Abstract
Context: Black women have lower 25-hydroxyvitamin D [25(OH)D] and higher PTH than white women. Recent evidence implicates PTH in adverse cardiovascular outcomes.
Objective: The objective of the study was to determine whether PTH increases at lower 25(OH)D levels (the threshold) in black compared with white women.
Design: Healthy black and white women, aged 20–80 yr were recruited to participate in a cross-sectional study of body-composition in black and white women. Measurement of serum 25(OH)D and PTH were carried out.
Setting: The study was a convenience sample recruited from a community setting.
Patients: Healthy black and white women were recruited by advertising and a direct mail campaign in a comparative study of body composition. Age ranged from 20-to 80 yr. There were 148 black and 129 white premenopausal participants and 87 black and 139 white postmenopausal participants.
Main Outcome: The main outcome was to determine whether the threshold for 25(OH)D/PTH differs in black and white women.
Results: A threshold of 37 nmol/liter (95% confidence interval 35–40) was found for black and 59 nmol/liter (95% confidence interval 56–63) for white women. These two values were significantly different (P < 0.001).
Conclusions: Black women have an increase in serum PTH at a lower 25(OH)D level than white women. Negative health outcomes of higher PTH should be investigated in black women.
Serum parathyroid hormone increases at lower levels of serum 25(OH)D in black women.
As a consequence of the relationship of skin pigmentation to synthesis of vitamin D, serum 25 hydroxyvitamin D [25(OH)D] levels are highest in Caucasians, lowest in black women, and intermediate in Hispanics (1). PTH levels show the predicted inverse relationship, being highest in black women and lowest in Caucasians (2). Several authors have sought a threshold value for serum 25(OH)D, a value of serum 25(OH)D below which there is a rise in PTH. The threshold has been considered an index of vitamin D sufficiency (3).
A systematic literature review in 2006 revealed optimal serum 25(OH)D based on a putative 25(OH)D/PTH threshold range between 25 and 122 nmol/liter (4). Half of the published studies reported estimates 50 nmol/liter or greater for 25(OH)D, and a third provided estimates between 40 and 50 nmol/liter. Calcium intake was found to have a significant effect on the threshold as did mean serum 25(OH)D. Serum 25(OH)D and dietary calcium accounted for 67% of the variance in thresholds of the various studies.
The literature on the relationship of 25(OH)D to PTH has a preponderance of studies in white men and women. The threshold for 25(OH)D was evaluated in a randomized controlled trial of vitamin D3 supplementation in 208 black women studied for 3 yr (5). Calcium intake of 1200 mg/d was ensured. Several statistical models were fitted to the data, and a threshold of 44 nmol/liter was found using a spline model. Because only black women were studied, these data could not be used to determine whether the 25(OH)D level is lower for the threshold in black vs. white women. In another study examining the determinants of serum PTH, race was a factor, but this was minimized when body mass index (BMI), age and calcium intake were considered (2).
Adult black women [despite lower 25(OH)D levels] have lower bone turnover, higher calcium absorption, higher calcium retention, less bone loss, and lower risk for fractures (6). Studies on PTH responsiveness have indicated skeletal resistance to PTH in black women (7). In childhood and adulthood, black women have lower urinary calcium excretion (higher calcium retention). Heaney (8) has estimated that in adults the calcium intake requirements for skeletal health are 300 mg/d less in black women than whites.
The importance of ethnic differences in the threshold seemed unimportant because there is no evidence that increasing 25(OH)D levels in black women have a beneficial effect on bone health. However, recent publications have revealed an influence of PTH on mortality and cardiovascular disorders, implicating the higher PTH in black women in conditions usually associated with health disparities (9,10,11,12,13). Consequently, we decided to reexamine the data from a study in which almost 500 healthy black and white women, aged 20–80 yr, had measurements of 25(OH)D and PTH. The question we wanted to explore was: is the 25(OH)D/PTH threshold different in black and white women?
Participants and Methods
Participants
Participants were recruited from advertising in the local media and through a direct mail campaign. Exclusion characteristics consisted of any chronic illness, medication know to affect bone metabolism, and any use of oral contraceptives or hormonal replacement therapy or hysterectomy. The study was approved by the Institutional Review Board of Winthrop-University Hospital, and written informed consent was obtained from each participant. The study sample and analyses were carried out between January 28, 1991, and November 16, 1994. A 3-d diet history was obtained and was reviewed with the study dietitian using food models to estimate portion size. A 24-h food recall form was also completed with the assistance of the dietitian. A BMI of 18–33 kg/m2 was considered acceptable for inclusion in the study. There were 148 black and 129 white premenopausal participants and 87 black and 139 white postmenopausal participants.
Laboratory studies
Serum PTH was measured by the Allegro intact PTH immunoassay, purchased from Nichols Institute (San Juan Capistrano, CA) (14). The intraassay coefficient of variation (CV) was 5.2%, and the interassay CV was 9.0%. Serum 25-hydroxyvitamin D was measured by a radioreceptor assay purchased from DiaSorin (previously named INCSTAR when we used the kits; Stillwater, MN). The intraassay CV was 4.1% and the interassay CV was 7.0%. Serum calcium was measured by atomic absorption spectrophotometry (PerkinElmer 560, Norwalk, CT). Serum creatinine was measured by the method of Heinegard and Tiderstrom (15).
Data analysis
We began our analyses by fitting a locally weighted regression smoothing scatterplot (LOESS) line to the data for both black and white women as shown in Figs. 1 and 2. The LOESS procedure is a nonparametric method for fitting a smooth curve that best characterizes the relationship between PTH and 25(OH)D (16). The LOESS lines supported the nonlinear shape for both black and white women. The SupF test was used to test whether a change point exist between PTH and 25(OH)D (17).
Figure 1.
The LOESS model curve for black women (unadjusted data).
Figure 2.
The LOESS model curve for white women (unadjusted data).
A piece-wise change point model was developed to determine and estimate the 25(OH)D threshold using a quasi-Newton search algorithm with box constraints (18). Data analyses were done in statistical package R, which is the gold standard for statistical data analysis and computing and is available at http://www.r-project.org.
Results
Race was self-declared. The age at menopause was the same for each group and menarche was 3 months earlier in black participants. Relevant demographics and laboratory values are given in Table 1.
Table 1.
Demographic and biochemical variables
| Mean (95% CI)
|
|||
|---|---|---|---|
| Black (n = 235) | White (n = 268) | P value | |
| Age (yr) | 43.7 (42.0, 45.4) | 51.4 (49.7, 53.1) | <0.01 |
| Age at menarche (yr) | 12.3 (12.1, 12.5) | 12.6 (12.4, 12.8) | 0.01 |
| Age at menopause (yr) | 50.1 (49.3, 50.9) | 51.0 (50.5, 51.5) | NS |
| Height (cm) | 164 (163, 165) | 164 (163, 165) | NS |
| Weight (kg) | 70.0 (68.4, 71.6) | 64.0 (62.8, 65.2) | <0.01 |
| Alcohol intake (mg/d) | 4.0 (2.3, 5.7) | 10.8 (8.8, 12.8) | <0.01 |
| Education (median) | 2 yr college | 4 yr college | NS |
| Number who fell/yr (%) | 20 | 30 | NS |
| Cigarette packs/yr | 199 (157, 241) | 290 (241, 339) | 0.01 |
| Calcium intake (mg) | 569.9 | 737.3 | <0.01 |
| PTH (pg/ml) | 39.0 | 35.9 | <0.01 |
| 25(OH)D (nmol/liter) | 32.7 | 67.8 | <0.01 |
NS, No significant difference.
The SupF value is 18.44 (P = 0.0220) for black women based on the whole range of 25(OH)D values from 2.75 to 107.75. The SupF value is 32.67 (P = 0.0004) for black women (n = 163) based on the 25(OH)D values from 11 to 54 (mean ± 1 sd). The SupF value is 21.49 (P = 0.0060) for white women based on the whole range of 25(OH)D values from 9.5 to 173.8. The SupF value is 26.32 (P = 0.0008) for white women (n = 181) based on the 25(OH)D values from 33 to 100 (mean ± 1 sd).
Mathematically the 25(OH)D/PTH threshold was modeled by an inflection point or a change-point analysis. In search for this threshold, we extensively investigated several different statistical smoothing techniques and also fitted different parametric statistical models. We concluded that only the piece-wise change-point model is useful based on the inherent variability in the data. This four-parameter change-point model can be described in mathematical form as follows:
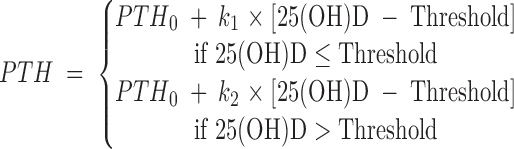 |
where parameter threshold is the 25(OH)D threshold level and PTH0 is the corresponding PTH level for the 25(OH)D threshold. Parameters k1 and k2 are the two slope parameters before/after the threshold.
We fitted this change-point model for each race separately, and with the combined data but with different parameters for each race. The best model we found is the change-point model combining both races’ data with different parameters for each race (Fig. 3).
Figure 3.
The best piece-wise change-point model to illustrate the thresholds for 25(OH)D for whites and blacks. The black dots are for blacks and the open circles for whites. The solid line is the fitted line for blacks and dashed line for whites based on an analysis of covariance model using rapid amplification of cDNA ends and 25(OH)D. PTH is adjusted by the regression model: PTH (picograms per milliliter) = A get BMI + calcium intake + total bone mineral density + serum creatinine.
The estimated thresholds were 37 mol/liter [95% confidence interval (CI) 35–40] for blacks and 59 nmol/liter (95% CI 56–63) for whites, and they are significantly different (P < 0.001). The associated PTH level at these thresholds are 38 (pg/ml) (95% CI 36.8–39.2) for blacks and 35 pg/ml (95% CI 33.5–36.5) for whites, and they are significantly different (P < 0.001).
In addition, the slopes before the thresholds are −0.31 (95% CI −0.33 to −0.29) for blacks and −0.37 (95% CI − 0.40 to −0.34) for whites, and they are also significantly different (P < 0.001). However, the slopes after the thresholds are not significantly different from zero and were set to zero for model fitting.
Discussion
Our data suggest a clearly lower threshold for PTH secretion in response to vitamin D status in black compared with white women. The threshold of 37 nmol/liter observed in black women are similar to a threshold of 40 nmol/liter we found in a prospective study of postmenopausal black women. The threshold for white women of 59 nmol/liter is consistent with some, but not other, studies with the literature clustered around 50 or 80 nmol/liter. There is an active debate about which inflection is correct in the white population because it has been proposed to make nutritional recommendations for vitamin D intake based on the threshold (4). Arguments can also be made against the relevance of the threshold to nutritional recommendations (3).
There are substantive ethnic differences in the calcium economy. Blacks have superior renal reabsorption of calcium, relative skeletal resistance to PTH and have been estimated to require 300 mg/d less of calcium intake per day than whites (19). Thus, despite having lower 25(OH)D due to reduced dermal synthesis of vitamin D, blacks have higher bone density and a reduced risk for osteoporotic fractures (20). It is reasonable to conclude that black women require less vitamin D as well as less calcium intake than white women to promote bone health. Black women are protected against their lower 25(OH)D by their lower threshold for PTH secretion. It may be speculated that this lower threshold may result in higher bone mass and protection from fractures.
However, recent studies have implicated higher PTH levels as having negative extraskeletal health implications. Patients with higher PTH levels with primary or secondary hyperparathyroidism have greater morbidity and mortality from cardiovascular disorders (9,10,12,13). A community-based prospective study of 958 men established that elevated levels of PTH are associated with an increased risk for cardiovascular mortality (12). Individuals with elevated PTH accounted for 20% of risk proportion for cardiovascular mortality so that reduction of PTH may have great public health implications, particularly if it can be accomplished with low cost by nutritional intervention. Because black women have a greater risk for cardiovascular morbidity and mortality, their elevated PTH levels, although not having an adverse effect on bone mass, may nevertheless be detrimental. Indeed, although there is little evidence from studies of the calcium economy to justify increasing recommendations for calcium and vitamin D intakes beyond the current adequate intake, black women seem to be more susceptible to extraskeletal illness that may be caused by insufficiency of these nutrients. Thus, whereas black women have adapted to their low 25(OH)D levels for skeletal health, their higher PTH levels may have adverse consequences in other systems. It will be necessary to consider this in making nutritional recommendations for vitamin D intake in black women; these recommendations can no longer consider skeletal health alone.
We recognize a number of limitations of our study. This was a convenience sample rather than a true population-based study. It did not include men: in recent studies in males, PTH has not been found to be higher in black men despite lower serum 25(OH)D (21). There are issues with the standardization and operator variability in the performance of 25(OH)D assays (22). The assay used for 25(OH)D preceded the current DiaSorin assay and was found on calibration to give levels only 2.5 nmol/liter lower than the DiaSorin assay (technical report, 1999; DiaSorin). However, further adjustment to these values would have to be made to adjust our 25(OH)D values to values in the National Health and Nutrition Examination Survey data set because of drifts in the DiaSorin assay due to reagent and calibration lot changes (National Health and Nutrition Examination Survey technical report 2009) (23). Our laboratory participates in Vitamin D External Quality Assessment Scheme, an international effort to standardize serum 25(OH)D measurements. The Allegro PTH assay has a correlation with newer assays in excess of 0.9 (24,25,26).
In summary, we present evidence that PTH levels will rise at a lower serum concentration of 25(OH)D in black women. The benefits to extraskeletal health of raising 25(OH)D to lower serum PTH levels in this population should be explored.
Footnotes
This work was supported by National Institutes of Health Grant IH R01AG015325.
Disclosure Summary: The authors have nothing to disclose.
First Published Online August 4, 2010
Abbreviations: BMI, Body mass index; CI, confidence interval; CV, coefficient of variation; LOESS, locally weighted regression smoothing scatterplot; 25(OH)D, 25 hydroxyvitamin D.
References
- Zadshir A, Tareen N, Pan D, Norris K, Martins D 2005 The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis 15(4 Suppl 5):97–101 [PubMed] [Google Scholar]
- Aloia JF, Feuerman M, Yeh JK 2006 Reference range for serum parathyroid hormone. Endocr Pract 12:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney RP 2005 Serum 25-hydroxyvitamin D and parathyroid hormone exhibit threshold behavior. J Endocrinol Invest 28:180–182 [DOI] [PubMed] [Google Scholar]
- Aloia JF, Talwar SA, Pollack S, Feuerman M, Yeh JK 2006 Optimal vitamin D status and serum parathyroid hormone concentrations in African American women. Am J Clin Nutr 84:602–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloia JF, Talwar SA, Pollack S, Yeh J 2005 A randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med 165:1618–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloia JF 2008 African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am J Clin Nutr 88:545S–550S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman F, Morgan DC, Nieves JW, Shen V, Luckey MM, Dempster DW, Lindsay R, Parisien M 1997 Resistance to bone resorbing effects of PTH in black women. J Bone Miner Res 12:958–966 [DOI] [PubMed] [Google Scholar]
- Heaney RP 2006 Low calcium intake among African Americans: effects on bones and body weight. J Nutr 136:1095–1098 [DOI] [PubMed] [Google Scholar]
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM 2004 Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15:2208–2218 [DOI] [PubMed] [Google Scholar]
- Garcia de la Torre N, Wass JA, Turner HE 2003 Parathyroid adenomas and cardiovascular risk. Endocr Relat Cancer 10:309–322 [DOI] [PubMed] [Google Scholar]
- Bibuld D 2009 Health disparities and vitamin D. Clin Rev Bone Miner Metab 7:63–76 [Google Scholar]
- Hagström E, Hellman P, Larsson TE, Ingelsson E, Berglund L, Sundström J, Melhus H, Held C, Lind L, Michaëlsson K, Arnlöv J 2009 Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation 119:2765–2771 [DOI] [PubMed] [Google Scholar]
- Andersson P, Rydberg E, Willenheimer R 2004 Primary hyperparathyroidism and heart disease: a review. Eur Heart J 25:1776–1787 [DOI] [PubMed] [Google Scholar]
- Kao PC, Jiang NS, Klee GG, Purnell DC 1982 Development and validation of a new radioimmunoassay for parathyrin (PTH). Clin Chem 28:69–74 [PubMed] [Google Scholar]
- Heinegård D, Tiderström G 1973 Determination of serum creatinine by a direct colorimetric method. Clin Chim Acta 43:305–310 [DOI] [PubMed] [Google Scholar]
- Cleveland W, Devlin S, 1988 Locally weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc 83:596–610 [Google Scholar]
- Chen H, Cohen P, Gordon K 2008 Using an econometric model of change points to locate turning points in individual time series. In: Cohen P, ed. Applied data analytic techniques for turning points research. Mahwah, NJ:Lawrence Erlbaum Associates; 183–194 [Google Scholar]
- Byrd R, Lu P, Nocedal J, Zhu C 1995 A limited memory algorithm for bound constrained optimization. SIAM J Sci Comput 16:1190–1208 [Google Scholar]
- Heaney R 2002 Ethnicity, bone status, and the calcium requirement. Nutr Res 22:153–178 [Google Scholar]
- Aloia JF, Vaswani A, Yeh JK, Flaster E 1996 Risk for osteoporosis in black women. Calcif Tissue Int 59:415–423 [DOI] [PubMed] [Google Scholar]
- Benjamin A, Moriakova A, Akhter N, Rao D, Xie H, Kukreja S, Barengolts E 2009 Determinants of 25-hydroxyvitamin D levels in African-American and Caucasian male veterans. Osteoporos Int 20:1795–1803 [DOI] [PubMed] [Google Scholar]
- Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, DeLuca HF, Drezner MK 2004 Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab 89:3152–3157 [DOI] [PubMed] [Google Scholar]
- 2009 Analytical note for NHANES, 2000–2006, and NHANES III (1988–1994) 25-hydroxyvitamin D analysis. (cited http://cdc.gov/nchs/data/nhanes/nhanes3/VitaminD_analyticnote.pdf; 1-7) [Google Scholar]
- Michelangeli V, Heyma P, Colman PG, Ebeling PR 1997 Evaluation of a new, rapid and automated immunochemiluminometric assay for the measurement of serum intact parathyroid hormone. Ann Clin Biochem 34(Pt 1):97–103 [DOI] [PubMed] [Google Scholar]
- Hermse D, Franzson L, Hoffmann JP, Ksaksson A, Kaufman JM, Leary E. Müller C, Nakatsuka K, Nishizawa Y, Reinauer H, Riesen W, Roth HJ, Steinmüller T, Troch T, Bergmann P 2002 Multicenter evaluation of a new immunoassay for intact PTH measurement on the Elecsys System 2010 and 1010. Clin Lab 48:131–141 [PubMed] [Google Scholar]
- Inaba M, Nakatsuka K, Imanishi Y, Watanabe M, Mamiya Y, Ishimura E, Nishizawa Y 2004 Technical and clinical characterization of the Bio-PTH (1-84) immunochemiluminometric assay and comparison with a second-generation assay for parathyroid hormone. Clin Chem 50:385–390 [DOI] [PubMed] [Google Scholar]





