Abstract
Context: The stress hormone cortisol has been linked with unfavorable cardiovascular risk factors, but longitudinal studies examining whether high levels of cortisol predict cardiovascular mortality are largely absent.
Objective: The aim of this study was to examine whether urinary cortisol levels predict all-cause and cardiovascular mortality over 6 yr of follow-up in a general population of older persons.
Design and Setting: Participants were part of the InCHIANTI study, a prospective cohort study in the older general population with 6 yr of follow-up.
Participants: We studied 861 participants aged 65 yr and older.
Main Outcome Measure: Twenty-four-hour urinary cortisol levels were assessed at baseline. In the following 6 yr, all-cause and cardiovascular mortality was ascertained from death certificates. Cardiovascular mortality included deaths due to ischemic heart disease and cerebrovascular disease.
Results: During a mean follow-up of 5.7 (sd = 1.2) yr, 183 persons died, of whom 41 died from cardiovascular disease. After adjustment for sociodemographics, health indicators, and baseline cardiovascular disease, urinary cortisol did not increase the risk of noncardiovascular mortality, but it did increase cardiovascular mortality risk. Persons in the highest tertile of urinary cortisol had a five times increased risk of dying of cardiovascular disease (hazard ratio = 5.00; 95% confidence interval = 2.02–12.37). This effect was found to be consistent across persons with and without cardiovascular disease at baseline (p interaction = 0.78).
Conclusions: High cortisol levels strongly predict cardiovascular death among persons both with and without preexisting cardiovascular disease. The specific link with cardiovascular mortality, and not other causes of mortality, suggests that high cortisol levels might be particularly damaging to the cardiovascular system.
High cortisol levels predict cardiovascular death among persons with and without pre-existing cardiovascular disease.
Cortisol is an important component of the stress system of the human body. In an acute physical or psychological stressful situation, the stress system becomes activated, with up-regulation of the hypothalamic-pituitary-adrenal (HPA) axis resulting in the secretion of cortisol. Direct effects of cortisol are, among others, mobilization of glucose and free fatty acids, a decrease of growth and sex hormone levels, an increase in cardiac output and blood pressure, and tempering of the activated immune system (1,2,3,4). The function of cortisol is to help the body recover from the stress and regain a status of homeostasis. However, chronically elevated cortisol can cause damage and dysregulation, and indeed, high levels of cortisol have been associated with cardiovascular risk factors, such as the metabolic syndrome and its components (5,6,7) and accelerated atherosclerosis (8,9). Accordingly, studies have suggested that a chronically dysregulated cortisol response is the link between stress-related disorders, such as depression, and subsequent cardiovascular morbidity and mortality (10,11). However, little direct evidence exists that cortisol actually increases the risk of cardiovascular end-points. A few studies among patients admitted to the hospital because of cardiac disease have shown that high cortisol levels predict subsequent events or death (12,13,14), but this could well be a reflection of acute stress or disease severity (15). Two studies reported a cortisol/testosterone ratio and a cortisol/dehydroepiandrosterone sulfate ratio to be predictive of incident ischemic heart disease and mortality, respectively, in middle-aged men (16) and male veterans (17). In an older general population, cortisol was associated with increased overall mortality (18), but because causes of death were not available, it remains unclear whether such an association was driven by cardiovascular death. Thus, although the existing literature suggests that cortisol might increase the risk of cardiovascular mortality, no study has directly tested this hypothesis. Therefore, we examined whether urinary cortisol levels predict all-cause and cardiovascular mortality over 6 yr of follow-up in a general population of older persons.
Subjects and Methods
Study sample
Participants were part of the InCHIANTI study, a prospective population-based study of older persons. From 1998 to 2000, the study sample was randomly selected from the population registry of two sites in Italy (Greve in Chianti, and Bagno a Ripoli) using a multistage stratified sampling method. Baseline data collection consisted of a home interview, a 24-h urine collection, and a medical evaluation at the study clinic. Follow-up visits were scheduled 3 and 6 yr after the baseline visit. The study complies with the Declaration of Helsinki. The Italian National Institute of Research and Care on Aging ethical committee approved the study protocol, and all respondents signed informed consent. A more detailed description of the study design is given elsewhere (19).
The InCHIANTI study included 1155 participants aged 65 and older. Because of possible profound effects on cortisol levels, persons using corticosteroids (n = 23; code H02 according to the World Health Organization Anatomical Therapeutic Chemical classification) and persons with severe renal function impairment [n = 30; a creatinine clearance rate of ≤30 ml/min based on serum creatinine, measured through a modified Jaffe method and calculated using the Cockcroft-Gault formula (20)] were excluded from our sample. In addition, 180 persons had missing data on urinary cortisol, and 61 had incomplete (<20 h) urine collection, leaving 861 persons for the present study. Excluded persons were significantly older (79.2 vs. 74.1 yr; P < 0.001), less educated (5.0 vs. 5.4 yr; P = 0.05), more often women (62.2 vs. 54.8%; P = 0.03), and more often deceased during follow-up from all causes (48.3 vs. 21.3%; P < 0.001) as well as from cardiovascular causes (11.6 vs. 4.8%; P < 0.001).
Urinary cortisol
The assessment of urinary cortisol over a 24-h period provides a rather stable indicator of the total cortisol excretion by the adrenals and measures the biologically active (unbound) cortisol. Before the in-clinic assessment, study participants were asked to collect at home all urine produced during a 24-h period starting after the first voided urine after awakening and including the first voided urine on the following day. At assessment, 10-ml aliquots of urine were prepared and stored at −80 C for later assaying at the Clinical Chemistry Laboratory of the Careggi Hospital (Florence, Italy). Urinary cortisol was measured by an immunochemiluminescence method and an ADVIA-Centaur immunoassay system (Bayer Diagnostics, Tarrytown, NY). The intraassay coefficient of variation was less then 2.0%, and the interassay coefficient of variation was less then 3.0%. Urinary cortisol level was defined as micrograms of cortisol excreted over 24 h, calculated as the concentration of cortisol (micrograms per milliliter) multiplied by the total amount of urine volume (milliliters) collected. The normal reference range was 28.5–213 μg cortisol excreted over 24 h. A continuous measure as well as a tertile categorization of urinary cortisol was used in the present study.
Mortality
Mortality data were obtained from the Mortality General Registry maintained by the Tuscany Region and from death certificates filed upon death at the registry office of the municipality of residence. Follow-up lasted from the baseline assessment until the day of death or the day of last contact and finished in 2006. Cause of death was derived from the official death certificates and coded according to the ninth revision of the International Classification of Diseases (ICD-9). Next to all-cause mortality, cardiovascular mortality was assessed and included death due to cerebrovascular disease (ICD codes 430–438) and ischemic heart disease (ICD codes 410–414). Noncardiovascular mortality included all other causes of death.
Covariates
Covariates were a priori selected based on previously shown association with both cortisol and mortality. Sociodemographic variables included age, sex, and years of education. Health indicators were smoking status (non, former, or current smoker), current alcohol intake (yes or no as to three or more drinks a day), and body mass index (weight in kilograms divided by height in meters squared). Waist circumference was measured by trained examiners at the largest midbody point. Three systolic and diastolic blood pressure measurements were taken using a standard mercury sphygmomanometer with the respondent in a supine position; the average of the last two readings of systolic as well as diastolic blood pressure was used in this analysis. Depressive symptoms were assessed using the original 20-item version of the self-report Center for Epidemiologic Studies-Depression Scale administered during the home interview (21). Cognitive functioning was assessed by means of the Mini-Mental State Examination score (22). Identification of cardiovascular disease (including angina pectoris, myocardial infarction, stroke, or transient ischemic attack) and diabetes at baseline was based on a standardized algorithm using information on self-reported history, pharmacological treatments, medical exam data, and hospital discharge records (23). As a global indicator of poor physical health, the number of other chronic diseases (including cancer, liver disease, gastrointestinal disease, congestive heart failure, Parkinson’s disease, peripheral arterial disease, lung disease, hip fracture, herniated disc, arthritis, osteoporosis, and cognitive deterioration) was calculated. From urinary samples, 24-h creatinine excretion was calculated as the concentration of creatinine (milligrams per milliliter) multiplied by the total amount of urine volume (milliliters) collected.
Statistical analyses
Descriptive statistics were used to present sample characteristics. Cox regression analyses were performed to assess whether urinary cortisol levels predict the risk of all-cause, noncardiovascular, and cardiovascular mortality in an age- and sex-adjusted and a fully adjusted (age, sex, education, smoking, alcohol intake, body mass index, waist circumference, systolic blood pressure, diastolic blood pressure, depressive symptoms, cognitive functioning, baseline cardiovascular disease, diabetes, number of other chronic diseases, 24-h urinary creatinine, and urine volume) model. The proportional hazards assumption was checked by including a time-to-event by cortisol interaction term. Next, a baseline cardiovascular disease by cortisol interaction term was included in the above-mentioned model to test whether associations were consistent across persons with and without preexisting cardiovascular disease. SPSS 15.0 (SPSS Inc., Chicago, IL) was used for all statistical analyses. A two-sided P value of ≤0.05 was considered statistically significant.
Results
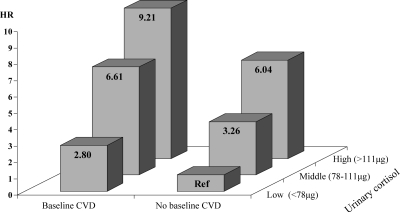
Sample characteristics are shown in Table 1. Mean age of the current sample was 74.1 (sd = 6.6) yr, 54.8% were women, and the mean 24-h urinary cortisol level was 99.3 (sd = 48.0) μg. During an average follow-up of 5.7 (sd = 1.2) yr, 183 (21.3%) persons died, including 41 (4.8%) who died from cardiovascular causes and 142 (16.5%) from other causes. Table 2 shows the results of both sex- and age-adjusted and fully adjusted Cox regression analyses assessing the association between 24-h urinary cortisol and all-cause, noncardiovascular, and cardiovascular death. The proportional hazards assumption was met in all analyses. After adjustment for sociodemographics, lifestyle, and health indicators, urinary cortisol was associated with increased risk of all-cause mortality [hazard ratio (HR) per sd increase = 1.17; 95% confidence interval (CI) = 1.01–1.36]. Persons in the highest tertile of urinary cortisol had a 1.74 (95% CI = 1.15–3.62) increased risk of dying within 6 yr. However, this increased risk of mortality was fully due to persons dying of cardiovascular disease because urinary cortisol was not associated with noncardiovascular mortality (HR per sd increase = 1.10; 95% CI = 0.93–1.31). In contrast, the risk of cardiovascular mortality increased with increasing levels of urinary cortisol (HR per sd increase = 1.42; 95% CI = 1.06–1.90). When examining urinary cortisol tertiles, persons in the highest tertile of urinary cortisol had a five times increased risk of dying of cardiovascular disease (HR = 5.00; 95% CI = 2.02–12.37). Next, a baseline cardiovascular disease by cortisol tertile interaction term was included in this Cox regression model but was not significant (P = 0.78). This indicated that the association between urinary cortisol and cardiovascular mortality existed for persons both with and without preexisting cardiovascular disease. These findings are illustrated in Fig. 1. Compared with persons without baseline cardiovascular disease in the lowest tertile of cortisol, those in the highest tertile had a 6.0 times increased risk of dying over 6 yr when cardiovascular disease was not present at baseline (HR = 6.04; 95% CI = 2.07–17.65) and a 9.2 times increased risk when cardiovascular disease preexisted at baseline (HR = 9.21; 95% CI = 2.48–34.25).
Table 1.
Sample characteristics (n = 861)
| Characteristic | Mean (sd) or % |
|---|---|
| Sociodemographics | |
| Age (yr) | 74.1 (6.6) |
| Women | 54.8 |
| Education (yr) | 5.4 (3.2) |
| Baseline health indicators | |
| Smoking status | |
| Never | 57.8 |
| Former | 27.5 |
| Current | 14.6 |
| Alcohol intake (≥3 drinks a day) | 10.7 |
| Body mass index | 27.5 (3.9) |
| Waist circumference (cm) | 92.8 (10.1) |
| Systolic blood pressure (mm Hg) | 150.5 (19.2) |
| Diastolic blood pressure (mm Hg) | 84.0 (8.4) |
| Depressive symptoms (CES-D) | 12.5 (8.6) |
| Cognitive functioning (MMSE)a | 26 [23–28] |
| Cardiovascular disease | 13.0 |
| Diabetes | 12.2 |
| Number of other chronic diseases | 1.1 (0.9) |
| Cortisol | |
| Urine volume (ml) | 1515 (570) |
| 24-h urinary creatinine (mg) | 964 (337) |
| 24-h urinary cortisol (μg) | 99.3 (48.0) |
| Mortality | |
| Deceased | 21.3 |
| Noncardiovascular death | 16.5 |
| Cardiovascular death | 4.8 |
CES-D, Center for Epidemiologic Studies Depression (scale); MMSE, Mini-Mental State Examination.
Because of non-normal distribution, median [interquartile range] is shown.
Table 2.
Urinary cortisol and 6-yr all-cause and cardiovascular mortality (n = 861)
| Sex- and age-adjusteda
|
Fully adjustedb
|
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| All-cause mortality (no. of deaths = 183) | ||||||
| 24-h urinary cortisolc | 1.07 | 0.92–1.24 | 0.37 | 1.17 | 1.01–1.36 | 0.03 |
| Tertiles of 24-h urinary cortisol | ||||||
| First tertile (<78 μg) | REF | REF | ||||
| Second tertile (78–111 μg) | 1.02 | 0.70–1.48 | 0.91 | 1.52 | 1.02–2.27 | 0.04 |
| Third tertile (>111 μg) | 1.12 | 0.78–1.61 | 0.54 | 1.74 | 1.15–2.62 | 0.009 |
| Noncardiovascular mortality (no. of deaths = 142) | ||||||
| 24-h urinary cortisolc | 0.99 | 0.84–1.19 | 0.95 | 1.10 | 0.93–1.31 | 0.27 |
| Tertiles of 24-h urinary cortisol | ||||||
| First tertile (<78 μg) | REF | REF | ||||
| Second tertile (78–111 μg) | 0.87 | 0.57–1.31 | 0.50 | 1.29 | 0.83–2.02 | 0.26 |
| Third tertile (>111 μg) | 0.84 | 0.55–1.27 | 0.41 | 1.28 | 0.80–2.05 | 0.30 |
| Cardiovascular mortality (no. of deaths = 41) | ||||||
| 24-h urinary cortisolc | 1.31 | 1.01–1.72 | 0.05 | 1.42 | 1.06–1.90 | 0.02 |
| Tertiles of 24-h urinary cortisol | ||||||
| First tertile (<78 μg) | REF | REF | ||||
| Second tertile (78–111 μg) | 1.93 | 0.81–4.57 | 0.14 | 2.91 | 1.18–7.19 | 0.02 |
| Third tertile (>111 μg) | 2.93 | 1.34–6.44 | 0.007 | 5.00 | 2.02–12.37 | 0.001 |
Based on Cox regression analyses adjusted for sex and age.
Additionally adjusted for education, smoking, alcohol intake, body mass index, waist circumference, systolic blood pressure, diastolic blood pressure, depressive symptoms, cognitive functioning, baseline cardiovascular disease, diabetes, number of other chronic diseases, 24-h urine creatinine, and urine volume.
HR per sd (=48 μg) increase.
Figure 1.
Risk of cardiovascular mortality across baseline cardiovascular disease (CVD) and urinary cortisol. Risk is based on Cox regression analyses adjusted for sex, age, education, smoking, alcohol intake, body mass index, waist circumference, systolic blood pressure, diastolic blood pressure, depressive symptoms, cognitive functioning, diabetes, number of other chronic diseases, 24-h urinary creatinine, and urine volume.
Discussion
The results of this study clearly show that basal cortisol levels in an older general population predict cardiovascular death, but not other causes of mortality. Persons in the highest tertile of urinary cortisol had a 5-fold increased risk of dying of cardiovascular disease over a 6-yr period. This finding strongly supports the frequently hypothesized link between hyperactivity of the HPA axis and cardiovascular disease.
The HPA axis controls a set of neuroendocrine reactions essential to life. When the HPA axis is activated, CRH is released by the hypothalamus and stimulates the pituitary to release ACTH, which in turn signals to the adrenal cortex to release cortisol. Cortisol, in turn, exerts negative feedback on the pituitary and hypothalamus to suppress ACTH and CRH production, respectively (2). This implies that hyperactivity of the HPA axis could be the result of a CRH overdrive, a strong ACTH response to CRH, a strong cortisol response to ACTH, and/or blunted negative feedback exerted by cortisol on central glucocorticoid receptors (24). It has been hypothesized that hyperactivity of the HPA axis may be a result of frequent or persistent stimulation (11). In particular, hyperactivity of the HPA axis has been found as a result of chronic stress (25) and is suggested to play a role in stress-related disorders such as depression (26). In addition, aging is associated with an increase in basal cortisol levels (27,28) and an increased cortisol response to challenge (29). Hyperactivity of the HPA axis in aging might in fact be the result of the “wear and tear” of a lifelong exposure to stress (27).
Hyperactivity of the HPA axis might exert damage to the human body in the long run. High levels of cortisol have been associated with several cardiovascular risk factors, such as those present in the metabolic syndrome and atherosclerosis (5,6,7,8,9). Because cortisol exerts a multitude of effects on glucose and free fatty acid metabolism, the autonomic nervous system, the inflammatory system, and sex and growth hormones (1,2,3,4), it is not surprising that continuous high levels of cortisol might lead to dysfunction of these systems, which are all implicated in cardiovascular disease (3,10,30). For instance, by activating lipoprotein lipase and inhibiting lipid mobilization, cortisol promotes the accumulation of visceral fat (1) because visceral fat is highly sensitive to cortisol owing to a high density of glucocorticoid receptors (31). Visceral fat, in turn, has been shown to be an important risk factor for cardiovascular disease (32,33). Also, hyperactivity of the HPA axis has been linked to the decline of immune functions during aging, particularly among chronically stressed older persons (34). This also may put persons at increased risk of cardiovascular disease (30). The mineralocorticoid receptor may play an important role in the detrimental effects of cortisol on cardiovascular health. This receptor is mainly occupied by cortisol, and activation of the mineralocorticoid receptor might promote proinflammatory effects (35) and has been shown to promote vascular cell calcification (36).
Thus, research has demonstrated that cortisol is associated with cardiovascular risk factors; however, little prospective evidence exists that cortisol actually predicts the onset of cardiovascular end-points. One previous study in the general population among middle-aged men found that a cortisol/testosterone ratio was predictive of incident ischemic heart disease over 16.5 yr of follow-up (16). No effects of either cortisol or testosterone alone were found. This study used a less reliable single measure of morning cortisol in blood that, unlike urinary or salivary cortisol, does not represent the biologically active cortisol and could possibly be increased by an acute stress reaction due to the blood draw itself. Our study within an older general population confirms that persons with high basal levels of cortisol are indeed at a greatly increased risk of cardiovascular death.
A few studies within heart patients have previously shown that cortisol predicts subsequent events or mortality (12,13,14). However, because these persons already have disease at the time of cortisol assessment, it is hard to determine whether cortisol is a cause or a consequence of disease status. The results of our study show that urinary cortisol predicted cardiovascular mortality in persons both with and without cardiovascular disease at baseline. This suggests that high cortisol levels are not merely a reflection of heart disease severity and might have a direct causal effect on cardiovascular death. On the other hand, it is possible that among those without preexisting cardiovascular disease, those with higher cortisol did have worse health status. However, we adjusted for several health indicators, and this only strengthened the association between cortisol and cardiovascular disease.
We found that cortisol was associated with cardiovascular, but not noncardiovascular, mortality. This indicates that the HPA axis might have specifically damaging effects on the cardiovascular system and has less profound consequences on other vital physiological systems. Another recent study in an older general population by Schoorlemmer et al. (18) did find salivary cortisol, but not serum cortisol, to be predictive of all-cause mortality over 7 yr of follow-up. This study was not able to differentiate between causes of death. Therefore, it is possible that the association of cortisol was only restricted to those with cardiovascular deaths and not noncardiovascular deaths, as in our study. The study by Schoorlemmer et al. (18), however, did not find an association between cortisol and the onset of nonfatal heart disease. Because heart disease was only measured by self-report, misclassification could have prevented the authors from detecting any association between cortisol and heart disease. On the other hand, it is possible that when cortisol levels are increased, a cardiac event is more likely to result in death, which is corroborated by the finding that high cortisol levels in heart patients seem to predict mortality (12,13,14).
Strengths of the present study include the use of urinary cortisol, which represents the biologically active cortisol and can be nonstressfully measured. Furthermore, we were able to examine the effects of cortisol on all-cause and cardiovascular mortality in a large randomly selected sample of the older general population, including persons with and without cardiovascular disease. A limitation of this study is that despite the large sample size, there were rather few cases of cardiovascular death. Nevertheless, even with this relatively small number of cases, strong clinically relevant significant effects of high urinary cortisol levels on cardiovascular mortality were found.
In conclusion, high cortisol levels in older persons strongly predict cardiovascular death among persons both with and without preexisting cardiovascular disease. The specific link with cardiovascular—and not all-cause—mortality suggests that hyperactivity of the HPA axis might be particularly damaging to the cardiovascular system.
Footnotes
The InCHIANTI study was supported as a targeted project (Grant ICS 110.1/RS97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (Grants 263 MD 916413 and 263 MD 821336). Data analyses were supported through Grant 1R01-HL972972 from the National Heart, Lung, and Blood Institute. Cortisol assays were supported by a professional services contract from the Laboratory of Epidemiology, Demography, and Biometry, National Institute on Aging.
Disclosure Summary: No conflict of interest is declared.
First Published Online August 25, 2010
Abbreviations: CI, Confidence interval; HPA, hypothalamic-pituitary-adrenal; HR, hazard ratio.
References
- Björntorp P 2001 Do stress reactions cause abdominal obesity and comorbidities? Obes Rev 2:73–86 [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW 1992 The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 267:1244–1252 [PubMed] [Google Scholar]
- Whitworth JA, Williamson PM, Mangos G, Kelly JJ 2005 Cardiovascular consequences of cortisol excess. Vasc Health Risk Manag 1:291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchimont D, Kino T, Galon J, Meduri GU, Chrousos G 2002 Glucocorticoids and inflammation revisited: the state of the art. NIH clinical staff conference. Neuroimmunomodulation 10:247–260 [DOI] [PubMed] [Google Scholar]
- Brunner EJ, Hemingway H, Walker BR, Page M, Clarke P, Juneja M, Shipley MJ, Kumari M, Andrew R, Seckl JR, Papadopoulos A, Checkley S, Rumley A, Lowe GD, Stansfeld SA, Marmot MG 2002 Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: nested case-control study. Circulation 106:2659–2665 [DOI] [PubMed] [Google Scholar]
- Fraser R, Ingram MC, Anderson NH, Morrison C, Davies E, Connell JM 1999 Cortisol effects on body mass, blood pressure, and cholesterol in the general population. Hypertension 33:1364–1368 [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Suthers K, Ferrucci L, Simonsick EM, Ble A, Schrager M, Bandinelli S, Lauretani F, Giannelli SV, Penninx BW 2007 Hypercortisolemic depression is associated with the metabolic syndrome in late-life. Psychoneuroendocrinology 32:151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker MJ, Koper JW, van Aken MO, Pols HA, Hofman A, de Jong FH, Kirschbaum C, Witteman JC, Lamberts SW, Tiemeier H 2008 Salivary cortisol is related to atherosclerosis of carotid arteries. J Clin Endocrinol Metab 93:3741–3747 [DOI] [PubMed] [Google Scholar]
- Matthews K, Schwartz J, Cohen S, Seeman T 2006 Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosom Med 68:657–661 [DOI] [PubMed] [Google Scholar]
- Björntorp P 2001 Heart and soul: stress and the metabolic syndrome. Scand Cardiovasc J 35:172–177 [DOI] [PubMed] [Google Scholar]
- Rosmond R 2005 Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology 30:1–10 [DOI] [PubMed] [Google Scholar]
- Güder G, Bauersachs J, Frantz S, Weismann D, Allolio B, Ertl G, Angermann CE, Störk S 2007 Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation 115:1754–1761 [DOI] [PubMed] [Google Scholar]
- Marklund N, Peltonen M, Nilsson TK, Olsson T 2004 Low and high circulating cortisol levels predict mortality and cognitive dysfunction early after stroke. J Intern Med 256:15–21 [DOI] [PubMed] [Google Scholar]
- Tenerz A, Nilsson G, Forberg R, Ohrvik J, Malmberg K, Berne C, Leppert J 2003 Basal glucometabolic status has an impact on long-term prognosis following an acute myocardial infarction in non-diabetic patients. J Intern Med 254:494–503 [DOI] [PubMed] [Google Scholar]
- Rotman-Pikielny P, Roash V, Chen O, Limor R, Stern N, Gur HG 2006 Serum cortisol levels in patients admitted to the department of medicine: prognostic correlations and effects of age, infection, and comorbidity. Am J Med Sci 332:61–67 [DOI] [PubMed] [Google Scholar]
- Smith GD, Ben-Shlomo Y, Beswick A, Yarnell J, Lightman S, Elwood P 2005 Cortisol, testosterone, and coronary heart disease: prospective evidence from the Caerphilly study. Circulation 112:332–340 [DOI] [PubMed] [Google Scholar]
- Boscarino JA 2008 Psychobiologic predictors of disease mortality after psychological trauma: implications for research and clinical surveillance. J Nerv Ment Dis 196:100–107 [DOI] [PubMed] [Google Scholar]
- Schoorlemmer RM, Peeters GM, van Schoor NM, Lips P 2009 Relationships between cortisol level, mortality and chronic diseases in older persons. Clin Endocrinol (Oxf) 71:779–786 [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM 2000 Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc 48:1618–1625 [DOI] [PubMed] [Google Scholar]
- National Kidney Foundation 2002 K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39:S1–S266 [PubMed] [Google Scholar]
- Radloff LS 1977 The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Measure 1:385–401 [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR 1975 Mini-mental state: a practical method for grading the state of patients for the clinician. J Psychiatr Res 12:189–198 [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME 1995 The Women’s Health and Aging Study. Health and social characteristics of older women with disability. Bethesda, MD: National Institute on Aging; NIH Publication no. 95-4009 [Google Scholar]
- Pasquali R, Vicennati V 2000 Activity of the hypothalamic-pituitary-adrenal axis in different obesity phenotypes. Int J Obes Relat Metab Disord 24(Suppl 2):S47–S49 [PubMed] [Google Scholar]
- Lightman SL 2008 The neuroendocrinology of stress: a never ending story. J Neuroendocrinol 20:880–884 [DOI] [PubMed] [Google Scholar]
- Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, Smit JH, Zitman FG, Penninx BW 2009 Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry 66:617–626 [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Leproult R, Kupfer DJ 1996 Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab 81:2468–2473 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Keenan DM, Roelfsema F, Iranmanesh A 2005 Aging-related adaptations in the corticotropic axis: modulation by gender. Endocrinol Metab Clin North Am 34:993–1014 [DOI] [PubMed] [Google Scholar]
- Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC 2005 A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology 30:80–91 [DOI] [PubMed] [Google Scholar]
- Willerson JT, Ridker PM 2004 Inflammation as a cardiovascular risk factor. Circulation 109:II2–II10 [DOI] [PubMed] [Google Scholar]
- Brönnegård M, Arner P, Hellström L, Akner G, Gustafsson JA 1990 Glucocorticoid receptor messenger ribonucleic acid in different regions of human adipose tissue. Endocrinology 127:1689–1696 [DOI] [PubMed] [Google Scholar]
- Gelber RP, Gaziano JM, Orav EJ, Manson JE, Buring JE, Kurth T 2008 Measures of obesity and cardiovascular risk among men and women. J Am Coll Cardiol 52:605–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas BJ, Penninx BW, Cesari M, Kritchevsky SB, Newman AB, Kanaya AM, Pahor M, Jingzhong D, Harris TB 2004 Association of visceral adipose tissue with incident myocardial infarction in older men and women: the Health, Aging and Body Composition Study. Am J Epidemiol 160:741–749 [DOI] [PubMed] [Google Scholar]
- Bauer ME, Jeckel CM, Luz C 2009 The role of stress factors during aging of the immune system. Ann NY Acad Sci 1153:139–152 [DOI] [PubMed] [Google Scholar]
- Rickard AJ, Young MJ 2009 Corticosteroid receptors, macrophages and cardiovascular disease. J Mol Endocrinol 42:449–459 [DOI] [PubMed] [Google Scholar]
- Jaffe IZ, Tintut Y, Newfell BG, Demer LL, Mendelsohn ME 2007 Mineralocorticoid receptor activation promotes vascular cell calcification. Arterioscler Thromb Vasc Biol 27:799–805 [DOI] [PubMed] [Google Scholar]