Abstract
Dehydroepiandrosterone (DHEA) and its sulfate form (DHEAS) have been the focus of considerable publicity because of their demonstrated associations with a broad range of health outcomes. Yet, knowledge about the effects of endogenous DHEA(S) on health in humans is limited and often inconclusive, largely because few of the studies have been based on prospective surveys of population-representative samples. This analysis uses a national longitudinal survey in Taiwan to investigate whether DHEAS is associated with subsequent changes (2000–2003) in functional limitations, cognitive impairment, depressive symptoms, and global self-rated health. Multivariate regression models based on this older Taiwanese sample show that among men, lower levels of DHEAS are related to declines in mobility and self-assessed health status and increases in depressive symptoms, while both low and very high levels of DHEAS are associated with poor cognitive function. There are no significant associations among women. These findings differ from those in a previous cross-sectional analysis based on the Taiwan study and underscore the importance of using prospective data to examine the effects of DHEAS on health. The evidence based on this and other longitudinal studies suggests that endogenous DHEAS is related to health outcomes for men, but not women, in both Western and non-Western populations.
Keywords: Dehydroepiandrosterone, Dehydroepiandrosterone sulfate, Health, Mental Health, Longitudinal Survey, Aged, Taiwan
INTRODUCTION
The adrenal androgen dehydroepiandrosterone (DHEA) and its sulfate form (DHEAS) have been the focus of considerable publicity in recent years because of their demonstrated associations with a broad range of health outcomes and extrapolated claims that, taken as nutritional supplements, they may enhance longevity. These inactive prohormones are secreted in large amounts by the adrenal cortex only in humans and in other primates and are converted to androgens and estrogens in peripheral tissues (Labrie et al., 2005). The steady decrease in serum levels of these steroids from early adulthood onwards suggests that they may be markers of aging and that high levels may protect against the pathological consequences of becoming old. Animal experiments reveal broad-ranging health effects of DHEA and DHEAS, and experimental and observational studies on humans demonstrate statistically significant (p<0.05) associations with survival and various measures of health status, including diabetes, cardiovascular disease, obesity, cognitive impairment, physical limitations and depressive symptoms (see, for example, Barrett-Connor and Goodman-Gruen, 1995; Berr et al., 1996; Cappola et al., 2006; Feldman et al., 2001; Mazat et al., 2001; Trivedi and Khaw, 2001).
In light of the fact that DHEA and DHEAS are precursors to sex hormones, it is not surprising that there appear to be important differences between men and women. Virtually all studies find that the concentration of these steroids is much higher in men than in women and many find that the associations between DHEA(S) and health or survival vary by sex. However, these latter findings are often contradictory, with some studies suggesting associations only among males, others finding no significant differences by sex, and two studies reporting stronger associations among women than men for selected outcomes (Berr et al., 1996; Glei et al., 2004).
Despite extensive research on these steroids, little is known about their specific functions or the mechanisms by which they affect health (Celec and Starka, 2003). Research suggests that DHEA may counterbalance the immunosuppressive effects of glucocorticoids, although the receptor for DHEA has not been fully characterized (Butcher and Lord, 2004). DHEA has been shown to have biological actions on hemostasis, cell proliferation, lipid metabolism, stress response, and immune function (Feldman et al., 2001; Thijssen and Nieuwenhuyse, 1999). Knowledge about the effects of DHEA(S) on health in humans is limited and often inconclusive for several reasons: many of the experimental studies have been performed on non-primates, which have far lower concentrations of these steroids than humans (e.g., Bovenberg et al., 2005; Dhatariya et al., 2005); experimental studies in humans have typically involved short-term administration of pharmacological doses, small samples, and subjects with various disorders such as adrenal insufficiency (e.g., Bovenberg et al., 2005; Dhatariya et al., 2005); the effects of DHEA(S) supplementation may not be generalizable to those of endogenous DHEA(S); and observational studies of humans have been primarily cross-sectional, preventing researchers from separating what are believed to be the effects of certain illnesses on DHEA(S) levels from the sought after effects of DHEA(S) on health status (Herbert, 1995; Kiechl et al., 2000).
Surprisingly few studies have been based on prospective surveys of population-representative samples, despite the advantage of this research design for investigating the effects of endogenous levels of DHEA(S) on health in the general population (e.g., high non-response rates, selection of healthy subjects, and non-random selection from larger samples; Berr et al., 1996; Feldman et al., 2001; Nafziger et al., 1991; Thijs et al., 2003). Moreover, virtually all of the longitudinal population-based surveys that have been carried out are based on community samples of (predominantly) whites in the United States or Western Europe, thereby limiting generalizations to broader populations because of racial and ethnic differences in concentrations of DHEAS and adrenal metabolism (Kiechl et al., 2000; Nafziger et al., 1991). Many have additional limitations, including restriction to one sex (Cappola et al., 2006; Feldman et al., 2001; Roth et al., 2002; Yaffe et al., 1998) and small, selective, or non-representative samples (e.g., high non-response rates, selection of healthy subjects, and non-random selection from larger samplesBerr et al., 1996; Birkenhager-Gillesse et al., 1994; Cappola et al., 2006; Kalmijn et al., 1998; Mazat et al., 2001; Roth et al., 2002). In addition, many of these surveys focus on survival, providing less information on the consequences of varying levels of DHEA(S) for other important outcomes related to physical and mental well-being.
In the present analysis, we use the 2000 Social Environment and Biomarkers of Aging Study (SEBAS) to explore the associations between DHEAS and declines over a 3-year period in functional limitations, cognitive impairment, depressive symptoms, and global self-rated health. This longitudinal survey is based on a random national sample of persons aged 54 and older in Taiwan, providing – to the best of our knowledge – the first set of population-based estimates of these associations for a non-Western society and one of the few prospective data sets to permit comparisons between men and women across a broad range of health outcomes. Because these data contain extensive information regarding health and socio-demographic characteristics at baseline, the resulting estimates are less likely than those from other studies to be plagued by biases due to confounding and reverse causality.
MATERIALS AND METHODS
Data
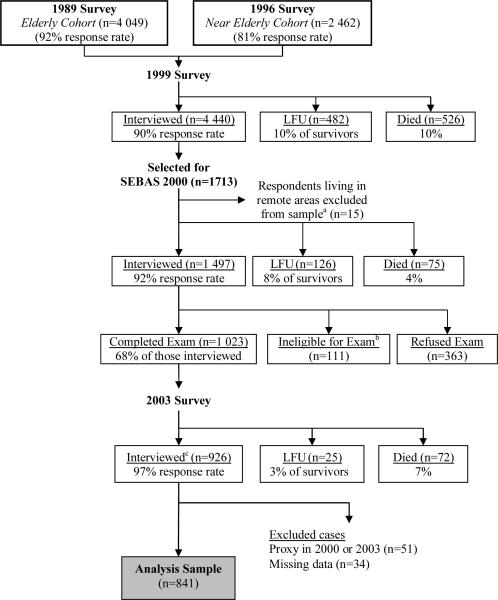
The data come from the 2000 Social Environment and Biomarkers of Aging Study (SEBAS), which comprises a national sub-sample of respondents interviewed as part of Survey of Health and Living Status of the Near Elderly and Elderly in Taiwan (Goldman et al., 2003a). The longitudinal survey began in 1989 with a national sample of 4 049 persons aged 60 and older and was expanded in 1996 to include 2 462 near elderly persons aged 50 to 66 in 1996 (see Figure 1). Both cohorts were interviewed in 1999. A random sub-sample of the 1999 survey was selected for the 2000 SEBAS, with an oversampling of persons 71 years and older (in 2000) and residents of urban areas.
Figure 1. Analysis sample for models of health outcomes in 2003.
LFU = Lost-to-followup
aA few respondents living in remote areas were excluded from the subsample because they lived too far from the hospitals contracted to do the physical examination portion of the study.
bSome respondents were not asked to participate in the hospital examination due to their health condition (i.e., living in an institution, seriously ill, catheter or diaper, kidney dialysis, other health condition that precludes blood drawing).
cAmong all survivors in 1999 (n=4 922), 800 died before the 2003 survey, 344 were LFU (including 137 of unknown vital status), and 3 778 were interviewed in 2003 (92% response rate).
In the 2000 SEBAS, 1 497 persons aged 54 and older completed an in-home interview, and 1 023 participated in a hospital examination (68 percent of those interviewed). Disproportionately high non-participation rates were found among the healthiest respondents as well as the least healthy, with persons who received the medical exam reporting the same average health status (measured on a five-point scale) as those who did not. Results presented elsewhere (Goldman et al., 2003b) suggest that, in the presence of controls for age, estimates derived from the medical exam portion of SEBAS are unlikely to be seriously biased.
SEBAS respondents who participated in the medical exams collected a 12-hour overnight urine sample (7pm to 7am, in order to obtain integrated values of cortisol and catecholamines), fasted overnight, and visited a nearby hospital the following morning. During the hospital visit, medical personnel drew a blood sample and took blood pressure and anthropometric measurements. Written informed consent was obtained for participation in the interview and physical examination, and the institutional review boards of the three participating institutions approved the survey procedures.
Almost all exam participants provided suitable blood and urine specimens. Blood and urine specimens were analyzed at Union Clinical Laboratories (UCL) in Taipei. In addition to the routine standardization and calibration tests performed by the laboratory, during the early stages of fieldwork nine individuals (outside of the target sample) contributed triplicate sets of specimens. In each case, two sets were submitted to UCL and a third was sent to Quest Diagnostics in the US. The results for DHEAS and urinary cortisol indicate high inter- and intra-lab reliability (intra-lab correlations ≥ 0.88; inter-lab correlations ≥ 0.95).
In 2003, 926 SEBAS exam participants were re-interviewed. We excluded from the analysis 51 respondents for whom the 2000 or 2003 interview was completed by proxy and 39 cases with missing data. The resulting analysis sample comprises 836 respondents (Figure 1).
Measures
We examine four outcomes capturing different dimensions of health status in 2003: depressive symptoms, cognitive impairment, mobility limitations and global self-rated health. Depressive symptoms are measured by a 10-item short form of the full CES-D (Center for Epidemiologic Studies Depression) scale, coded according to standard practice based on both the number and severity of symptoms (potential range from 0 to 30). Previous studies have demonstrated that a shortened form of the CES-D yields similar internal consistency, factor structure, and accuracy in detecting depressive symptoms as the full 20-item CES-D among elderly Chinese (Boey, 1999). Cognitive impairment is based on items from the modified Short Portable Mental Status Questionnaire (Pfeiffer, 1975), the modified Rey Auditory Verbal Learning Test (Lezak, 1983), and a modification of the Digits Backward test (Wechsler, 1981). The measure is a count of the number of cognitive tasks completed incorrectly, including basic orientation questions, a series of four subtractions, and immediate memory recall (potential range from 0 to 24). The measure of mobility limitations counts the number of physical tasks, out of the following nine, that the respondent reports difficult performing without aid: standing continuously for 15 minutes and for two hours, squatting, raising both hands over his or her head, grasping or turning objects with his or her fingers, lifting or carrying an object weighing 11–12 kg., running a short distance (20–30 meters), walking 200–300 meters, and climbing two or three flights of stairs. Global SRH is based on the following question: “Regarding your current state of health, do you feel it is excellent, good, average, not so good, or poor?” This five-point ordinal measure is scored so that five indicates “poor” health.
The key independent variable is serum DHEAS in 2000, assayed using radioimmunoassay (Dudley et al., 1985), with an inter-assay coefficient of variation of 12.9 and sensitivity of 1.1μg/dL. We chose to measure DHEAS rather than DHEA because DHEAS is cleared less rapidly from the bloodstream and has less diurnal variation (Kroboth et al., 1999). We include urinary cortisol as a control variable because DHEAS is believed to act as an antagonist to the effects of cortisol, extreme levels of cortisol are related to numerous chronic conditions, and concentrations of these two steroids may be correlated (Butcher and Lord, 2004; Carlson et al., 1999; Folan et al., 2001; Kalmijn et al., 1998; Kroboth et al., 2003). Cortisol is based on the 12-hour urine sample and measured in micrograms per gram creatinine to adjust for body size. Assays of urinary cortisol were made by high pressure liquid chromatography (Krstulovic, 1982), with a lower detection limit of 4μg/L and inter-assay coefficient of variation of 10.8. Because outliers may have undue influence on the estimates, we trimmed several values (n=3 for DHEAS; n=1 for cortisol) to five standard deviations above the mean. To allow for possible non-linear effects, we include quadratic terms for DHEAS and cortisol in the statistical models if the corresponding coefficients are significantly different from zero (p<0.05).
Additional control variables comprise age, sex, residence in an urban area, and baseline health status. Age is modeled as a continuous variable; a quadratic term is also included when it is statistically significant. Measures of the four outcome variables assessed in 2000 provide controls for baseline health status.
Cardiovascular risk factors (in 2000) include smoking, alcohol consumption, diastolic and systolic blood pressure, total and HDL cholesterol, glycosylated hemoglobin, BMI, and waist-hip ratio. Smoking status distinguishes those who did not smoke, those who smoked fewer than 20 cigarettes daily, and those who smoked 20 or more cigarettes daily during the previous six months. Although the mechanisms are not understood, numerous studies have found smoking to be positively related to DHEAS (Barrett-Connor et al., 1986; Barrett-Connor and Goodman-Gruen, 1995; Kroboth et al., 1999; Porsova-Dutoit et al., 2000; Ravaglia et al., 2002; Trivedi and Khaw, 2001). A categorical variable indicates whether the respondent drank alcohol never, sometimes, or frequently/everyday during the past six months.
Analytical Strategy
Descriptive statistics for all variables used in the analysis are weighted to compensate for the over-sampling by age and urban residence. Multivariate models are based on unweighted data, but control for age and urban residence to adjust for the sampling design. Stata 9.0 was used for all analyses. Because of the multistage sampling design, we use a robust estimator of variance and adjust for clustering by primary sampling units to produce correct standard errors (StataCorp, 2005).
For each outcome, we use a statistical model that is appropriate for the level of measurement of that health outcome. For depressive symptoms, a continuous variable, we estimate a linear regression model. SRH, an ordinal variable, is modeled using ordered logit regression. We fit Poisson models for the two count measures (cognitive impairment and mobility limitations). Because a large proportion of respondents (34%) had zero mobility limitations, we model the number of mobility limitations with a zero-inflated Poisson model (ZIP) (Long and Freese, 2006), which allows for a greater than expected number of zero counts and provides a much better fit than the standard Poisson model. Given the skewed distribution of DHEAS, we explored alternative specifications (i.e., log-transformed levels of DHEAS, exclusion of outliers) in preliminary analyses and found that the results were generally the same.
For each health outcome, we estimate two models. The first model regresses DHEAS on health in 2003 with controls for age, sex, urban residence, cortisol, and baseline health status. In the second model, we add the set of cardiovascular risk factors to the explanatory variables in the first model. By including the lagged dependent variable (health in 2000) in these models, we are implicitly modeling the effects of DHEAS (in 2000) on the change in health outcomes between 2000 and 2003.
Because findings from earlier research suggest that the effects of DHEAS levels on health may be significant for men but not for women, we include interaction terms between DHEAS levels and sex in all models. In exploratory analyses, we also tested interaction terms between sex and cortisol level, but because these terms were not significantly different from zero, they are excluded in the final models.
For health outcomes that are significantly associated with DHEAS, we use predicted values to assess the magnitude of the associations. These predictions are obtained by successively assigning the 10th, 25th, 75th and 90th percentile values of DHEAS (for a given sex) to all observations, assigning the value of sex, retaining other explanatory variables at their observed values, and using the coefficients from the multivariate models to obtain the predictions.
RESULTS
Table 1 provides descriptive statistics of the four outcome variables measured in 2003, DHEAS, the baseline health variables, cortisol, other control variables, and cardiovascular risk factors, all measured in 2000. Values for the health variables indicate that, on average, respondents reported poorer health in 2003 than in 2000 (e.g., 2.2 vs. 1.8 mobility limitations). Levels of DHEAS are substantially higher among men than women, declining progressively for men throughout this age range and for women below age 75. These values are comparable to those for some, but not all, samples in Western populations (Glei et al., 2004). The percentage of the sample that is female (44 percent) is atypically low because of the substantial migration of Nationalists, primarily soldiers, from Mainland China in the late 1940s.
TABLE 1.
Descriptive statistics for all measures, weighted estimates
| Variable | Observed Range | Mean (SD) or % | ||
|---|---|---|---|---|
| Total | Females | Males | ||
| DHEAS in 2000 (μg/dl)a | 0.7–388 | 83.0 (60.0) | 60.2 (43.8) | 100.6 (64.7) |
| Age 54–59 | 1.1–388 | 101.9 (70.8) | 73.5 (46.2) | 124.9 (78.7) |
| Age 60–64 | 1.1–271 | 83.2 (53.9) | 59.1 (43.2) | 106.6 (53.4) |
| Age 65–69 | 1.1–386 | 75.8 (61.0) | 54.9 (45.0) | 93.8 (67.3) |
| Age 70–74 | 1.1–361 | 72.9 (51.2) | 47.6 (39.4) | 85.7 (51.8) |
| Age 75+ | 0.7–302 | 70.7 (47.3) | 55.7 (37.2) | 81.1 (50.8) |
| Control variables in 2000 | ||||
| Female | 0, 1 | 43.5% | -- | -- |
| Age (years) | 54–91 | 65.6 (7.6) | 65.1 (7.4) | 66.0 (7.7) |
| Urban residence | 0,1 | 44.1% | 44.8% | 43.6% |
| Cortisol (μg/g creatinine)b | 2–282 | 26.3 (28.1) | 29.4 (25.0) | 23.9 (30.1) |
| Number of mobility limitations | 0–9 | 1.8 (2.2) | 2.5 (2.4) | 1.2 (1.8) |
| CES-Depression scale | 0–28 | 5.2 (5.2) | 6.2 (5.9) | 4.5 (4.6) |
| Number of cognitive impairments | 0–21 | 7.0 (3.2) | 7.7 (3.7) | 6.4 (2.6) |
| Self-assessed health status | 1–5 | |||
| Excellent (1) | 13.0% | 10.4% | 15.0% | |
| Good (2) | 12.9% | 12.1% | 13.6% | |
| Average (3) | 48.4% | 46.8% | 49.5% | |
| Not so good (4) | 23.0% | 27.3% | 19.6% | |
| Poor (5) | 2.8% | 3.4% | 2.3% | |
| Cardiovascular risk factors in 2000 | ||||
| Smoking status past six months: | ||||
| Did not smoke | 0,1 | 76.8% | 97.7% | 60.6% |
| Smoked < 20 cigarettes/day | 0,1 | 11.1% | 2.0% | 18.1% |
| Smoked ≥ 20 cigarettes/day | 0,1 | 12.1% | 0.3% | 21.3% |
| Alcohol consumption past six months: | ||||
| Never | 0,1 | 74.6% | 93.6% | 60.0% |
| Sometimes | 0,1 | 18.1% | 5.7% | 27.6% |
| Frequently or everyday | 0,1 | 7.3% | 0.6% | 12.4% |
| Systolic blood pressure (mmHg)b | 83–237 | 136.8 (19.8) | 138.0 (20.4) | 135.8 (19.2) |
| Diastolic blood pressure (mmHg)b | 50–137 | 82.5 (11.0) | 82.2 (11.1) | 82.7 (11.0) |
| Total cholesterol (mg/dL) | 83–355 | 201.7 (38.7) | 210.6 (39.2) | 194.9 (37.0) |
| HDL cholesterol (mg/dL) | 14–110 | 49.3 (13.7) | 52.3 (13.8) | 47.0 (13.1) |
| Glycosylated hemoglobin (%)b | 3.8–12.5 | 5.8 (1.4) | 6.0 (1.7) | 5.6 (1.1) |
| BMI | 16.2–42.2 | 24.6 (3.6) | 25.1 (3.8) | 24.2 (3.3) |
| Waist-hip ratiob | 0.6–1.2 | 0.9 (0.1) | 0.8 (0.1) | 0.9 (0.1) |
| Health Outcomes in 2003 | ||||
| Number of mobility limitations | 0–9 | 2.2 (2.5) | 2.9 (2.7) | 1.7 (2.2) |
| CES-Depression scale | 0–29 | 5.2 (5.5) | 6.0 (5.7) | 4.5 (5.2) |
| Number of cognitive impairments | 0–24 | 8.5 (3.8) | 9.2 (4.4) | 7.9 (3.2) |
| Self-assessed health status | 1–5 | |||
| Excellent (1) | 10.9% | 8.3% | 12.9% | |
| Good (2) | 22.3% | 21.6% | 22.9% | |
| Average (3) | 34.9% | 33.8% | 35.6% | |
| Not so good (4) | 28.2% | 32.3% | 25.1% | |
| Poor (5) | 3.7% | 4.0% | 3.5% | |
|
| ||||
| Number of cases | 836 | |||
Values of DHEAS are based on the average of duplicate assays. The value was assigned to the detection limit (1.1 ug/dl) if either of the two assays were below assay sensitivity (n=8). Three outliers on DHEAS (416.2, 440.9, and 496.6 μg/dl) were trimmed to five standard deviations above the mean.
Outliers were trimmed to five standard deviations above the mean: cortisol (n=1; 1291 μg/g creatinine), systolic blood pressure (n=1; 243 mmHg), diastolic blood pressure (n=1; 141 mmHg), glycoslyated hemoglobin (n=3, 12.8–13.3%), and waist-hip ratio (n=2, 1.3–1.4).
Results from the multivariate models are presented in Table 2. To facilitate comparisons by sex, we show separate DHEAS estimates for men and women, derived from the main effect of DHEAS and the interaction between DHEAS and sex. After adjustment for control variables (Model 1), DHEAS is significantly associated with three of four health outcomes among men, whereas there is no significant relationship among women. For men, the coefficients indicate that higher levels of DHEAS are associated with fewer mobility limitations and depressive symptoms (p<0.05). The results reveal a non-linear relationship for cognitive impairment: higher levels of DHEAS are associated with fewer cognitive impairments up to a value of 160 μg/dl, after which higher levels are associated with very modest increases in cognitive impairments. Thus, the relationship between DHEAS and cognitive impairment is negative for the vast majority (86%) of men in our sample (those with values of DHEAS below 160 μg/dl). Auxiliary analyses (not shown) suggest that the non-linear effect may be driven by a small number (n=6) of respondents with very high levels of DHEAS (325–388 μg/dl). The results suggest a non-linear relationship between cortisol and mobility limitations, but cortisol is not significantly associated with the other health outcomes.
TABLE 2.
Coefficients from regression models of health outcomes in 2003 on DHEAS in 2000a, unweighted analyses
| Mobility Limitations | CES-D | Cognitive Impairment | Poor Self-Assessed Health | |||||
|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (1) | (2) | (1) | (2) | (1) | (2) | |
| DHEAS (μg/dl): Femalesb | 0.0009 | 0.0010 | 0.0044 | 0.0041 | −0.0016 | −0.0017 | 0.0006 | 0.0006 |
| DHEAS squared: Femalesb | -- | -- | -- | -- | 0.000008 | 0.000008 | -- | -- |
| DHEAS (μg/dl): Maleb | −0.0030**c | 0.0032**c | −0.0082* | −0.0088* | −0.0016*** | −0.0016*** | −0.0019 | −0.0022* |
| DHEAS squared: Maleb | -- | -- | -- | -- | 0.000005*** | 0.00005*** | -- | -- |
| Cortisol (μg/g creatinine) | −0.0014 | −0.0012 | 0.0016 | −0.0008 | −0.0002 | −0.0002 | 0.0016 | 0.0011 |
| Cortisol squared | 0.00001* | 0.00001* | -- | -- | -- | -- | -- | -- |
p<0.05;
p<0.01;
p<0.001
Models are fitted using linear regression for the depressive symptom score, Poisson regression for cognitive impairment, zero-inflated Poisson regression for mobility limitations, and ordered logistic regression for self-assessed health status. Both models includes the control variables, while Model 2 also adjusts for cardiovascular risk factors (see Table 1).
For variables with sex interactions, we show the total effect for males (equivalent to the main effect) and females (equivalent to the sum of the main and the interaction effect).
Sex difference in the parameter estimate is significant (p<0.05).
Researchers have speculated that estimated effects of DHEAS on health may be biased because of failure to include information on cardiovascular risk factors that may be correlated with DHEAS, although it is unclear whether these factors are potential confounders or whether they are mediators linking DHEAS and health (Khaw, 1996; Kiechl et al., 2000; Nafziger et al., 1991; Thijs et al., 2003). Model 2 adjusts for a set of cardiovascular markers. The estimated coefficients of DHEAS remain virtually unchanged for both sexes with one exception: the association between DHEAS and self-assessed health increases slightly in magnitude and reaches statistical significance (p<0.05).
The predicted values presented in Table 3 underscore the substantial variation in mobility limitations and depressive symptoms for varying concentrations of DHEAS among men: values of DHEAS at the 90th percentile are associated with 0.9 fewer mobility limitations and 1.3 fewer depressive symptoms than values at the 10th percentile. The predicted cognitive impairment score decreases from 8.7 at the 10th percentile of DHEAS to 8.0 at the 90th percentile. The curvilinear relationship with cognitive impairment is evident only at very high levels of DHEAS: at the 95th percentile (218 μg/dl), the predicted value for cognitive impairment increases to 8.2 (not shown).
TABLE 3.
Predicted valuesa for health outcomes at the 10th, 25th, 75th, and 90th percentile values of DHEAS for males
| Mobility Limitations | CES-D | Cognitive Impairment | Self-Assessed Health | |||||
|---|---|---|---|---|---|---|---|---|
| Excellent | Good | Average | Not so good | Poor | ||||
| 10th percentile (DHEAS=33.2 μg/dl) | 2.3 | 5.7 | 8.7 | 8.1 | 19.6 | 34.9 | 32.0 | 5.3 |
| 25th percentile (DHEAS=55.9 μg/dl) | 2.1 | 5.5 | 8.5 | 8.8 | 20.5 | 35.0 | 30.8 | 4.9 |
| 75th percentile (DHEAS=129.1 μg/dl) | 1.7 | 4.9 | 8.0 | 11.3 | 23.0 | 35.0 | 27.0 | 3.7 |
| 90th percentile (DHEAS=180.0 μg/dl) | 1.4 | 4.4 | 8.0 | 13.3 | 24.6 | 34.7 | 24.4 | 3.0 |
Predicted values are calculated by setting DHEAS to the specified values, assigning sex as male, leaving all other covariates at their observed values in the sample, and using the coefficients from Model 2 (Table 2).
DISCUSSION
Our estimates suggest that, for the older Taiwanese population, DHEAS is related to subsequent declines in various dimensions of men's (but not women's) psychological and physical health. These findings differ from those in a previous cross-sectional analysis based on the Taiwan study that found larger associations between DHEAS and mobility limitations and cognitive function for women than for men (Glei et al., 2004). Although the present analysis uses the same measures of DHEAS as the earlier study, health outcomes in the earlier study were assessed at roughly the same time as the biological measures.
If DHEAS is related to survival, as some previous studies suggest, then mortality attrition may have biased our results. To explore this possibility, we recoded each health outcome variable as binary, where the value one indicates poor health or deceased by 2003. Then, we estimated two sets of logit models (with the same covariates as Model 2): one that included and one that excluded the 72 respondents who died between 2000 and 2003. The inclusion of deceased respondents had virtually no effect on the results (data not shown), suggesting that mortality attrition does not explain the inconsistencies between the cross-sectional and the longitudinal results.
Another possible explanation relates to sex differences in the extent to which a correlation between DHEAS and health results from reverse causality (that is, from the impact of disease on levels of DHEAS). Although there is some evidence that DHEA(S) levels decrease during illness (Herbert, 1995; Kiechl et al., 2000), there are few insights into how or whether this process differs by sex. Numerous correlation analyses relating health status to measures of DHEAS for men and women shed little light on the issue, both because the time sequence of cause and effect is unclear and because the patterns by sex vary enormously across studies. For example, Berr et al. (1996) found that measures of functional and psychological status had stronger associations with levels of DHEAS in women, whereas Tilvis et al. (1999) identified stronger associations between various diseases and levels of DHEAS in men. We used data from the earlier rounds of the Taiwan survey to examine the effects of changes in health during 1999–2000 and 1996–2000 on levels of DHEAS in 2000 and found evidence that an increase in mobility limitations over these periods was associated with lower levels of DHEAS for women but not men - a finding that is consistent with the stronger (but spurious) DHEAS associations identified for women in the cross-sectional analysis (Glei et al., 2004).
These findings underscore the importance of using prospective rather than cross-sectional data to examine the effects of DHEAS on health. In Table 4, we summarize the few prospective studies that examine all-cause mortality or one of the four broad health outcomes explored in this paper. Note that the length of follow-up varies substantially across these studies—from one year in (Birkenhager-Gillesse et al., 1994) to 19 years in (Barrett-Connor and Goodman-Gruen, 1995). The studies, most of which examine survival or cognitive impairment, offer mixed evidence on the protective effects of DHEAS on men's health. Findings for women are more clear-cut: most studies find no significant effect, although one study (Yaffe et al., 1998) suggests that lower levels of DHEAS are related to depression, and another study (Cappola et al., 2006) finds that both low and high values of DHEAS are associated with increased mortality. Several review articles on sex differences in the effects of DHEA(S) on fatal and nonfatal cardiovascular outcomes (Khaw, 1996; Porsova-Dutoit et al., 2000; Thijs et al., 2003) also indicate that DHEAS is associated with health outcomes more frequently for men than for women. Except for SEBAS, all of the studies listed in Table 4 are based on Western populations. Thus, it is not clear whether differences in results between the Taiwanese and the Western samples represent true variation in the relationship between DHEAS and health across different social contexts or whether they result from other factors that vary across studies (e.g., sample selection, length of follow-up).
TABLE 4.
Summary of results from longitudinal studies with population-based samples regarding the relationship between DHEAS and health or survival
| All-cause Mortality | Functional Limitations | Depressive Symptoms | Cognitive Impairment | Poor Self-Assessed Health Status | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Reference | Dataset/Cohort, Length of Follow-up | M | F | M | F | M | F | M | F | M | F |
| Current Study | SEBAS (Taiwan), 3 years | ↓ | N.S. | ↓ | N.S. | ⋃ | N.S. | ↓ | N.S. | ||
| Birkenhager-Gillesse et al., 1994 | Leiden (Netherlands), 1 year | N.S. | N.S. | ||||||||
| Glei and Goldman, 2006 | SEBAS (Taiwan), 3 years | N.S. | N.S. | ||||||||
| Tilvis et al., 1999 | Helsinki Aging Study (Finland), 5 years | N.S. | N.S. | ||||||||
| Barrett-Connor et al., 1986 | Rancho Bernardo, CA (US), 12 years | ↓ | |||||||||
| Berr et al., 1996 a | PAQUID (France), 4 years | ↓ | N.S. | N.S. | N.S. | ||||||
| Mazat et al., 2001 a | PAQUID (France), 9 years | ↓ | N.S. | N.S. | N.S. | ↓ | N.S. | N.S. | N.S. | ||
| Trivedi and Khaw, 2001 | Cambridge General Practice Health Study (England), 5–9 years | ↓ | N.S. | ||||||||
| Cappola et al., 2006 | WHAS (US), 5 years | ⋃ | |||||||||
| Barrett-Connor and Goodman-Gruen, 1995 | Rancho Bernardo, CA (US), 19 years | ↑ | ↑ | ||||||||
| Yaffe et al., 1998 | Study of Osteoporotic Fractures (US), 2 years | ↓ | |||||||||
| Barrett-Connor and Edelstein, 1994b | Rancho Bernardo, CA (US), 16 years | N.S. | N.S. | ||||||||
↓ Higher levels of DHEAS associated with lower risk (p<0.05); ↑ Higher levels of DHEAS associated with greater risk (p<0.05); ⋃ Both low and high levels of DHEAS are associated with greater risk (p<0.05); N.S. No significant relationship.
The original cohort (n=2792) comprises a population-based sample, but analyses are based on a sub-sample (n=622) of volunteers who agreed to blood sampling.
Study examined five measures of cognitive function; DHEAS was significant for only one of these outcomes and only for females.
The evidence to date suggests that endogenous DHEAS is related to health outcomes for men, but not women, in both Western and non-Western populations. Numerous researchers have speculated that the apparent sex differences may result from differences between men and women in health-related behaviors (e.g., smoking or drinking) or cardiovascular risk factors (Barrett-Connor et al., 1986; Mazat et al., 2001). However, we find no evidence to support these hypotheses. Thus, we are left with vague explanations, similar to those proposed by others, pertaining to potential sex differences in DHEAS concentration and excretion rates, as well as differences between men and women in hormonal metabolism and sex steroid environments (Mazat et al., 2001; Nafziger et al., 1991; Ravaglia et al., 1996; Trivedi and Khaw, 2001).
Limitations of the present study include the shortness of the follow-up period and reliance on serum DHEAS measured at one point in time. The latter issue is of particular concern because concentrations of DHEAS differ from those of DHEA and because measured concentrations of DHEA(S) are likely to depend on the type of assay and vary over time (Nafziger et al., 1991; Yaffe et al., 1998). Future waves of SEBAS will provide a second measurement of DHEAS in 2006–2007, additional biomarkers, and information pertaining to health and survival in subsequent years. These data will permit more sophisticated analyses that distinguish between the effects of DHEAS on changes in health and those of illness on changes in DHEAS over a longer time interval. Despite the potential advantages of this type of study, more in-depth biomedical analysis will be required to enhance our understanding of the marked reductions in adrenal secretion of DHEA(S) with advancing age, the mechanisms that result in such a broad range of physiological effects, and how and why these effects differ between men and women.
ACKNOWLEDGMENTS
This work has been supported by the Demography and Epidemiology Unit of the Behavioral and Social Research Program of the National Institute of Aging, under grant numbers R01AG16790 and R01AG16661, and by the National Institute of Child Health and Human Development under grant number 5P30HD32030. We thank Maxine Weinstein for her helpful comments on this paper.
The survey procedures were approved by the institutional review boards at Princeton University, Georgetown University, and the Bureau of Health Promotion, Department of Health, Taiwan and conformed to the principles embodied in the Declaration of Helsinki.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Barrett-Connor E, Goodman-Gruen D. The epidemiology of DHEAS and cardiovascular disease. Ann. N. Y. Acad. Sci. 1995;774:259–270. doi: 10.1111/j.1749-6632.1995.tb17386.x-i1. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Khaw KT, Yen SS. A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N. Engl. J. Med. 1986;315:1519–1524. doi: 10.1056/NEJM198612113152405. [DOI] [PubMed] [Google Scholar]
- Berr C, Lafont S, Debuire B, Dartigues JF, Baulieu EE. Relationships of dehydroepiandrosterone sulfate in the elderly with functional, psychological, and mental status, and short-term mortality: a French community-based study. Proc Natl Acad Sci U S A. 1996;93:13410–13415. doi: 10.1073/pnas.93.23.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenhager-Gillesse EG, Derksen J, Lagaay AM. Dehydroepiandrosterone sulphate (DHEAS) in the oldest old, aged 85 and over. Ann. N. Y. Acad. Sci. 1994;719:543–552. doi: 10.1111/j.1749-6632.1994.tb56858.x. [DOI] [PubMed] [Google Scholar]
- Boey KW. Cross-validation of a short form of the CES-D in Chinese elderly. Int. J. Geriatr. Psychiatry. 1999;14:608–617. doi: 10.1002/(sici)1099-1166(199908)14:8<608::aid-gps991>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Bovenberg SA, van Uum SH, Hermus AR. Dehydroepiandrosterone administration in humans: evidence based? Neth. J. Med. 2005;63:300–304. [PubMed] [Google Scholar]
- Butcher SK, Lord JM. Stress responses and innate immunity: aging as a contributory factor. Aging Cell. 2004;3:151–160. doi: 10.1111/j.1474-9728.2004.00103.x. [DOI] [PubMed] [Google Scholar]
- Cappola AR, Xue QL, Walston JD, Leng SX, Ferrucci L, Guralnik J, Fried LP. DHEAS levels and mortality in disabled older women: the Women's Health and Aging Study I. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:957–962. doi: 10.1093/gerona/61.9.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson LE, Sherwin BB, Chertkow HM. Relationships between dehydroepiandrosterone sulfate (DHEAS) and cortisol (CRT) plasma levels and everyday memory in Alzheimer's disease patients compared to healthy controls. Horm. Behav. 1999;35:254–263. doi: 10.1006/hbeh.1999.1518. [DOI] [PubMed] [Google Scholar]
- Celec P, Starka L. Dehydroepiandrosterone - is the fountain of youth drying out? Physiol. Res. 2003;52:397–407. [PubMed] [Google Scholar]
- Dhatariya K, Bigelow ML, Nair KS. Effect of dehydroepiandrosterone replacement on insulin sensitivity and lipids in hypoadrenal women. Diabetes. 2005;54:765–769. doi: 10.2337/diabetes.54.3.765. [DOI] [PubMed] [Google Scholar]
- Dudley RA, Edwards P, Ekins RP, Finney DJ, McKenzie IG, Raab GM, Rodbard D, Rodgers RP. Guidelines for immunoassay data processing. Clin. Chem. 1985;31:1264–1271. [PubMed] [Google Scholar]
- Feldman HA, Johannes CB, Araujo AB, Mohr BA, Longcope C, McKinlay JB. Low dehydroepiandrosterone and ischemic heart disease in middle-aged men: prospective results from the Massachusetts Male Aging Study. Am. J. Epidemiol. 2001;153:79–89. doi: 10.1093/aje/153.1.79. [DOI] [PubMed] [Google Scholar]
- Folan MM, Stone RA, Pittenger AL, Stoffel JA, Hess MM, Kroboth PD. Dehydroepiandrosterone, dehydroepiandrosterone-sulfate, and cortisol concentrations in intensive care unit patients. Crit. Care Med. 2001;29:965–970. doi: 10.1097/00003246-200105000-00012. [DOI] [PubMed] [Google Scholar]
- Glei DA, Goldman N, Weinstein M, Liu IW. Dehydroepiandrosterone sulfate (DHEAS) and health: does the relationship differ by sex? Exp. Gerontol. 2004;39:321–331. doi: 10.1016/j.exger.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Goldman N, Weinstein M, Chang MC, Lin HS, Chuang YL, Lin YH, Lin SH, Liu IW, Liu HY. 2000 Social Environment and Biomarkers of Aging Study in Taiwan (SEBAS): Main documentation for SEBAS public use data. ICPSR; Ann Arbor, MI: 2003a. [Google Scholar]
- Goldman N, Lin IF, Weinstein M, Lin YH. Evaluating the quality of self-reports of hypertension and diabetes. J. Clin. Epidemiol. 2003b;56:148–154. doi: 10.1016/s0895-4356(02)00580-2. [DOI] [PubMed] [Google Scholar]
- Herbert J. The age of dehydroepiandrosterone. Lancet. 1995;345:1193–1194. doi: 10.1016/s0140-6736(95)91987-2. [DOI] [PubMed] [Google Scholar]
- Kalmijn S, Launer LJ, Stolk RP, de Jong FH, Pols HA, Hofman A, Breteler MM, Lamberts SW. A prospective study on cortisol, dehydroepiandrosterone sulfate, and cognitive function in the elderly. J. Clin. Endocrinol. Metab. 1998;83:3487–3492. doi: 10.1210/jcem.83.10.5164. [DOI] [PubMed] [Google Scholar]
- Khaw KT. Dehydroepiandrosterone, dehydroepiandrosterone sulphate and cardiovascular disease. J. Endocrinol. 1996;150(Suppl):S149–53. [PubMed] [Google Scholar]
- Kiechl S, Willeit J, Bonora E, Schwarz S, Xu Q. No association between dehydroepiandrosterone sulfate and development of atherosclerosis in a prospective population study (Bruneck Study) Arterioscler. Thromb. Vasc. Biol. 2000;20:1094–1100. doi: 10.1161/01.atv.20.4.1094. [DOI] [PubMed] [Google Scholar]
- Kroboth PD, Amico JA, Stone RA, Folan M, Frye RF, Kroboth FJ, Bigos KL, Fabian TJ, Linares AM, Pollock BG, Hakala C. Influence of DHEA administration on 24-hour cortisol concentrations. J. Clin. Psychopharmacol. 2003;23:96–99. doi: 10.1097/00004714-200302000-00014. [DOI] [PubMed] [Google Scholar]
- Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. DHEA and DHEA-S: a review. J. Clin. Pharmacol. 1999;39:327–348. doi: 10.1177/00912709922007903. [DOI] [PubMed] [Google Scholar]
- Krstulovic AM. Investigations of catecholamine metabolism using high-performance liquid chromatography: analytical methodology and clinical applications. J. Chromatogr. 1982;229:1–34. doi: 10.1016/s0378-4347(00)86033-8. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Belanger A, Lin SX, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J. Endocrinol. 2005;187:169–196. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. Second Edition Oxford University Press; New York: 1983. [Google Scholar]
- Long JS, Freese J. Regression Models for Categorical Dependent Variables using Stata. Stata Press; College Station, TX: 2006. [Google Scholar]
- Mazat L, Lafont S, Berr C, Debuire B, Tessier JF, Dartigues JF, Baulieu EE. Prospective measurements of dehydroepiandrosterone sulfate in a cohort of elderly subjects: relationship to gender, subjective health, smoking habits, and 10-year mortality. Proc. Natl. Acad. Sci. U. S. A. 2001;98:8145–8150. doi: 10.1073/pnas.121177998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafziger AN, Herrington DM, Bush TL. Dehydroepiandrosterone and dehydroepiandrosterone sulfate: their relation to cardiovascular disease. Epidemiol. Rev. 1991;13:267–293. doi: 10.1093/oxfordjournals.epirev.a036071. [DOI] [PubMed] [Google Scholar]
- Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Porsova-Dutoit I, Sulcova J, Starka L. Do DHEA/DHEAS play a protective role in coronary heart disease? Physiol. Res. 2000;49(Suppl 1):S43–56. [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Boschi F, Bernardi M, Pratelli L, Pizzoferrato A, Gasbarrini G. The relationship of dehydroepiandrosterone sulfate (DHEAS) to endocrine-metabolic parameters and functional status in the oldest-old. Results from an Italian study on healthy free-living over-ninety-year-olds. J. Clin. Endocrinol. Metab. 1996;81:1173–1178. doi: 10.1210/jcem.81.3.8772596. [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Sacchetti L, Nativio V, Scali CR, Mariani E, Zanardi V, Stefanini A, Macini PL. Dehydroepiandrosterone-sulfate serum levels and common age-related diseases: results from a cross-sectional Italian study of a general elderly population. Exp. Gerontol. 2002;37:701–712. doi: 10.1016/s0531-5565(01)00232-7. [DOI] [PubMed] [Google Scholar]
- Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter EJ. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;297:811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- StataCorp . Stata User's Guide: Release 9.0. Stata Corporation; College Station, TX: 2005. [Google Scholar]
- Thijs L, Fagard R, Forette F, Nawrot T, Staessen JA. Are low dehydroepiandrosterone sulphate levels predictive for cardiovascular diseases? A review of prospective and retrospective studies. Acta Cardiol. 2003;58:403–410. doi: 10.2143/AC.58.5.2005304. [DOI] [PubMed] [Google Scholar]
- Thijssen JHH, Nieuwenhuyse H. DHEA: A Comprehensive Review. Parthenon; Pearl River, NY: 1999. [Google Scholar]
- Tilvis RS, Kahonen M, Harkonen M. Dehydroepiandrosterone sulfate, diseases and mortality in a general aged population. Aging (Milano) 1999;11:30–34. [PubMed] [Google Scholar]
- Trivedi DP, Khaw KT. Dehydroepiandrosterone sulfate and mortality in elderly men and women. J. Clin. Endocrinol. Metab. 2001;86:4171–4177. doi: 10.1210/jcem.86.9.7838. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R Manual. Psychological Corporation; New York: 1981. [Google Scholar]
- Yaffe K, Ettinger B, Pressman A, Seeley D, Whooley M, Schaefer C, Cummings S. Neuropsychiatric function and dehydroepiandrosterone sulfate in elderly women: a prospective study. Biol. Psychiatry. 1998;43:694–700. doi: 10.1016/s0006-3223(97)00303-x. [DOI] [PubMed] [Google Scholar]