen: only 45% of the surface receptors are occupied by antigen (9). Upon antigen stimulation, the B cells fail to proliferate and differentiate into antibody-secreting cells, either during immunization with exogenous HEL or in response to the innate Toll-like receptor (TLR) agonists CpG-containing DNA and lipopolysaccharide(10, 11).
Subsequent studies utilized transgenesis to generate mice in which B cells were specific for endogenous antigens against which tolerance is often broken in autoimmunity. Anti-DNA antibody formation is the hallmark of the autoimmune disease in systemic lupus erythematosus (SLE) and some autoimmune mouse strains, e.g. MRL.Faslpr/lpr or NZB/NZW mice (12, 13). A model developed by Shlomchik et al. uses an anti-DNA heavy chain variable region (3H9) derived from an autoimmune MRL.Faslpr/lpr mouse (2, 14, 15). The tg heavy chain pairs with endogenous light chains to generate a polyclonal B-cell repertoire enriched in cells specific for single-stranded DNA (ssDNA) and double-stranded DNA (dsDNA), in addition to a population of non-DNA-specific B cells. These VH3H9 mice (on a BALB/c background) were further crossed with Vκ8 tg mice to generate VH3H9Vκ8 mice, in which specificity of peripheral B cells to ssDNA was assessed using immunofluorescence and production and characterization of hybridomas. Over 60% of IgM+ splenic B cells bound ssDNA. More than 50% of hybridomas generated using this mouse produced antibodies that bound ssDNA. These mice produce normal number of IgM+ B cells in the periphery yet do not produce DNA-specific antibodies, indicating anergy of DNA-specific B cells. Discrepant results were seen in a second anti-DNA model. The heavy chain variable region from an anti-DNA antibody (D42) derived from NZB/NZW mouse was knocked in to the Ig locus to allow class switch and somatic mutation (16, 17). Even when on a non-autoimmune prone genetic background, these mice produce autoantibodies that bind ssDNA and dsDNA with a wide range of affinity.
Additional insight regarding mechanisms operative in anergy have been learned from Ig transgenic mice in which B cells react with the hapten p-azophenlyarsonate (Ars) and cross-react with endogenous autoantigen (ssDNA) (18). These mice were originally generated to provide freedom to control the affinity and avidity of the antigen in studies of BCR signaling. These Ars/A1 mice produce mIgM and mIgD that bind Ars with moderate affinity of 2 × 10−5 M and ssDNA with a low affinity of 2 × 10−3 (19). B cells in the mouse progress though T1 and T2 stage, but both follicular and marginal zone (MZ) cells are greatly reduced in number. B cells in this mouse are anergic, as judged by inability to mount immune responses to thymus-dependent and -independent Ars immunogens. The ability of the Ars-specific BCR to cross-react with ssDNA provided the opportunity to study reversibility of anergy by competitive dissociation of autoantigen using monovalent Arsonate. These experiments showed that most indicators of anergy are reversed within a few minutes of removal of autoantigen from the BCR (20). These results indicate that anergy is maintained primarily by biochemical signaling circuitry activated by chronic BCR occupancy, rather than by durable mechanisms such as genetic reprogramming.
Use of the transgenic models described above left open the question of the importance of anergy to silencing self-reactive cells under physiologic conditions. Merrell et al. (21) and Teague et al. (22) noted that anergic B cells seen in both the Ars/A1 and MD4 by ML5 models exhibit a similar surface marker phenotype. These cells express mature B-cell markers such as CD23, CD21, mIgD, and mIgM, but also express the immature B-cell marker CD93. Although less extensively examined, a similar phenotype was reported for 3H9Vλ2 anergic B cells (15, 23). In all cases, mIgM is reduced relative to T1, T2, and follicular B cells. We explored the occurrence of cells of this phenotype in wildtype (wt) C57BL/6 mice and MD4 tg C57BL/6 mice (21). Five to eight percent of B cells in C57BL/6 mice were found to exhibit this phenotype, while this cell population was absent in the foreign antigen specificity-biased repertoire of MD4 anti-HEL mice. We further showed that these cells display defects in BCR-mediated signaling similar to anergic transgenic B cells, fail to mount immune responses, and are enriched in autoreactive cells. Finally, these cells, which were previously referred to as T3 cells, exhibit a short half-life reminiscent of transgenic anergic B cells (24). We concluded that this population represents a physiologic cohort of anergic B cells, and thus we refer to them as An1 (anergic population one) cells. Interestingly, the frequency of these cells considered in the context of their short lifespan leads to the prediction that nearly 50% of newly produced B cells are destined to become anergic. This conclusion is consistent with the suggested 70% frequency of autoreactive B cells among the earliest immature B cells in BM, and reports that approximately 25% of peripheral B cells have edited (1, 25). As discussed earlier, anergic B cells from transgenic models as well as An1 cells generally exhibit a transitional phenotype, suggesting that these cells may simply be arrested at an immature developmental stage. Alternatively, the An1 phenotype may represent a unique compartment that is accessed by B cells at any developmental stage following induction of anergy. To address this question, we adoptively transferred mature MD4 B cells into ML5 mice and monitored subsequent changes in their surface phenotype. These mature B cells were found to adopt the An1 phenotype within 48 h of transfer to an antigen-sufficient animal (21). These findings support the notion that anergic B cells are not developmentally arrested but may enter the An1 compartment from mature stages. Since T cells in ML5 mice are anergic (26), acquisition of this anergic phenotype can be viewed as the consequence of receiving signal one in the absence of signal two, as hypothesized by Bretcher and Cohn (27).
Given the reversible nature of B-cell anergy, a logical question is whether anergic B cells form a source of autoreactive B cells, which under certain circumstances could give rise to autoimmunity. Some lines of evidence suggest that this could be the case. Teague et al. showed that the An1 population is reduced in autoimmune NZB × NZWF1 mice consistent with loss of anergy in these mice (22). In another study, the D42 heavy chain transgene on NZB background was crossed with Vκ8 light chain transgenic NZW mice to create a mouse that has monoclonal B cells with low affinity anti-DNA BCR on the NZB × NZW F1 background (28). D42 × Vκ8 B cells were found to adopt the An1 surface and calcium signaling phenotype. However, the mice developed SLE-like autoimmune disease. Thus, in this model it would appear that a small proportion of anti-DNA B cells leave (or fail to enter) the anergic compartment and mount an antoantibody response. These findings further indicate that the NZB/NZW genetic predisposition alone is not sufficient to drive autoreactive B cells from the An1 compartment. Escape may depend on a second ‘hit’, such as an innate immune signal.
While our understanding of B-cell anergy in the mice is increasing due to intense study, much less is known about the basis of anergy in human and its role in maintaining immune tolerance. Based on findings in the mouse, Duty and Wilson (29) examined human peripheral blood for B cells subsets in an effort to define an anergic compartment. The CD19+IgD+IgMlo/negCD27− (CD27 is expressed on human memory and plasma cells) B cells were found to be unresponsive to BCR stimulation based on calcium mobilization and protein tyrosine phosphorylation. This population represents 2.5% of peripheral blood B cells. Ig heavy and light chains isolated from these cells are in the germline configuration, indicating lack of previous immune experience, and the population is enriched in B cells that recognize ssDNA and HEp-2 cell antigens. These cells appear to represent an anergic population, possibly the human equivalent of An1 cells.
Anergy in the context Bretcher and Cohn’s two signal model of lymphocyte activation
Nearly four decades ago, Peter Bretscher and Mel Cohn (27) suggested that lymphocyte activation during immune responses required two signals: the first, i.e. signal one, being derived from antigen and a second coming from other sources. We now believe that in the case of B-cell activation, this second signal can come from cognate T-cell help or TLR ligands. Bretscher and Cohn (27) also suggested that immune tolerance results when cells receive signal one without receiving signal two. It appears that not only is signal two required to avoid tolerance, in this case anergy, but that this signal, e.g. CD40L, must arrive within a certain period of time (30). Signal one, by driving transient upregulation of CD86 and antigen processing, sets the stage for effective antigen presentation to CD4+ T cells leading to expression of CD40L and back-signaling to the B cell. An additional effect of signal one is the desensitization of antigen receptors, such that subsequent re-exposure to antigen, e.g. an autoantigen mimetic microbial immunogen, does not lead to a response, e.g. upregulation of CD86. In effect, this unresponsiveness is anergy. Anergic B cells have received signal one in the absence of properly timed signal two and consequently are unresponsive to exogenous antigens. Since most autoreactive T cells should be eliminated by negative selection in the thymus, the two-signal model may explain how B cells distinguish between self and non-self antigens.
Consequences of receiving signal one without signal two
Reduced lifespan
Multiple features of An1 B cells are likely to reduce their participation in immune responses to immunogens that may carry autoantigen mimetic epitopes. One such feature is reduced lifespan. It is known that the half-life of follicular B cell is about 40 days, significantly longer than 5 days for anergic B cells (31, 32). However, the basis of the reduced life span of anergic cells is poorly understood. Initial work using the MD4 × ML5 model, where no competing naive cells were present, showed the life span of anergic cells to be normal (7). It was later shown that when non-anergic cells are present to compete with anergic cells in the same mouse, anergic cells displayed a shorter life span. This discrepancy was subsequently shown to reflect competition for B-cell activating factor belonging to the tumor necrosis factor family (BAFF), also known as B-lymphocyte stimulator, an important survival factor for peripheral B cells (33). Essentially, naive B cells out-compete anergic B cells for limiting amounts of BAFF present in vivo. When there is no competition, anergic B cells receive sufficient BAFF signals to survive. This appears to be a function of reduced expression of BAFF receptors and/or reduced competence of BAFF receptors on anergic cells to signal (34). Importantly, overexpression of BAFF by transgenesis leads to autoimmunity (35).
While BAFF competition is an important piece of the puzzle, it is unlikely to be the only factor contributing to premature death of anergic cells. For example, removal of autoantigen from anergic Ars/A1 B cells increases their lifespan in cultures containing little or no BAFF (20). Thus, they must be predisposed to die by other mechanisms. One such mechanism is high expression of the proapoptotic BCL family member BIM seen in anergic B cells (36). The likely importance of BIM in this regard is underscored by observations that anergy is lost in MD4 × ML5 B cells lacking BIM.
An additional factor contributing to death of anergic B cells may be apoptotic signals initiated by FAS signaling on anergic B cells that encounter FASL+ CD4+ T cells. This conclusion is based on works showing that mice deficient for FAS (MRL.faslpr/lpr) or FASL (B6.fasgld/gld) tend to develop autoimmunity with age (37, 38). Most compelling, however, are studies showing that the interaction of anergic anti-HEL B cells with CD4+ T cells specific for HEL results in apoptosis, rather than B-cell activation, and this involves FASL and FAS (39). However, not all anergic B cells express FAS, and although Ars/A1 anergic B cell express FAS, they do not die upon FAS stimulation (Gauld, unpublished data). Thus, a role of CD4+ T-cell-induced FAS-mediated death of reducing half-life of anergic cells may be restricted to the HEL model.
Follicular exclusion
As discussed earlier, B cells receive signal one by virtue of contact with their specific antigen in BM, circulation, or in secondary lymphoid organs. This signal induces them to enter T-cell-rich cortical areas in quest of appropriate T-helper cell, with whom they undergo cognate interactions. In a normal thymus-dependent immune response, productive interaction with Th cells lead to delivery of signal two, stimulating B-cell activation and entry into the follicule where the germinal center reaction is initiated. Like antigen-stimulated naive cells, anergic B cells enter T-cell zones as a consequence of receiving signal one and become arrested there, presumably because they do not encounter or cannot interact productively with an appropriate Th cell (40, 41). This phenomenon, referred to as follicular exclusion, has been seen in both the MD4 by ML5 and the Ars/A1 transgenic models of anergy (JCC and AG, unpublished data)
Antigen receptor signaling and its regulation in anergy B cells
Antigen receptor signaling in naive B cells
The BCR is composed of mIg that is non-covalently associated with a heterodimer of the signal transducing molecules Igα (CD79a) and Igβ (CD79b) (42). Some proportion of these receptors are probably associated constitutively with a Src-family tyrosine kinase, usually Lyn, via interaction of the Lyn SH4 domain with Igα/β tails (43) and simultaneous lipid modification-dependent Lyn anchoring in the plasma membrane (44). Below we describe BCR signaling events that intervene between antigen stimulation and calcium mobilization. We focus on this proximal BCR signaling circuitry because it is disrupted in anergic B cells.
Aggregation of mIg and associated Igα/Igβ on naive B cells by polyvalent antigen leads to activation of Lyn (45) [or alternate Src-family kinase (46)], which triggers downstream responses by phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) on both Igα and Igβ (47, 48). Aggregation of multiple BCRs by multivalent antigen is both sufficient and necessary to bring about dual phosphorylation of Igα/Igβ ITAMs (49). The dual phosphorylation of ITAM tyrosine residues is necessary for docking and subsequent activation of the cytosolic tyrosine kinase Syk, which is in turn necessary for initiation of several downstream signaling pathways involved in activation, survival, and proliferation (50, 51). Syk contains two SH2 domains and undergoes a stable physical and functional interaction with the BCR only when both SH2 of its domains engage phosphotyrosines in an ITAM. Once engaged, Syk is activated by tyrosine phosphorylation (52).
In parallel with activation of Syk is stimulation of PI3K and resultant production of phosphatidylinositol (3–5) triphosphate [PtdIns(3–5)P3]. PI3K activation can occur by multiple mechanisms including direct interactions with Lyn (53), the adapter BCAP (54), and CD19 (55). Phosphorylation of BCAP and CD19 on YxxM motifs suffices for most PI3K activation following BCR stimulation (54). CD19 also mediates the further, apparently processive, activation of Lyn (56) and promotes signaling by recruitment of positive signal mediators Btk and Vav (57).
PtdIns(3–5)P3 is a crucial lipid second messenger that functions at multiple levels in BCR signaling. PtdIns(3–5)P3 function involves recruitment of effectors that bind the phospholipid’s head-group via their pleckstrin homology (PH) domains. Thus, PtdIns(3–5)P3 mediates membrane association of multiple effectors, including VAV, Btk, PLCγ, Akt, and SOS to BCR proximal areas of plasma membrane where they are accessible to their upstream regulators including tyrosine kinases, e.g. Syk, and downstream targets such as the PLCγ substrate PtdIns(4, 5)P2. PLCγ cleavage of PtdIns(4, 5)P2 yields diacylglycerol (DAG), which stimulates protein kinase C (PKC) of various isoforms. The Ins(1, 4, 5)P3 byproduct engages its receptor on the endoplasmic reticulum (ER), triggering intracellular calcium release and store release-dependent influx of extracellular calcium (58, 59).
Another major signaling circuit emanating from antigen receptor aggregation involves Ras, which is coupled to the activation of Erk. It has been known for some time that this pathway is also dependent upon PtdIns(3–5)P3 generation (60), but it has been unclear precisely why. The Ras GTP exchange factor (GEF) SOS possesses a PtdIns(3–5)P3-binding PH domain and was implicated in this process. Recently, however, it was shown that RasGRP1 and RasGRP3 GEFs are critical intermediaries in Ras activation following BCR aggregation, apparently acting redundantly (61). These GEFs contain C1 domains that mediate their recruitment to the plasma membrane via binding to DAG. They are regulated by the localization and by DAG regulated kinases, presumably PKC isoforms. Finally, it has been shown very recently that RasGRPs function cooperatively with SOS (62). Following receptor aggregation, RasGRP generates the initial RasGTP pool. This RasGTP binds to SOS catalyzing its GEF activity and forward feeding generation of additional RasGTP.
It appears that all of the principle downstream signaling pathways emanating from the BCR are dependent on PI3K and the accumulation of PtdIns (3–5)P3. This will become important in the context of the role of inositol phospholipid and phosphotyrosine phosphatases as regulators of BCR signaling.
Antigen receptor signaling is regulated by multiple feedback mechanisms involving effectors as inositol lipid and phosphotyrosine phosphatases. Briefly, PtdIns(3–5)P3 accumulation is inhibited by the SH2 domain-containing phosphatidylinositol 5-phosphatases (SHIP-1), and by phosphatase and tensin homologue deleted on chromosome 10 (PTEN) (63, 64). SHIP-1 is phosphorylated upon BCR stimulation, indicating a role in feedback regulation (65). It is apparently recruited to Lyn via its adapter, downstream of kinase (Dok) (66–68). SHIP-1 can also be recruited via binding to co-aggregated FcγRIIB (69, 70). Upon BCR and FcγRIIB co-aggregation, the FcR immunoreceptor tyrosine-based inhibitory motif (ITIM) (71) is phosphorylated by Lyn, leading to recruitment of SHIP-1 and Dok. It is important to note that FcγRIIB-dependent activation of the SHIP-1/Dok circuit is much more robust than its activation in BCR feedback. The SH2-containing phosphotyrosine phosphatase SHP-1 also plays a central role in negative feedback regulation. SHP-1 action involves Lyn phosphorylation membrane adapters, e.g. CD22 and CD72 (72), which contain ITIMs. Phosphorylated ITIMs recruit SHP-1, which limits BCR signaling by dephosphorylation of it substrates, including Igα, Igβ, Syk, Vav, and SLP-65/BLNK (73). These molecules are discussed in greater detail below in the context of signaling in anergic B cells.
BCR signaling by chronically occupied antigen receptors
It has been shown in both anti-HEL and Ars/A1 models that maintenance of anergy requires constant binding of antigen to BCR (20, 74). This indicates that on anergic cells autoantigen-occupied BCR transduce signals that somehow enforce the unresponsive state. Consistent with this concept of chronic signaling by occupied receptors are the facts that anergic B cells exhibit elevated intracellular free calcium and activation of the Erk kinase pathway (75). Disengagement of the anergic BCR resulted in restoration of basal calcium levels to those seen in naive B cells, reduction of Erk phosphorylation, extension of lifespan, and restoration the ability to response to antigen by calcium mobilization and CD86 up-regulation (20). It therefore seems that chronicity of BCR occupancy in anergic cells leads to biased transduction of inhibitory signals.
We have begun to look more closely at mechanisms of signaling by chronically occupied BCR on anergic cells. Anergic B cells display subtle but significant increases in Igα/β ITAM tyrosine phosphorylation, but only monophosphorylation is detectable (S. O’Neill et al., unpublished data). This result is suggestive of low-level activation of Lyn, but not Syk, by chronically liganded receptors. ITAM monophosphorylation is unable to support Syk binding and activation, but Lyn can bind to monophosphorylated ITAMs (51, 76). This could generate signaling bias, with Lyn propagating the inhibitory signal. Consistent with this possibility, Lyn-deficient mice display an autoimmune phenotype, suggesting that the inhibitory role of Lyn is dominant over its activating role in BCR signaling (77, 78). Lyn is a likely candidate as a driver of inhibitory signaling based on its known substrates. Consistent with this possibility the Lyn substrates SHIP-1 and Dok-1 are constitutively phosphorylated in anergic B cells (21, 79, S. O’Neill, unpublished data)
Trans-inhibition of BCR and CxCR4 signaling in anergic cells
Signals that are generated by chronic BCR occupancy and enforce unresponsiveness act globally in the cell to inhibit signaling by remotely stimulated receptors (79). These include but may not be limited to antigen receptors and certain chemokine receptors. Inhibition is seen at the level of calcium mobilization and CxCL12 induced migration. These trans-inhibitory signals are rapidly terminated by removal of autoantigen, suggesting that they are mediated by a non-durable biochemical mechanism.
Some evidence exists relative to the site of action of these trans-inhibitory signals. Stimulation of unoccupied BCR on anergic B cells leads to ITAM monophosphorylation but no detectable biphosphorylation (S. O’Neill, unpublished data). Consistent with these findings, stimulation does not result in activation of Syk or broad scale protein tyrosine phosphorylation seen in naive cells (80, 81). Calcium mobilization is blunted as is activation of NF-κB and all other downstream responses (75). These results indicate that trans-inhibitory signals disrupt the earliest events in BCR signaling. Some evidence suggests that this may involve disruption of the physical integrity of the antigen receptor, resulting in inability of the BCR to transduce signals efficiently (82).
PTEN, SHIP-1, and SHP-1 as mediators of anergy
Significant evidence supports a role for SHIP-1, SHP-1, and PTEN in enforcing the unresponsiveness of anergic cells. SHIP-1 is composed of an amino-terminal SH2 domain, a phosphatase domain, and an extended C-terminal region containing two NPxY motifs and proline-rich region. These domains enable interactions with a several proteins, including Shc, SHP-2, Gab, CD150, and Dok (83). SHIP-1 associates with Dok-1 via binding of a phosphorylated NPxY to the Dok PTB domain. BCR stimulation leads to transient translocation of SHIP-1 to the plasma membrane (84). We hypothesize that this translocation is mediated by associated Dok, which contains a PH domain that binds PtdIns(3–5)P3 (85). SHIP-1 and its adapter Dok are tyrosine phosphorylated in transgenic and naturally occurring anergic cells (21, 79, S. O’Neill, unpublished dat), and Dok is the primary phosphoprotein that coprecipitates with SHIP-1 in these cells (JCC and P. Waterman, unpublished data).
Based on evidence suggesting that a SHIP-1/Dok inhibitory circuit is active in anergic B cells, we addressed SHIP-1 requirements for maintenance anergy. B-cell-targeted knockout of SHIP-1 was found to result in an autoimmune phenotype comparable in onset kinetics and severity to that seen in NZB/NZW and MRL models (S. O’Neill and AG and JCC, unpublished data). By 8 weeks of age, animals display easily detectable IgG anti-chromatin antibodies followed by onset of kidney disease and premature death. As noted earlier, SHIP-1 is also the primary mediator of inhibitory signaling by FcγRIIB, and on C57BL/6 background, FcγRIIB−/− mice develop autoimmunity (86). Thus, it is possible that disease in SHIP-1−/− mice actually reflects loss of FcγRIIB signaling function. However, in B-cell-targeted SHIP-1−/− mice autoimmunity is more rapid in onset and more severe than in FcγRIIB−/− mice and does not appear to be strain dependent (S. O’Neill, AG and JCC, unpublished data).
As a negative regulator of BCR signaling, SHP-1 is also a candidate mediator of the antigen unresponsiveness of anergic B cells. SHP-1 is a cytosolic tyrosine phosphatase. SHP-1 contains two adjacent amino-terminal SH2 domains that regulate its catalytic activity. Specifically, engagement of these SH2 domains by ITIM phosphotyrosines reduces repression of the phosphatase activity (87, 88). SHP-1 then binds, dephosphorylates, and therefore inactivates CD22 (89), Syk (90), and BLNK (by homology to SLP-76 in T cells) (91). Pao et al. (92) constructed B-cell-targeted SHP-1 knockout mice and studied their phenotype. Cellular analysis showed elevated B-1a cell numbers, impaired BCR-induced calcium mobilization in B-1a cells and reduced CD40-induced proliferation. Young mice displayed elevated serum Ig, and older mice displayed anti-DNA autoantibodies, immune complex deposition that led to glomerulonephritis and premature death. Onset and severity of disease in mice appears comparable with that seen in the B-cell-targeted SHIP-1 knockout.
There is an interesting and potentially important difference in regulation of SHP-1 and SHIP-1 that may be important to consider in the context of their potential function in anergy. Studies of signaling by inhibitory KIR and FcγRIIB have shown that SHP-1 acts only locally to inhibit signaling by receptors with which it is coaggregated (93). This is consistent with the concept that its SH2 domains must be engaged to upregulate its activity. Thus SHP-1, while an important mediator of inhibition of signaling mediated by receptors with which it is associated, seems an unlikely candidate for mediation of trans-inhibitory signals. However, these authors found that once activated by FcγRIIB (and presumably BCR), SHIP-1 is able to inhibit remotely stimulated receptors. Consistent with a possible role for SHIP-1, anergic B cells contain reduced cellular levels of PtdIns(3–5)P3 as detected by immunofluorescence staining (94), and signaling by both BCR and CxCR4 is dependent on PtdIns(3–5)P3. Finally, we have shown that trans-inhibition of CxCR4 signaling in anergic cells is largely dependent on SHIP-1 expression (79). Thus, we suspect that SHIP-1 is the primary mediator of trans-inhibitory signaling in anergic cells.
It was recently reported that anergic anti-HEL B cells express increased levels of the PTEN (94). PTEN mediates dephosphorylation of PtdIns(3–5)P3 yielding PtdIns(4, 5)P2, therefore antagonizing PI3K signaling. In addition to its phosphatase domain, PTEN contains a calcium-independent C2 domain that enables it to bind lipids, allowing association with the plasma membrane and correct orientation of the catalytic domain (95–98). PTEN activity seems to be regulated, at least in part, by protein levels (99). Clearly increased PTEN expression could contribute to trans-inhibitory signaling. Our laboratory has been able to confirm the report that PTEN is upregulated in anergic anti-HEL B cells, however, such upregulation is not seen in Ars/A1 B cells (S. O’Neill, unpublished data). Based on these limited studies, it seems likely that very high avidity autoantigen reactivity may uniquely upregulate PTEN expression. In this context, it is curious that PTEN knockout does not lead to significant autoimmunity. This may be because PTEN is required for activation of immunoglobulin class switch recombination (100, 101). Thus, PTEN knockout would prevent generation of pathogenic IgG autoantibodies. While this may explain lack of production of class switched autoantibodies, IgM autoantibody production is much greater in B-cell-targeted SHIP-1 and SHP-1 mice than in PTEN targeted mice. It seems most likely that the former play a more important role in maintaining anergy. Finally, it is surprising since both would be expected to result in increased levels of PtdIns(3–5)P3, that knockout of PTEN but not SHIP-1 impairs class switch recombination. This apparent paradox will require further study.
These findings underscore the important role of the PI3K pathway modulators in enforcing anergy. As shown in Fig. 1, stimulation of antigen receptors on anergic B cells may lead to inefficient activation of PI3K and the limited PtdIns(3–5)P3 that is produced under these circumstances may be immediately dephosphorylated by SHIP-1 or PTEN.
Fig. 1.
Signaling pathways operative in naive and anergic B cells.
Both SHIP-1 and PTEN reduce PtdIns(3–5)P3 levels, but there are important distinctions. While PTEN-mediated hydrolysis results in the formation of the PI3K and PLCγ substrate PtdIns(4, 5)P2, SHIP-1-mediated hydrolysis generates PtdIns(3, 4)P2 that acts as a secondary messenger by recruiting a unique set of PH domain-containing adapter proteins, including TAPP1 and TAPP2 (102). The function of TAPP proteins is poorly understood.
How are the activities of SHIP-1/Dok, SHP-1, and PTEN brought into play in anergic B cells? As noted earlier, functional activity of PTEN is thought to be determined primarily by its expression level, and it is uniquely upregulated in the MD4 × ML5 anergic cells. It is not known whether this upregulation reflects transcriptional, translation, or post-translational regulation. SHIP and Dok are substrates of Lyn suggesting direct interaction. However, it is not known whether some intermediary adapter is involved in enriching the substrates in the vicinity of the kinase. The activity of SHP-1 can apparently be regulated by phosphorylation of Y536 and Y564 in its C-terminal unique region (103). Based on the sequence surrounding these residues, they are likely sites of Lyn-mediated phosphorylation. As discussed below, available evidence indicates that SHP-1 can be ushered to the BCR signaling complex by multiple membrane adapter proteins that contain ITIMs.
In the mouse, there are two ITIM-containing membrane adapters that have been shown to participate in maintaining B-cell anergy by recruiting SHP-1 to sites of antigen receptor signaling: CD22 and CD72. Additional ITIM-containing membrane adapters, e.g. FcRL5 and PIR-B, are expressed by B cells, but their functions are less defined.
CD22
CD22 is a transmembrane receptor/adapter that binds specifically to α 2–6 sialic acid in appropriate amino acid context. It is expressed on the surface of B cells starting at the Pre-B stage in low levels, with high expression levels in mature B cells. Expression is lost during differentiation to the plasma cell stage (104, 105). It appears that CD22 must associate with the BCR to inhibit its signaling. CD22 inhibition of BCR signaling requires its sialic acid binding domain (106), suggesting direct CD22 interaction with carbohydrate on mIg (107). In addition, antigens that contain α2–6 in proper context induce enhanced CD22-mediated inhibition of BCR (108–111). After stimulation of the BCR by antigen, CD22 is tyrosine phosphorylated on three ITIMs in its intracellular tail by the Src family kinase Lyn (112). SHP-1 is recruited to the phosphorylated ITIMs and is activated (113). SHP-1 then dephosphorylates BCR signal transduction proteins, thereby inhibiting B-cell activation. Phosphorylation of CD22 can also recruit SHIP-1 via the adapter molecules Grb2 and Shc (114).
The role of CD22 in B-cell anergy was assessed using gene knockout mice (115, 116). Compared with wildtype littermates, B cells from CD22−/− mice are hyper-responsive to receptor signaling, as seen by higher concentration of free calcium in the cytosol, increased proliferation, and upregulation of CD86. CD22 also seems to play a role in preventing B-cell response to autoantigen-mimetic T-independent type 2 immunogens (108). Many extracellular autoantigens contain α2–6 sialic acid moieties and thus could bind CD22 and invoke its function.
The relationship of Lyn, CD22, and SHP-1 function in anergy was dissected using a haplo-insufficiency at each locus (89). Using this genetic approach, negative regulation was found in this pathway to be a quantitative trait, where each component plays a limiting role. These studies indicate that Lyn, CD22, and SHP1 function in a linear pathway contributing to the maintenance of anergy in the anti-HEL anergy model.
CD72
CD72 (Lyb-2) has also been advanced as an SHP-1 adapter involved in maintenance of anergy. CD72 is expressed from the pro-B through the mature stage and is downregulated in plasma cells (117, 118). The CD72 ligand is CD100, a semaphorin family receptor expressed on T cells and activated B cells (119). The cytoplasmic domain of CD72 contains two ITIMs that are tyrosine phosphorylated by Lyn, leading to recruitment of SHP-1, Grb-2, and Cbl-b (120–122).
The function of CD72 was investigated using genetic ablation (123). BM from CD72−/− mice showed increased numbers of pre-B cells and decreased numbers of recirculating mature B cells. Analysis of spleen and lymph nodes shows decreased numbers of total B cells. This finding suggests that lack of CD72 reduces the efficiency of transition from pre-B to immature stages. While the number of follicular B cells was decreased in these animals, the number of B-1 cells was increased. The role of CD72 in maintaining anergy was studied by crossing CD72−/− mice with MD4 and MD4 × ML5 mice (122). Sera from CD72−/− MD4 × ML5 mice were found to contain modestly increased levels of anti-HEL antibody relative to sera from MD4 × ML5 mice. Splenic B cells from CD72−/− MD4 × ML5 mounted greater calcium mobilization responses, survived longer, proliferated more, and produced more antibody in response to HEL stimulation relative to cells from MD4 × ML5 mice. Interestingly, CD72 was found to associate with and regulate Cbl-b (see below). Thus, CD72 may contribute the regulation of anergy in B cells.
Fc receptor-like molecules
Fc receptor-like (FcRL), also known as Ig-superfamily receptor translocation associated (IRTA), glycoproteins are members of the Ig-superfamily and homologues of the Fc receptor (FcR) family (124, 125). This homology includes linked genomic location, similar gene structure and content of ITIMs and ITAMs (126, 127). FcRL differ from FcRs in their preferential B-lineage expression and dual signaling properties, e.g. FcRL-1 possesses an ITAM, while FcRL-5 possesses both an ITAM and an ITIM (127). Furthermore, there is no evidence that FcRL function as Fc receptors, and their ligands are unknown. Only FcRL-1 and FcRL-5 are expressed in mice. While they can be detected in low levels in the pre-B stage, and expression peaks in the periphery. FcRL-1 is expressed predominantly on follicular, MZ, and B-1 cells. FcRL-5 is expressed in splenic MZ B cells and their precursors (128) and peritoneal cavity B-1 cells. FcRL-1 ligation induces modest B-cell proliferation. Cross-linking BCR and FcRL-1 leads to enhanced Ca mobilization, upregulation of CD69 and CD86, and does not result in apoptosis. These results demonstrate a costimulatory role for FcRL-1 (126). FcRL-5 possesses both an ITAM and an ITIM in its cytoplasmic tail. Co-aggregation of FcRL-5 with BCR causes inhibition of calcium mobilization. This inhibition indicates a dominant role for the ITIM, relative to the ITAM, in FcRL-5 signaling (126). A role for FcRL in autoimmunity is likely, given their expression on B cells, their signaling capacity and relation to classical FcγRIIB (that is implicated in autoimmune disorders) (129, 130). The FcRL are located in human chromosome 1, in a locus linked to several autoimmune disorders, including SLE and multiple sclerosis (131–133). FcRL-3 in humans is linked to rheumatoid arthritis in Japanese patients (134).
PIR-b
Paired Ig-like receptor-b (PIR-b), also called Lilrb3, is an ITIM-containing membrane adapter expressed by dendritic cells, mast cells, natural killer cells, monocytes, and B cells (135). PIR-b binding to its ligand major histocompatibility complex class I (MHC-I) (136) leads to phosphorylation of its ITIM (137) by Lyn (138). PIR-b phosphorylation creates a docking site for SHP-1, leading to dephosphorylation of BCR signaling molecules (139). Genetic ablation of PIR-b results in expansion of the B-1 population. Sera from 40-week-old PIR-b−/− mice displays elevated levels of rheumatoid factor (RF) (140). Intraperitoneal injection of the TLR-9 ligand CpG into 8-week-old PIR-b−/− mice leads to increased serum titers of RF compared with C57BL/6 mice. Additional studies of PIR-b−/− mice revealed increased susceptibility to salmonella infection (141) and impaired dendritic cell maturation (142). While these findings do implicate PIR-b in autoimmunity, it is probably not among the critical players in anergy.
Other potential contributors to antigen unresponsiveness of anergic cells
Genetic reprogramming
As discussed earlier, multiple features that distinguish anergic B cells are quickly lost upon removal of the autoantigen from receptors. While the rapidity of these changes is inconsistent with the possibility that anergy is maintained by genetic reprogramming, a contribution of such a mechanism cannot be excluded. It is especially tempting to entertain a role for genetic reprogramming in B-cell anergy because this mechanism has been shown to be important in T-cell anergy and would be durable.
Elevated intracellular free calcium level is a hallmark of both T and B-cell anergy, and the level of this elevation has been shown to be sufficient to activate the Ca-regulated transcription factor nuclear factor of activated T cells (NFAT) (143). In T cells, NFAT plays a critical role both in cell activation in the context of second signals and in inactivation due to TCR signaling alone, i.e. signal one (144). NFAT cooperates with the transcription factor activator protein-1 (AP-1) to upregulate genes involved in productive activation. AP-1 activation depends on costimulation. Stimulation of NFAT alone upregulates a unique set of genes that enforce T-cell unresponsiveness (145).
To test the potential function of NFAT activation in B-cell anergy, NFAT1−/− mice were crossed to MD4.ML5 mice and the consequences studied (144). The loss of NFAT1 resulted in increased life span of B cells, as suggested by a 20-fold increase in the number mature peripheral anti-HEL B cells. Anti-HEL autoantibody levels increased by 23-fold. Although not definitive, these findings suggest a role for NFAT1 in the maintenance of anergy in B cells.
c-CBL
Cbl family of proteins is E3 ubiquitin ligases (146) that interact with E2 ubiquitin-conjugating enzymes to regulate the signaling of broad range of receptors (147). This function is achieved by ubiquitination of components of the receptor’s signal transduction machinery. Cbl-b ablated mice develop spontaneous autoimmunity characterized by autoantibody production, B and T infiltration into multiple organs, and these cells hyperproliferate following antigen receptor stimulation (148). B-cell-specific ablation of both Cbl and Cbl-b results in developmental changes and autoimmune manifestations. These changes include increased numbers of T1, B1, and MZ B cells. The autoimmune manifestations include occurrence of anti-dsDNA antibodies, ANA antibodies, multi-organ infiltration, and glomerulonephritis. In Cbl/Cbl-b knockout, MD4 × ML5 mice anergy is lost as indicated by increased numbers of HEL-specific mature B cells and autoantibody production. These cells upregulate CD86 and MHC-II and mobilize Ca after stimulation, and increase tyrosine phosphorylation of BCR signal transduction molecules (149).
STIM-1
A consistent feature of anergic T and B cells is elevated basal intracellular free calcium and failure to mobilize calcium upon antigen receptor stimulation. We considered the possibility that this failure could reflect inactivation of signaling intermediaries that are required for calcium influx via store-operated calcium (SOC) entry mechanisms. Stromal interacting molecule 1 (STIM1) is an 84 kDa ER transmembrane protein that was first identified as a growth suppressor protein implicated in several malignancies (150, 151). STIM1 was also found to be part of the calcium release-activated calcium (CRAC) current (152), a type of SOC pathway entry found in a variety of mammalian cells, including white blood cells (153). In lymphocytes, TCR or BCR ligation-mediated generation of Ins(1, 4, 5)P3 leads to calcium release from the ER (72). The resultant local reduction in calcium concentration is sensed by STIM1, leading to conformational change of STIM1 and its ‘activation’ (154, 155). Triggering calcium entry is achieved by activated STIM1 association with a calcium channel pore subunit ORAI1 (CRACM1) (156–159). STIM1 aggregates in the ER membrane to form puncta, which induce ORAI1 dimers to dimerize creating a functional channel pore, consisting of four units of ORAI1 (160).
Genetic ablation was utilized to explore the role of STIM1 in cell development and activation. STIM1 deficiency is embryonic lethal in mice, therefore cells were isolated from the embryos and differentiated in culture. STIM1-deficient mast cells show impaired calcium influx when stimulated through the high affinity Fc receptor for IgE, FcεR, as well as impaired activation of NFAT and NF-κB transcription factors. In addition, degranulation and cytokine production are impaired (161). STIM1-deficient T cells show reduced calcium influx, reduced cytokine production, and reduced nuclear translocation of NFAT (162). A STIM1 mutation has been reported in a human family in which three siblings suffered from immunodeficiency, heptosplenomegaly, autoimmune hemolytic anemia, thrombocytopenia, and muscular hypotonia (163). The STIM1 mutation was found to be a nonsense mutation, creating a truncated protein that is not expressed. The phenotype of murine and the human STIM1 ablation underscores the important role STIM1 plays in cell activation.
It was recently shown that STIM1 function is regulated by posttranslational modification. While STIM1 has long known to be phosphorylated (153), the physiological relevance of this phosphorylation was unknown. In a recent work, phosphorylation was found to inhibit STIM1 function, and this modification was found to occur during mitosis. Phosphorylation therefore functions to prevent calcium signaling calcium during cell division (164). Phosphorylation of STIM1 serine residues 486 and 668 were found to mediate to inhibition of STIM1-mediated calcium influx.
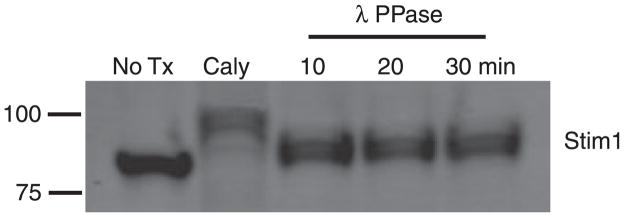
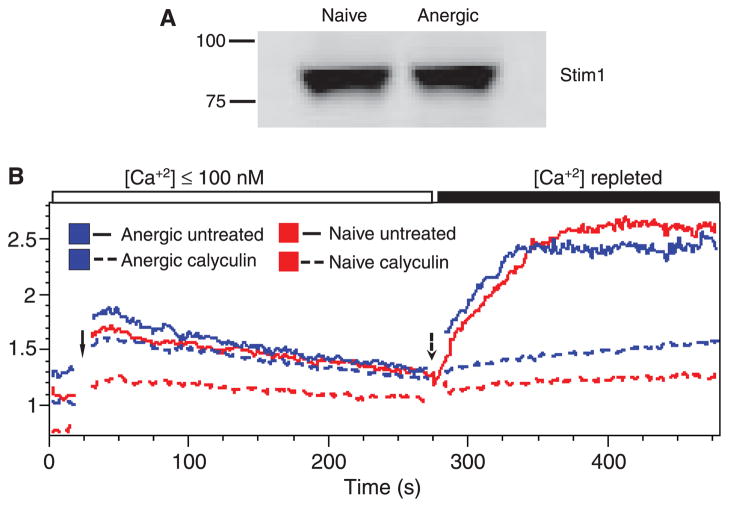
To explore the role of STIM1 phosphorylation in modulating calcium signaling in B cells, we first assessed its phosphorylation in the Bal-17 murine mature B-cell line (165). To determine whether a cycle of phosphorylation and dephosphorylation might be ongoing, we incubated cells with the serine/threonine phosphatase inhibitor Calyculin A and analyzed effects on apparent molecular weight. The treatment resulted in a molecular weight shift consistent with phosphorylation (Fig. 2). Treatment of the cell lysate with λ protein phosphatase, a broad-spectrum phosphatase, reduced the Mr of STIM1, indicating that the shift is indeed due to phosphorylation. We next determined, based on molecular weight shift, whether STIM1 is phosphorylated in anergic cells, using ex vivo B cells from the Ars/A1 mice (19). No molecular weight shift was seen (Fig. 3A).
Fig. 2. Stim1 phosphorylation in naive cells.
Bal-17 B-lymphoma cells (5 × 107 cells/ml) were treated with 50 nM Calyculin A or vehicle, and whole cell lysates were prepared using an NP-40 lysis buffer. 100 μl of lysate containing 5 × 106 cell equivalents of Calyculin A-treated cells was incubated with λ PPase (400 u) for the indicated time. The lysate was fractionated on SDS-PAGE gels, transferred to PVDF membrane, and blotted using rabbit anti-Stim1 antibody followed by rat anti-rabbit-680 nm secondary antibody. Blots were analyzed using a LYCOR fluorescence analyzer.
Fig. 3. Stim1 is functional in anergic B cells.
(A) Splenocytes isolated from κ tg (naive) and Ars/A1 (anergic) mice were CD43 depleted to yield B cells. Naive and anergic B cells were cell lysed using NP-40, separated on SDS-PAGE gels, transferred to PVDF membrane, and blotted with rabbit anti-Stim1 antibody followed by rat anti-rabbit-680 nm secondary antibody. (B) Naive and anergic splenocytes were loaded with Indo-AM and [Ca+2]i measured by flow cytometry. EGTA (4 mM final concentration) was added to each sample immediately before recording to chelate calcium. After the baseline was established, cells were stimulated with ionomycin (1 μM final concentration) (solid arrow) and analysis continued. Calcium was then repleted (dashed arrow) by addition of calcium chloride (4 mM final concentration) and analysis resumed.
A caveat in interpretation of this experiment is that phosphorylation of only the two critical serine residues STIM1 might not be sufficient cause a detectable molecular weight shift (164). To better answer the question of whether STIM1 function is altered in anergy, we compared the ability of splenocytes from Ars/A1 anergic and control κ transgenic mice to undergo store depletion induced calcium influx (Fig. 3B). Intracellular free calcium levels were recorded before and after cells in <100 nM calcium (EGTA buffer) were stimulated with the calcium ionophore ionomycin to deplete intracellular calcium stores. Calcium was then repleted, and calcium influx was measured. Anergic and naive B cells underwent equivalent influx responses indicating the STIM1 function is equivalent in anergic and naive cells. In further experiments Calyculin A pretreatment blocked calcium influx in both cells (Fig. 3B). These results are consistent with the hypothesis that STIM1 function is not impaired in anergic B cells.
References
- 1.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 2.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 5.Halverson R, Torres RM, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat Immunol. 2004;5:645–650. doi: 10.1038/ni1076. [DOI] [PubMed] [Google Scholar]
- 6.Nossal GJ, Pike BL. Clonal anergy: persistence in tolerant mice of antigen-binding B lymphocytes incapable of responding to antigen or mitogen. Proc Natl Acad Sci USA. 1980;77:1602–1606. doi: 10.1073/pnas.77.3.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodnow CC, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 8.Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat Rev Immunol. 2007;7:633–643. doi: 10.1038/nri2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodnow CC, Crosbie J, Jorgensen H, Brink RA, Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989;342:385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- 10.Rui L, Healy JI, Blasioli J, Goodnow CC. ERK signaling is a molecular switch integrating opposing inputs from B cell receptor and T cell cytokines to control TLR4-driven plasma cell differentiation. J Immunol. 2006;177:5337–5346. doi: 10.4049/jimmunol.177.8.5337. [DOI] [PubMed] [Google Scholar]
- 11.Rui L, Vinuesa CG, Blasioli J, Goodnow CC. Resistance to CpG DNA-induced autoimmunity through tolerogenic B cell antigen receptor ERK signaling. Nat Immunol. 2003;4:594–600. doi: 10.1038/ni924. [DOI] [PubMed] [Google Scholar]
- 12.Watson ML, et al. Genetic analysis of MRL-lpr mice: relationship of the Fas apoptosis gene to disease manifestations and renal disease-modifying loci. J Exp Med. 1992;176:1645–1656. doi: 10.1084/jem.176.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aarons I. Renal immunofluorescence in Nzb-Nzw mice. Nature. 1964;203:1080–1081. doi: 10.1038/2031080a0. [DOI] [PubMed] [Google Scholar]
- 14.Shlomchik MJ, Aucoin AH, Pisetsky DS, Weigert MG. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci USA. 1987;84:9150–9154. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erikson J, Radic MZ, Camper SA, Hardy RR, Carmack C, Weigert M. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991;349:331–334. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- 16.Eilat D, Hochberg M, Pumphrey J, Rudikoff S. Monoclonal antibodies to DNA and RNA from NZB/NZW F1 mice: antigenic specificities and NH2 terminal amino acid sequences. J Immunol. 1984;133:489–494. [PubMed] [Google Scholar]
- 17.Pewzner-Jung Y, Friedmann D, Sonoda E, Jung S, Rajewsky K, Eilat D. B cell deletion, anergy, and receptor editing in ‘knock in’ mice targeted with a germline-encoded or somatically mutated anti-DNA heavy chain. J Immunol. 1998;161:4634–4645. [PubMed] [Google Scholar]
- 18.Wysocki LJ, Sato VL. The strain A anti-p-azophenylarsonate major cross-reactive idiotypic family includes members with no reactivity toward p-azophenylarsonate. Eur J Immunol. 1981;11:832–839. doi: 10.1002/eji.1830111016. [DOI] [PubMed] [Google Scholar]
- 19.Benschop RJ, Aviszus K, Zhang X, Manser T, Cambier JC, Wysocki LJ. Activation and anergy in bone marrow B cells of a novel immunoglobulin transgenic mouse that is both hapten specific and autoreactive. Immunity. 2001;14:33–43. doi: 10.1016/s1074-7613(01)00087-5. [DOI] [PubMed] [Google Scholar]
- 20.Gauld SB, Benschop RJ, Merrell KT, Cambier JC. Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat Immunol. 2005;6:1160–1167. doi: 10.1038/ni1256. [DOI] [PubMed] [Google Scholar]
- 21.Merrell KT, et al. Identification of anergic B cells within a wild-type repertoire. Immunity. 2006;25:953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Teague BN, et al. Cutting edge: transitional T3 B cells do not give rise to mature B cells, have undergone selection, and are reduced in murine lupus. J Immunol. 2007;178:7511–7515. doi: 10.4049/jimmunol.178.12.7511. [DOI] [PubMed] [Google Scholar]
- 23.Roark JH, Bui A, Nguyen KA, Mandik L, Erikson J. Persistence of functionally compromised anti-double-stranded DNA B cells in the periphery of non-autoimmune mice. Int Immunol. 1997;9:1615–1626. doi: 10.1093/intimm/9.11.1615. [DOI] [PubMed] [Google Scholar]
- 24.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 25.Casellas R, et al. Contribution of receptor editing to the antibody repertoire. Science. 2001;291:1541–1544. doi: 10.1126/science.1056600. [DOI] [PubMed] [Google Scholar]
- 26.Adelstein S, et al. Induction of self-tolerance in T cells but not B cells of transgenic mice expressing little self antigen. Science. 1991;251:1223–1225. doi: 10.1126/science.1900950. [DOI] [PubMed] [Google Scholar]
- 27.Bretscher P, Cohn M. A theory of self-non-self discrimination. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 28.Kat I, Makdasi E, Fischel R, Eilat D. B-cell anergy is maintained in anti-DNA transgenic NZB/NZW mice. Int Immunol. 2010;22:101–111. doi: 10.1093/intimm/dxp120. [DOI] [PubMed] [Google Scholar]
- 29.Duty JA, et al. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J Exp Med. 2009;206:139–151. doi: 10.1084/jem.20080611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fulcher DA, et al. The fate of self-reactive B cells depends primarily on the degree of antigen receptor engagement and availability of T cell help. J Exp Med. 1996;183:2313–2328. doi: 10.1084/jem.183.5.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fulcher DA, Basten A. Reduced life span of anergic self-reactive B cells in a double-transgenic model. J Exp Med. 1994;179:125–134. doi: 10.1084/jem.179.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tough DF, Sprent J. Lifespan of lymphocytes. Immunol Res. 1995;14:1–12. doi: 10.1007/BF02918494. [DOI] [PubMed] [Google Scholar]
- 33.Mackay F, Schneider P, Rennert P, Browning J. BAFF and APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 34.Lesley R, et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 35.Mackay F, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliver PM, Vass T, Kappler J, Marrack P. Loss of the proapoptotic protein, BIM, breaks B cell anergy. J Exp Med. 2006;203:731–741. doi: 10.1084/jem.20051407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weintraub JP, Godfrey V, Wolthusen PA, Cheek RL, Eisenberg RA, Cohen PL. Immunological and pathological consequences of mutations in both Fas and Fas ligand. Cell Immunol. 1998;186:8–17. doi: 10.1006/cimm.1998.1290. [DOI] [PubMed] [Google Scholar]
- 38.Cohen PL, Eisenberg RA. The lpr and gld genes in systemic autoimmunity: life and death in the Fas lane. Immunol Today. 1992;13:427–428. doi: 10.1016/0167-5699(92)90066-G. [DOI] [PubMed] [Google Scholar]
- 39.Rathmell JC, et al. CD95 (Fas)-dependent elimination of self-reactive B cells upon interaction with CD4 + T cells. Nature. 1995;376:181–184. doi: 10.1038/376181a0. [DOI] [PubMed] [Google Scholar]
- 40.Cyster JG, Goodnow CC. Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity. 1995;3:691–701. doi: 10.1016/1074-7613(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 41.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 42.Reth M, et al. The B-cell antigen receptor complex. Immunol Today. 1991;12:196–201. doi: 10.1016/0167-5699(91)90053-V. [DOI] [PubMed] [Google Scholar]
- 43.Johnson SA, Pleiman CM, Pao L, Schneringer J, Hippen K, Cambier JC. Phosphorylated immunoreceptor signaling motifs (ITAMs) exhibit unique abilities to bind and activate Lyn and Syk tyrosine kinases. J Immunol. 1995;155:4596–4603. [PubMed] [Google Scholar]
- 44.Honda Z, et al. Sequential requirements of the N-terminal palmitoylation site and SH2 domain of Src family kinases in the initiation and progression of FcεRI signaling. Mol Cell Biol. 2000;20:1759–1771. doi: 10.1128/mcb.20.5.1759-1771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamanashi Y, Kakiuchi T, Mizuguchi J, Yamamoto T, Toyoshima K. Association of B cell antigen receptor with protein tyrosine kinase Lyn. Science. 1991;251:192–194. doi: 10.1126/science.1702903. [DOI] [PubMed] [Google Scholar]
- 46.Wechsler RJ, Monroe JG. Src-family tyrosine kinase p55fgr is expressed in murine splenic B cells and is activated in response to antigen receptor cross-linking. J Immunol. 1995;154:3234–3244. [PubMed] [Google Scholar]
- 47.Lin J, Justement LB. The MB-1/B29 heterodimer couples the B cell antigen receptor to multiple src family protein tyrosine kinases. J Immunol. 1992;149:1548–1555. [PubMed] [Google Scholar]
- 48.Campbell KS, Cambier JC. B lymphocyte antigen receptors (mIg) are non-covalently associated with a disulfide linked, inducibly phosphorylated glycoprotein complex. EMBO J. 1990;9:441–448. doi: 10.1002/j.1460-2075.1990.tb08129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeFranco AL, et al. Signal transduction by the B-cell antigen receptor. Ann N Y Acad Sci. 1995;766:195–201. doi: 10.1111/j.1749-6632.1995.tb26662.x. [DOI] [PubMed] [Google Scholar]
- 50.van Oers NS, Weiss A. The Syk/ZAP-70 protein tyrosine kinase connection to antigen receptor signalling processes. Semin Immunol. 1995;7:227–236. doi: 10.1006/smim.1995.0027. [DOI] [PubMed] [Google Scholar]
- 51.Kurosaki T, Johnson SA, Pao L, Sada K, Yamamura H, Cambier JC. Role of the Syk autophosphorylation site and SH2 domains in B cell antigen receptor signaling. J Exp Med. 1995;182:1815–1823. doi: 10.1084/jem.182.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keshvara LM, Isaacson C, Harrison ML, Geahlen RL. Syk activation and dissociation from the B-cell antigen receptor is mediated by phosphorylation of tyrosine 130. J Biol Chem. 1997;272:10377–10381. doi: 10.1074/jbc.272.16.10377. [DOI] [PubMed] [Google Scholar]
- 53.Pleiman CM, Hertz WM, Cambier JC. Activation of phosphatidylinositol-3′ kinase by Src-family kinase SH3 binding to the p85 subunit. Science. 1994;263:1609–1612. doi: 10.1126/science.8128248. [DOI] [PubMed] [Google Scholar]
- 54.Aiba Y, Kameyama M, Yamazaki T, Tedder TF, Kurosaki T. Regulation of B-cell development by BCAP and CD19 through their binding to phosphoinositide 3-kinase. Blood. 2008;111:1497–1503. doi: 10.1182/blood-2007-08-109769. [DOI] [PubMed] [Google Scholar]
- 55.Buhl AM, Pleiman CM, Rickert RC, Cambier JC. Qualitative regulation of B cell antigen receptor signaling by CD19: selective requirement for PI3-kinase activation, inositol-1,4,5-trisphosphate production and Ca2+ mobilization. J Exp Med. 1997;186:1897–1910. doi: 10.1084/jem.186.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujimoto M, et al. CD19 regulates Src family protein tyrosine kinase activation in B lymphocytes through processive amplification. Immunity. 2000;13:47–57. doi: 10.1016/s1074-7613(00)00007-8. [DOI] [PubMed] [Google Scholar]
- 57.Tedder TF, Zhou LJ, Engel P. The CD19/CD21 signal transduction complex of B lymphocytes. Immunol Today. 1994;15:437–442. doi: 10.1016/0167-5699(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 58.Fruman DA, Bismuth G. Fine tuning the immune response with PI3K. Immunol Rev. 2009;228:253–272. doi: 10.1111/j.1600-065X.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- 59.Hikida M, Kurosaki T. Regulation of phospholipase C-γ2 networks in B lymphocytes. Adv Immunol. 2005;88:73–96. doi: 10.1016/S0065-2776(05)88003-4. [DOI] [PubMed] [Google Scholar]
- 60.Hashimoto A, et al. Involvement of guanosine triphosphatases and phospholipase Cγ2 in extracellular signal-regulated kinase, c-Jun NH2-terminal kinase, and p38 mitogen-activated protein kinase activation by the B cell antigen receptor. J Exp Med. 1998;188:1287–1295. doi: 10.1084/jem.188.7.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coughlin JJ, Stang SL, Dower NA, Stone JC. RasGRP1 and RasGRP3 regulate B cell proliferation by facilitating B cell receptor-Ras signaling. J Immunol. 2005;175:7179–7184. doi: 10.4049/jimmunol.175.11.7179. [DOI] [PubMed] [Google Scholar]
- 62.Roose JP, Mollenauer M, Ho M, Kurosaki T, Weiss A. Unusual interplay of two types of Ras activators, RasGRP and SOS, establishes sensitive and robust Ras activation in lymphocytes. Mol Cell Biol. 2007;27:2732–2745. doi: 10.1128/MCB.01882-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leung WH, Tarasenko T, Bolland S. Differential roles for the inositol phosphatase SHIP in the regulation of macrophages and lymphocytes. Immunol Res. 2009;43:243–251. doi: 10.1007/s12026-008-8078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leslie NR, Downes CP. PTEN: The down side of PI 3-kinase signalling. Cell Signal. 2002;14:285–295. doi: 10.1016/s0898-6568(01)00234-0. [DOI] [PubMed] [Google Scholar]
- 65.Fong DC, et al. Mutational analysis reveals multiple distinct sites within Fcγ receptor IIB that function in inhibitory signaling. J Immunol. 2000;165:4453–4462. doi: 10.4049/jimmunol.165.8.4453. [DOI] [PubMed] [Google Scholar]
- 66.Songyang Z, Yamanashi Y, Liu D, Baltimore D. Domain-dependent function of the rasGAP-binding protein p62Dok in cell signaling. J Biol Chem. 2001;276:2459–2465. doi: 10.1074/jbc.M005504200. [DOI] [PubMed] [Google Scholar]
- 67.Yamanashi Y, et al. Role of the rasGAP-associated docking protein p62(dok) in negative regulation of B cell receptor-mediated signaling. Genes Dev. 2000;14:11–16. [PMC free article] [PubMed] [Google Scholar]
- 68.Fong DC, Malbec O, Arock M, Cambier JC, Fridman WH, Daeron M. Selective in vivo recruitment of the phosphatidylinositol phosphatase SHIP by phosphorylated Fcγ receptor IIB during negative regulation of IgE-dependent mouse mast cell activation. Immunol Lett. 1996;54:83–91. doi: 10.1016/s0165-2478(96)02654-5. [DOI] [PubMed] [Google Scholar]
- 69.Malbec O, et al. Fc epsilon receptor I-associated lyn-dependent phosphorylation of Fcγ receptor IIB during negative regulation of mast cell activation. J Immunol. 1998;160:1647–158. [PubMed] [Google Scholar]
- 70.D’Ambrosio D, Fong DC, Cambier JC. The SHIP phosphatase becomes associated with FcγRIIB1 and is tyrosine phosphorylated during ‘negative’ signaling. Immunol Lett. 1996;54:77–82. doi: 10.1016/s0165-2478(96)02653-3. [DOI] [PubMed] [Google Scholar]
- 71.Daeron M, et al. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of FcγRIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity. 1995;3:635–646. doi: 10.1016/1074-7613(95)90134-5. [DOI] [PubMed] [Google Scholar]
- 72.Dal Porto JM, Gauld SB, Merrell KT, Mills D, Pugh-Bernard AE, Cambier J. B cell antigen receptor signaling 101. Mol Immunol. 2004;41:599–613. doi: 10.1016/j.molimm.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 73.Plas DR, Thomas ML. Negative regulation of antigen receptor signaling in lymphocytes. J Mol Med. 1998;76:589–595. doi: 10.1007/s001090050254. [DOI] [PubMed] [Google Scholar]
- 74.Rathmell JC, Fournier S, Weintraub BC, Allison JP, Goodnow CC. Repression of B7.2 on self-reactive B cells is essential to prevent proliferation and allow Fas-mediated deletion by CD4(+) T cells. J Exp Med. 1998;188:651–659. doi: 10.1084/jem.188.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Healy JI, et al. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419–428. doi: 10.1016/s1074-7613(00)80285-x. [DOI] [PubMed] [Google Scholar]
- 76.Pao LI, Famiglietti SJ, Cambier JC. Asymmetrical phosphorylation and function of immunoreceptor tyrosine-based activation motif tyrosines in B cell antigen receptor signal transduction. J Immunol. 1998;160:3305–3314. [PubMed] [Google Scholar]
- 77.Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 78.Hibbs ML, et al. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 79.Brauweiler A, Merrell K, Gauld SB, Cambier JC. Cutting edge: acute and chronic exposure of immature B cells to antigen leads to impaired homing and SHIP1-dependent reduction in stromal cell-derived factor-1 responsiveness. J Immunol. 2007;178:3353–3357. doi: 10.4049/jimmunol.178.6.3353. [DOI] [PubMed] [Google Scholar]
- 80.Cooke MP, et al. Immunoglobulin signal transduction guides the specificity of B cell-T cell interactions and is blocked in tolerant self-reactive B cells. J Exp Med. 1994;179:425–438. doi: 10.1084/jem.179.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vilen BJ, Burke KM, Sleater M, Cambier JC. Transmodulation of BCR signaling by transduction-incompetent antigen receptors: implications for impaired signaling in anergic B cells. J Immunol. 2002;168:4344–4451. doi: 10.4049/jimmunol.168.9.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vilen BJ, Nakamura T, Cambier JC. Antigen-stimulated dissociation of BCR mIg from Igα/Igβ: implications for receptor desensitization. Immunity. 1999;10:239–248. doi: 10.1016/s1074-7613(00)80024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dorner T, Lipsky PE. Signalling pathways in B cells: implications for autoimmunity. Curr Top Microbiol Immunol. 2006;305:213–240. doi: 10.1007/3-540-29714-6_11. [DOI] [PubMed] [Google Scholar]
- 84.Petrie RJ, Schnetkamp PP, Patel KD, Awasthi-Kalia M, Deans JP. Transient translocation of the B cell receptor and Src homology 2 domain-containing inositol phosphatase to lipid rafts: evidence toward a role in calcium regulation. J Immunol. 2000;165:1220–1227. doi: 10.4049/jimmunol.165.3.1220. [DOI] [PubMed] [Google Scholar]
- 85.Zhao M, Schmitz AA, Qin Y, Di Cristofano A, Pandolfi PP, Van Aelst L. Phosphoinositide 3-kinase-dependent membrane recruitment of p62(dok) is essential for its negative effect on mitogen-activated protein (MAP) kinase activation. J Exp Med. 2001;194:265–274. doi: 10.1084/jem.194.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bolland S, Ravetch JV. Spontaneous autoimmune disease in FcγRIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 87.Yang J, Liang X, Niu T, Meng W, Zhao Z, Zhou GW. Crystal structure of the catalytic domain of protein-tyrosine phosphatase SHP-1. J Biol Chem. 1998;273:28199–28207. doi: 10.1074/jbc.273.43.28199. [DOI] [PubMed] [Google Scholar]
- 88.Frank C, Keilhack H, Opitz F, Zschornig O, Bohmer FD. Binding of phosphatidic acid to the protein-tyrosine phosphatase SHP-1 as a basis for activity modulation. Biochemistry. 1999;38:11993–12002. doi: 10.1021/bi982586w. [DOI] [PubMed] [Google Scholar]
- 89.Cornall RJ, et al. Polygenic autoimmune traits: Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity. 1998;8:497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
- 90.Dustin LB, et al. Expression of dominant-negative src-homology domain 2-containing protein tyrosine phosphatase-1 results in increased Syk tyrosine kinase activity and B cell activation. J Immunol. 1999;162:2717–2724. [PubMed] [Google Scholar]
- 91.Binstadt BA, et al. SLP-76 is a direct substrate of SHP-1 recruited to killer cell inhibitory receptors. J Biol Chem. 1998;273:27518–27523. doi: 10.1074/jbc.273.42.27518. [DOI] [PubMed] [Google Scholar]
- 92.Pao LI, et al. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27:35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 93.Blery M, et al. Reconstituted killer cell inhibitory receptors for major histocompatibility complex class I molecules control mast cell activation induced via immunoreceptor tyrosine-based activation motifs. J Biol Chem. 1997;272:8989–8996. doi: 10.1074/jbc.272.14.8989. [DOI] [PubMed] [Google Scholar]
- 94.Browne CD, Del Nagro CJ, Cato MH, Dengler HS, Rickert RC. Suppression of phosphatidylinositol 3,4,5-trisphosphate production is a key determinant of B cell anergy. Immunity. 2009;31:749–760. doi: 10.1016/j.immuni.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee JO, et al. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- 96.Gelb MH, Min JH, Jain MK. Do membrane-bound enzymes access their substrates from the membrane or aqueous phase: interfacial versus non-interfacial enzymes. Biochim Biophys Acta. 2000;1488:20–27. doi: 10.1016/s1388-1981(00)00106-2. [DOI] [PubMed] [Google Scholar]
- 97.Georgescu MM, Kirsch KH, Kaloudis P, Yang H, Pavletich NP, Hanafusa H. Stabilization and productive positioning roles of the C2 domain of PTEN tumor suppressor. Cancer Res. 2000;60:7033–7038. [PubMed] [Google Scholar]
- 98.Leslie NR, Bennett D, Gray A, Pass I, Hoang-Xuan K, Downes CP. Targeting mutants of PTEN reveal distinct subsets of tumour suppressor functions. Biochem J. 2001;357:427–435. doi: 10.1042/0264-6021:3570427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sly LM, Rauh MJ, Kalesnikoff J, Buchse T, Krystal G. SHIP, SHIP2, and PTEN activities are regulated in vivo by modulation of their protein levels: SHIP is up-regulated in macrophages and mast cells by lipopolysaccharide. Exp Hematol. 2003;31:1170–1181. doi: 10.1016/j.exphem.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 100.Omori SA, et al. Regulation of class-switch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signaling. Immunity. 2006;25:545–557. doi: 10.1016/j.immuni.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 101.Kishimoto H, et al. Physiological functions of PTEN in mouse tissues. Cell Struct Funct. 2003;28:11–21. doi: 10.1247/csf.28.11. [DOI] [PubMed] [Google Scholar]
- 102.Marshall AJ, Krahn AK, Ma K, Duronio V, Hou S. TAPP1 and TAPP2 are targets of phosphatidylinositol 3-kinase signaling in B cells: sustained plasma membrane recruitment triggered by the B-cell antigen receptor. Mol Cell Biol. 2002;22:5479–5491. doi: 10.1128/MCB.22.15.5479-5491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Z, Shen K, Lu W, Cole PA. The role of C-terminal tyrosine phosphorylation in the regulation of SHP-1 explored via expressed protein ligation. J Biol Chem. 2003;278:4668–4674. doi: 10.1074/jbc.M210028200. [DOI] [PubMed] [Google Scholar]
- 104.Stoddart A, Ray RJ, Paige CJ. Analysis of murine CD22 during B cell development: CD22 is expressed on B cell progenitors prior to IgM. Int Immunol. 1997;9:1571–1579. doi: 10.1093/intimm/9.10.1571. [DOI] [PubMed] [Google Scholar]
- 105.Torres RM, et al. Identification and characterization of the murine homologue of CD22, a B lymphocyte-restricted adhesion molecule. J Immunol. 1992;149:2641–2649. [PubMed] [Google Scholar]
- 106.Jin L, McLean PA, Neel BG, Wortis HH. Sialic acid binding domains of CD22 are required for negative regulation of B cell receptor signaling. J Exp Med. 2002;195:1199–1205. doi: 10.1084/jem.20011796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nitschke L. CD22 and Siglec-G: B-cell inhibitory receptors with distinct functions. Immunol Rev. 2009;230:128–143. doi: 10.1111/j.1600-065X.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 108.Duong BH, et al. Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo. J Exp Med. 2010;207:173–187. doi: 10.1084/jem.20091873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Courtney AH, Puffer EB, Pontrello JK, Yang ZQ, Kiessling LL. Sialylated multivalent antigens engage CD22 in trans and inhibit B cell activation. Proc Natl Acad Sci USA. 2009;106:2500–2505. doi: 10.1073/pnas.0807207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kelm S, Gerlach J, Brossmer R, Danzer CP, Nitschke L. The ligand-binding domain of CD22 is needed for inhibition of the B cell receptor signal, as demonstrated by a novel human CD22-specific inhibitor compound. J Exp Med. 2002;195:1207–1213. doi: 10.1084/jem.20011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Engel P, Wagner N, Miller AS, Tedder TF. Identification of the ligand-binding domains of CD22, a member of the immunoglobulin superfamily that uniquely binds a sialic acid-dependent ligand. J Exp Med. 1995;181:1581–1586. doi: 10.1084/jem.181.4.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Conrad FJ, Rice JS, Cambier JC. Multiple paths to loss of anergy and gain of autoimmunity. Autoimmunity. 2007;40:418–424. doi: 10.1080/08916930701464723. [DOI] [PubMed] [Google Scholar]
- 113.Blasioli J, Paust S, Thomas ML. Definition of the sites of interaction between the protein tyrosine phosphatase SHP-1 and CD22. J Biol Chem. 1999;274:2303–2307. doi: 10.1074/jbc.274.4.2303. [DOI] [PubMed] [Google Scholar]
- 114.Poe JC, Fujimoto M, Jansen PJ, Miller AS, Tedder TF. CD22 forms a quaternary complex with SHIP, Grb2, and Shc: a pathway for regulation of B lymphocyte antigen receptor-induced calcium flux. J Biol Chem. 2000;275:17420–17427. doi: 10.1074/jbc.M001892200. [DOI] [PubMed] [Google Scholar]
- 115.O’Keefe TL, Williams GT, Batista FD, Neuberger MS. Deficiency in CD22, a B cell-specific inhibitory receptor, is sufficient to predispose to development of high affinity autoantibodies. J Exp Med. 1999;189:1307–1313. doi: 10.1084/jem.189.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.O’Keefe TL, Williams GT, Davies SL, Neuberger MS. Hyperresponsive B cells in CD22-deficient mice. Science. 1996;274:798–801. doi: 10.1126/science.274.5288.798. [DOI] [PubMed] [Google Scholar]
- 117.Yakura H, Shen FW, Kaemmer M, Boyse EA. Lyb-2 system of mouse B cells. Evidence for a role in the generation of antibody-forming cells. J Exp Med. 1981;153:129–135. doi: 10.1084/jem.153.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Parnes JR, Pan C. CD72, a negative regulator of B-cell responsiveness. Immunol Rev. 2000;176:75–85. doi: 10.1034/j.1600-065x.2000.00608.x. [DOI] [PubMed] [Google Scholar]
- 119.Kumanogoh A, et al. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity. 2000;13:621–631. doi: 10.1016/s1074-7613(00)00062-5. [DOI] [PubMed] [Google Scholar]
- 120.Adachi T, Flaswinkel H, Yakura H, Reth M, Tsubata T. The B cell surface protein CD72 recruits the tyrosine phosphatase SHP-1 upon tyrosine phosphorylation. J Immunol. 1998;160:4662–4665. [PubMed] [Google Scholar]
- 121.Wu Y, et al. The B-cell transmembrane protein CD72 binds to and is an in vivo substrate of the protein tyrosine phosphatase SHP-1. Curr Biol. 1998;8:1009–1017. doi: 10.1016/s0960-9822(07)00421-6. [DOI] [PubMed] [Google Scholar]
- 122.Li DH, et al. Modulation of peripheral B cell tolerance by CD72 in a murine model. Arthritis Rheum. 2008;58:3192–3204. doi: 10.1002/art.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pan C, Baumgarth N, Parnes JR. CD72-deficient mice reveal nonredundant roles of CD72 in B cell development and activation. Immunity. 1999;11:495–506. doi: 10.1016/s1074-7613(00)80124-7. [DOI] [PubMed] [Google Scholar]
- 124.Davis RS, Wang YH, Kubagawa H, Cooper MD. Identification of a family of Fc receptor homologs with preferential B cell expression. Proc Natl Acad Sci USA. 2001;98:9772–9777. doi: 10.1073/pnas.171308498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hatzivassiliou G, et al. IRTA1 and IRTA2, novel immunoglobulin superfamily receptors expressed in B cells and involved in chromosome 1q21 abnormalities in B cell malignancy. Immunity. 2001;14:277–289. doi: 10.1016/s1074-7613(01)00109-1. [DOI] [PubMed] [Google Scholar]
- 126.Davis RS. Fc receptor-like molecules. Annu Rev Immunol. 2007;25:525–560. doi: 10.1146/annurev.immunol.25.022106.141541. [DOI] [PubMed] [Google Scholar]
- 127.Davis RS, Dennis G, Jr, Kubagawa H, Cooper MD. Fc receptor homologs (FcRH1-5) extend the Fc receptor family. Curr Top Microbiol Immunol. 2002;266:85–112. doi: 10.1007/978-3-662-04700-2_7. [DOI] [PubMed] [Google Scholar]
- 128.Srivastava B, Quinn WJ, III, Hazard K, Erikson J, Allman D. Characterization of marginal zone B cell precursors. J Exp Med. 2005;202:1225–1234. doi: 10.1084/jem.20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nieto A, Caliz R, Pascual M, Mataran L, Garcia S, Martin J. Involvement of Fcγ receptor IIIA genotypes in susceptibility to rheumatoid arthritis. Arthritis Rheum. 2000;43:735–739. doi: 10.1002/1529-0131(200004)43:4<735::AID-ANR3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 130.Kimberly RP, et al. Diversity and duplicity: human Fcγ receptors in host defense and autoimmunity. Immunol Res. 2002;26:177–189. doi: 10.1385/IR:26:1-3:177. [DOI] [PubMed] [Google Scholar]
- 131.Marrack P, Kappler J, Kotzin BL. Autoimmune disease: why and where it occurs. Nat Med. 2001;7:899–905. doi: 10.1038/90935. [DOI] [PubMed] [Google Scholar]
- 132.Dai KZ, et al. The T cell regulator gene SH2D2A contributes to the genetic susceptibility of multiple sclerosis. Genes Immun. 2001;2:263–268. doi: 10.1038/sj.gene.6363774. [DOI] [PubMed] [Google Scholar]
- 133.Tsao BP. The genetics of human systemic lupus erythematosus. Trends Immunol. 2003;24:595–602. doi: 10.1016/j.it.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 134.Kochi Y, et al. A functional variant in FCRL3, encoding Fc receptor-like 3, is associated with rheumatoid arthritis and several autoimmunities. Nat Genet. 2005;37:478–485. doi: 10.1038/ng1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Arm JP, Nwankwo C, Austen KF. Molecular identification of a novel family of human Ig superfamily members that possess immunoreceptor tyrosine-based inhibition motifs and homology to the mouse gp49B1 inhibitory receptor. J Immunol. 1997;159:2342–2349. [PubMed] [Google Scholar]
- 136.Borges L, Hsu ML, Fanger N, Kubin M, Cosman D. A family of human lymphoid and myeloid Ig-like receptors, some of which bind to MHC class I molecules. J Immunol. 1997;159:5192–5196. [PubMed] [Google Scholar]
- 137.Maeda A, Kurosaki M, Ono M, Takai T, Kurosaki T. Requirement of SH2-containing protein tyrosine phosphatases SHP-1 and SHP-2 for paired immunoglobulin-like receptor B (PIR-B)-mediated inhibitory signal. J Exp Med. 1998;187:1355–1360. doi: 10.1084/jem.187.8.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ho LH, Uehara T, Chen CC, Kubagawa H, Cooper MD. Constitutive tyrosine phosphorylation of the inhibitory paired Ig-like receptor PIR-B. Proc Natl Acad Sci USA. 1999;96:15086–15090. doi: 10.1073/pnas.96.26.15086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Maeda A, Scharenberg AM, Tsukada S, Bolen JB, Kinet JP, Kurosaki T. Paired immunoglobulin-like receptor B (PIR-B) inhibits BCR-induced activation of Syk and Btk by SHP-1. Oncogene. 1999;18:2291–2297. doi: 10.1038/sj.onc.1202552. [DOI] [PubMed] [Google Scholar]
- 140.Kubo T, et al. Augmented TLR9-induced Btk activation in PIR-B-deficient B-1 cells provokes excessive autoantibody production and autoimmunity. J Exp Med. 2009;206:1971–1982. doi: 10.1084/jem.20082392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Torii I, et al. PIR-B-deficient mice are susceptible to Salmonella infection. J Immunol. 2008;181:4229–4239. doi: 10.4049/jimmunol.181.6.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ujike A, Takeda K, Nakamura A, Ebihara S, Akiyama K, Takai T. Impaired dendritic cell maturation and increased T(H)2 responses in PIR-B(-/-) mice. Nat Immunol. 2002;3:542–548. doi: 10.1038/ni801. [DOI] [PubMed] [Google Scholar]
- 143.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 144.Borde M, Barrington RA, Heissmeyer V, Carroll MC, Rao A. Transcriptional basis of lymphocyte tolerance. Immunol Rev. 2006;210:105–119. doi: 10.1111/j.0105-2896.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 145.Soto-Nieves N, et al. Transcriptional complexes formed by NFAT dimers regulate the induction of T cell tolerance. J Exp Med. 2009;206:867–876. doi: 10.1084/jem.20082731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Joazeiro CA, Wing SS, Huang H, Leverson JD, Hunter T, Liu YC. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science. 1999;286:309–312. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]
- 147.Duan L, Reddi AL, Ghosh A, Dimri M, Band H. The Cbl family and other ubiquitin ligases: destructive forces in control of antigen receptor signaling. Immunity. 2004;21:7–17. doi: 10.1016/j.immuni.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 148.Bachmaier K, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 149.Kitaura Y, et al. Control of the B cell-intrinsic tolerance programs by ubiquitin ligases Cbl and Cbl-b. Immunity. 2007;26:567–578. doi: 10.1016/j.immuni.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sotelo-Avila C, Gooch WM., III Neoplasms associated with the Beckwith-Wiedemann syndrome. Perspect Pediatr Pathol. 1976;3:255–272. [PubMed] [Google Scholar]
- 151.Hu RJ, et al. A 2.5-Mb transcript map of a tumor-suppressing subchromosomal transferable fragment from 11p15.5, and isolation and sequence analysis of three novel genes. Genomics. 1997;46:9–17. doi: 10.1006/geno.1997.4981. [DOI] [PubMed] [Google Scholar]
- 152.Roos J, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Manji SS, et al. STIM1: a novel phosphoprotein located at the cell surface. Biochim Biophys Acta. 2000;1481:147–155. doi: 10.1016/s0167-4838(00)00105-9. [DOI] [PubMed] [Google Scholar]
- 154.Baba Y, et al. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci USA. 2006;103:16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: an initiation mechanism for capacitive Ca2+ entry. J Biol Chem. 2006;281:35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 156.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 157.Vig M, et al. CRACM1 is a plasma membrane protein essential for storeoperated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Penna A, et al. The CRAC channel consists of a tetramer formed by STIM-induced dimerization of Orai dimers. Nature. 2008;456:116–120. doi: 10.1038/nature07338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol. 2008;9:81–88. doi: 10.1038/ni1546. [DOI] [PubMed] [Google Scholar]
- 162.Oh-Hora M, et al. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Picard C, et al. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N Engl J Med. 2009;360:1971–1980. doi: 10.1056/NEJMoa0900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Smyth JT, et al. Phosphorylation of STIM1 underlies suppression of store-operated calcium entry during mitosis. Nat Cell Biol. 2009;11:1465–1472. doi: 10.1038/ncb1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mond JJ, Feuerstein N, Finkelman FD, Huang F, Huang KP, Dennis G. B-lymphocyte activation mediated by anti-immunoglobulin antibody in the absence of protein kinase C. Proc Natl Acad Sci USA. 1987;84:8588–8592. doi: 10.1073/pnas.84.23.8588. [DOI] [PMC free article] [PubMed] [Google Scholar]