Abstract
Context: Vitamin D may be important in the pathogenesis of severe preeclampsia. Given the few effective preventive strategies for severe preeclampsia, studies establishing this link are needed so that effective interventions can be developed.
Objective: Our objective was to assess whether midgestation vitamin D deficiency is associated with development of severe preeclampsia.
Design and Setting: We conducted a nested case-control study of pregnant women who had previously given blood for routine genetic multiple marker screening and subsequently delivered at a tertiary hospital between January 2004 and November 2008.
Patients: Participants included women with singleton pregnancies in the absence of any chronic medical illnesses. From an overall cohort of 3992 women, 51 cases of severe preeclampsia were matched by race/ethnicity with 204 women delivering at term with uncomplicated pregnancies. Banked maternal serum was used to measure maternal 25-hydroxyvitamin D [25(OH)D].
Main Outcome Measure: The main outcome was severe preeclampsia.
Results: Midgestation maternal 25(OH)D concentration was lower in women who subsequently developed severe preeclampsia compared with controls [median (interquartile range), 75 (47–107) nmol/liter vs. 98 (68–113) nmol/liter; P = 0.01]. Midgestation maternal 25(OH)D of less than 50 nmol/liter was associated with an almost 4-fold odds of severe preeclampsia (unadjusted odds ratio, 3.63; 95% confidence interval, 1.52–8.65) compared with midgestation levels of at least 75 nmol/liter. Adjustment for known confounders strengthened the observed association (adjusted odds ratio, 5.41; 95% confidence interval, 2.02–14.52).
Conclusion: Maternal midgestation vitamin D deficiency was associated with increased risk of severe preeclampsia. Vitamin D deficiency may be a modifiable risk factor for severe preeclampsia.
Maternal midgestation vitamin D deficiency is an independent risk factor for severe preeclampsia.
Preeclampsia is a pregnancy-specific syndrome characterized by high blood pressure and proteinuria after 20 wk gestation that occurs in up to 8% of pregnant women (1,2,3). Women who develop preeclampsia are at increased risk for development of pulmonary edema, coagulation defects, hepatic and/or renal failure, seizures, cerebral hemorrhage, blindness, and death (2,4,5,6,7,8). Infants born to mothers with preeclampsia are at increased risk of prematurity and more likely to be small for gestational age (9,10). In addition, authors have reported that women with a history of preeclampsia are at an elevated risk for cardiovascular disease later in life (11,12).
The etiology of preeclampsia is unknown. Abnormal trophoblastic invasion, inflammatory changes, oxidative stress, and immunological factors are all potential contributing factors (13). An emerging area of study that has garnered significant attention is the role of vitamin D and its active metabolite, 1,25-dihydroxyvitamin D. Increased production of inflammatory cytokines, such as TNF-α, has been reported in pregnancies complicated by vitamin D deficiency (14). Furthermore, 1,25-dihydroxyvitamin D stimulates the activity of T-regulatory cells, which are vital in supporting placental implantation through immune tolerance (15). In preeclampsia, the metabolism of vitamin D in placental tissue is altered, and these differences may play a role in the abnormal trophoblastic invasion found in these pregnancies (16).
Researchers have previously reported lower maternal serum vitamin D concentrations in women after diagnosis of preeclampsia (17,18), and a single study has demonstrated lower levels before clinical diagnosis of preeclampsia at term (19). However, the clinically significant morbidity associated with preeclampsia occurs when the diagnosis is made preterm. Early-onset (preterm) or severe preeclampsia is characterized by more pronounced clinical symptoms, worse maternal-fetal outcomes, and higher risk of recurrence in future pregnancies (20,21). Consequently, our objective was to assess whether midgestation vitamin D deficiency is more prevalent in women with severe preeclampsia. We hypothesized that midgestation levels of vitamin D are lower among women who later develop severe preeclampsia, compared with healthy women delivering at term.
Subjects and Methods
Study design
We conducted a nested, case-control study in a cohort of 3992 women. All women who had previously given blood for routine genetic multiple marker screening and subsequently delivered at the University of North Carolina-Chapel Hill between January 2004 and November 2008 were eligible. All women, regardless of risk status or payer status are offered this screening as part of routine prenatal care. Nonfasting blood samples were collected for routine genetic multiple marker screening between 15 and 20 wk gestation, and serum aliquots were barcoded and frozen at −70 C. Maternal demographic and medical data were chart abstracted. This study was approved by the Institutional Review Board before data collection, and permission was obtained to use banked serum from these women for research purposes.
Severe preeclampsia was defined as a systolic blood pressure of at least 160 mm Hg and/or a diastolic blood pressure of at least 110 mm Hg, recorded on at least two occasions 6 h apart, plus proteinuria (≥300 mg in a 24-h collection or 1+ on a urine dipstick) or a systolic blood pressure of at least 140 mm Hg and/or a diastolic blood pressure of at least 90 mm Hg, recorded on at least two occasions 6 h apart plus 5 g of proteinuria in a 24-h period. We further classified cases of preeclampsia as severe in the setting of pulmonary edema, seizures, oliguria (<500 ml/24 h), elevated liver enzymes accompanied by right upper quadrant pain, thrombocytopenia (<100,000/mm3), or persistent cerebral symptoms such as headache or blurry vision (22). Healthy women with term deliveries (≥37 wk) were used as controls. For the present study, for both cases and controls, we excluded women with multiple gestation, major congenital fetal anomalies, pregestational hypertension, kidney disease, diabetes mellitus, known thrombophilias, or any other significant preexisting chronic medical disease. A single investigator reviewed all patient charts retrospectively, and only patients meeting the strict definitions described above were included in the study.
From the total cohort of 3992 women, we identified 51 cases during the study period who met all inclusion and exclusion criteria. These cases were matched by race/ethnicity, in 4:1 ratio, to a random, computer-generated referent group of 204 healthy women delivering at term. Of the 51 cases, 44 had an adequate volume of serum available for analysis. Of the 204 controls, 201 had an adequate volume of serum available for analysis. Four subjects (one case; three controls) were excluded due to a technical problem with 25-hydroxyvitamin D [25(OH)D] measurement.
Based on definitions of vitamin D status in the literature (23,24), we categorized 25(OH)D as at least 75 nmol/liter (reference group), 50–74.9 nmol/liter, and less than 50 nmol/liter.
Laboratory analyses
Serum aliquots for each enrolled subject were shipped on dry ice to Massachusetts General Hospital (MGH, Boston, MA) for serum 25(OH)D measurement by liquid chromatography-tandem mass spectrometry (25). The method used is an isotope dilution, liquid chromatography-tandem mass spectrometry assay optimized in the MGH laboratory based on published procedures (26). The limit of detection is 5 nmol/liter for vitamin D2 and 7.5 nmol/liter for vitamin D3. The between-run coefficient of variation for a quality control serum containing a total vitamin D concentration of 57 nmol/liter is 7.5%.
Statistical analysis
Statistical analyses were performed using Stata 10.1 (StataCorp, College Station, TX) and summarized data using basic descriptive statistics. We performed the unadjusted analyses using Wilcoxon-Mann Whitney, χ2, and Fisher’s exact tests to compare differences between cases and controls. All P values were two-tailed, with P < 0.05 considered statistically significant. To further describe the bivariate relationship between serum 25(OH)D level and risk of severe preeclampsia, the locally weighted scatterplot smoothing (Lowess) technique was used. Lowess plots are designed to produce a smooth fit to the data that also reduces the influence of extreme outliers. Multivariable logistical regression was performed to evaluate independent predictors of severe preeclampsia, with results reported as odds ratios (ORs) with 95% confidence intervals (CIs).
Results
We successfully analyzed 25(OH)D levels from 241 samples (43 cases, 198 controls). The median gestational age of serum collection for both groups was 17 wk (interquartile range, 16–19 wk). The median 25(OH)D level for all subjects was 95 nmol/liter (interquartile range, 65–112 nmol/liter), and levels were lower among black vs. white women [median (interquartile range), 93 (61–112) nmol/liter vs. 99 (82–113) nmol/liter; P = 0.08). As shown in Table 1, women who developed severe preeclampsia may have been slightly older than controls, but this difference was not statistically significant (P = 0.07). All other demographic and clinical characteristics between the groups at midgestation were similar. Women with severe preeclampsia were delivered earlier when compared with controls (33 vs. 40 wk; P < 0.001).
Table 1.
Clinical and demographic characteristics of women who developed severe preeclampsia (cases) and race/ethnicity-matched women who did not (controls)
| Variables | Controls | Cases | P value |
|---|---|---|---|
| n | 198 | 43 | |
| Age (yr) | 28 (23–32) | 30 (25–34) | 0.07 |
| Race/ethnicity | |||
| White | 29 (58) | 30 (13) | |
| Black | 40 (79) | 40 (17) | |
| Hispanic | 26 (51) | 26 (11) | |
| Other | 5 (10) | 5 (2) | |
| Multiparous | 47 (93) | 42 (18) | 0.54 |
| Body mass index (kg/m2) | 31 (27–35) | 30 (28–35) | 0.84 |
| Gestational age at serum collection (wk) | 17 (16–19) | 17 (16–19) | 0.79 |
| Gestational age at delivery (wk) | 40 (39–41) | 33 (31–35) | <0.001 |
| Season of blood draw | 0.46 | ||
| Winter | 26 (52) | 28 (12) | |
| Spring | 30 (59) | 28 (12) | |
| Summer | 31 (62) | 23 (10) | |
| Fall | 13 (25) | 21 (9) | |
| 25(OH)D (nmol/liter) | 98 (68–113) | 75 (47–107) | 0.01a |
| Serum 25(OH)D level | 0.01 | ||
| <50 nmol/liter | 10 (19) | 26 (11) | |
| 50–74.9 nmol/liter | 21 (41) | 23 (10) | |
| 75+ nmol/liter | 70 (138) | 51 (22) |
Data are expressed as percentage (number) or median (interquartile range). Wilcoxon-Mann Whitney test was used for continuous variables, and Fisher’s exact test for categorical variables.
Wilcoxon rank sum test.
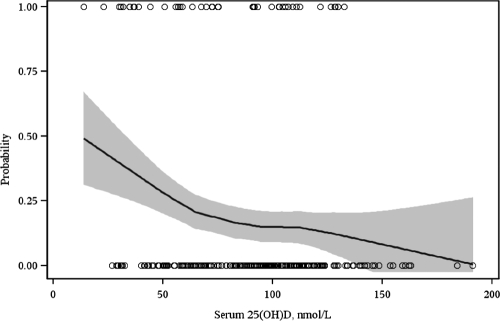
Median maternal serum 25(OH)D concentration at midgestation was 23% lower in women who subsequently developed severe preeclampsia compared with healthy women delivering at term (Table 1). Midgestation vitamin D deficiency [defined as 25(OH)D <50 nmol/liter] was more common in women who developed severe preeclampsia compared with controls (26 vs. 10%; P = 0.01). In unadjusted models, midgestation maternal 25(OH)D of less than 50 nmol/liter was associated with an almost 4-fold odds of severe preeclampsia (OR, 3.63; 95% CI, 1.52–8.65) compared with midgestation levels of at least 75 nmol/liter (Table 2). This association was strengthened by adjustment for season of blood draw, maternal age, multiparity, body mass index, and gestational age at serum collection. In the covariate-adjusted models, we found more than a 5-fold increase in the odds of severe preeclampsia (adjusted OR, 5.41; 95% CI, 2.02–14.52) among women with midgestation 25(OH)D of less than 50 nmol/liter compared with women with midgestation 25(OH)D of at least 75 nmol/liter (Table 2). As demonstrated in Fig. 1, there was an inverse association between maternal 25(OH)D concentration at midgestation and risk of severe preeclampsia. There were no cases of severe preeclampsia among women with serum 25(OH)D concentrations greater than 135 nmol/liter.
Table 2.
Unadjusted and adjusted ORs for severe preeclampsia according to vitamin D status
| Serum 25(OH)D level (nmol/liter) | Controls (n) | Severe preeclampsia (n) | Unadjusted OR (95% CI) | P value | Adjusted OR (95% CI)a | P value |
|---|---|---|---|---|---|---|
| 75+ | 138 | 22 | 1.00 (Reference) | 1.00 (Reference) | ||
| 50–74.9 | 41 | 10 | 1.53 (0.67–3.49) | 0.31 | 2.16 (0.86–5.40) | 0.10 |
| <50 | 19 | 11 | 3.63 (1.52–8.65) | 0.004 | 5.41 (2.02–14.52) | 0.001 |
Adjusted for season of blood draw, maternal age, multiparity, body mass index, and gestational age at serum collection.
Figure 1.
Lowess plot of the association between maternal 25(OH)D at 15–20 wk gestation and the risk of severe preeclampsia.
Discussion
In the present study, we sought to determine whether midgestation vitamin D deficiency is associated with an increased risk of severe preeclampsia. Our results confirmed our hypothesis that women who developed severe preeclampsia had significantly lower maternal serum 25(OH)D early in pregnancy compared with healthy women with uncomplicated term pregnancies. Furthermore, the proportion of midgestation vitamin D deficiency [defined as serum 25(OH)D level <50 nmol/liter] was higher in women with severe preeclampsia.
Our findings confirm and extend earlier work by Bodnar et al. (19), where vitamin D deficiency early in pregnancy was associated with preeclampsia. The investigators reported a dose-response relationship, with 25(OH)D levels of less than 37.5 nmol/liter being associated with a 5-fold increase in the odds of preeclampsia (adjusted OR, 5.0; 95% CI, 1.7–14.1). Interestingly, the majority of the patients in this study reported regular prenatal vitamin use. An important difference in our study was that we focused on women with severe preeclampsia. Although Bodnar et al. (19) do not report how many of their patients had severe preeclampsia, the women in the preeclampsia arm of their study delivered at a median gestational age of 39 wk, suggesting that most of their cases were likely mild. Finally, the vitamin D concentrations in the study by Bodnar et al. are quite lower than those reported in our study. There are several potential reasons for this difference. Most importantly, Bodnar et al. (19) do not report the seasons when their blood samples were collected. In our study, approximately 75% of our samples were collected during spring, summer, and fall when sunlight is plentiful. Perhaps the patients in Bodnar’s study had more of their samples collected in winter. Also, the patients in Bodnar’s study were located at a higher latitude (Pennsylvania) than the patients in our study (North Carolina). Finally, there are likely to be unmeasured lifestyle differences between the two study groups (i.e. amount of time spent outside) that could also contribute to the differences in vitamin D concentrations between the studies.
Several studies have provided evidence that vitamin D supplementation may reduce the risk of preeclampsia. In a prospective cohort study by Haugen et al. (27) involving more than 20,000 nulliparous women, a 29% reduced risk of preeclampsia was observed in women who reported supplementing with 400–600 IU of vitamin D during the first half of their pregnancies. Marya et al. (28) conducted a randomized controlled trial of calcium and vitamin D supplementation (1200 IU/d) beginning at 20–24 wk gestation in 400 women. Although the supplemented group experienced a significant reduction in blood pressure and their incidence of preeclampsia was lower than the control group (6 vs. 9%), the preeclampsia results were not statistically significant. In a controlled trial of 5644 women in the United Kingdom, those receiving a dietary supplement of halibut liver oil containing 900 IU of vitamin D per day starting at 20 wk gestation demonstrated a 32% decreased odds of preeclampsia compared with women who did not receive the supplement (29). However, the supplement also contained other vitamins and minerals, making it difficult to determine whether vitamin D was the main reason for this decrease.
Our findings must be interpreted in the context of our study design. When using a case-control design, there is potential for selection bias, especially in the selection of controls. It is challenging to match for all potential covariates, and it is possible that unmeasured differences exist between the groups that may explain the apparent association. However, our controls were nested within a large cohort of women who provided serum samples as part of routine prenatal screening, reducing selection bias. It is also possible that unmeasured confounding explains the apparent association between vitamin D deficiency and preeclampsia. However, we found that adjustment for some known potential confounders strengthened the observed association. To establish a causal association between vitamin D deficiency and preeclampsia, a randomized controlled trial of vitamin D supplementation among women with vitamin D deficiency is needed.
Our study does have several methodological strengths. The maternal serum was collected well before delivery at a time when no clinical manifestations of preeclampsia were evident, reducing the likelihood that subclinical disease affected vitamin D levels. In addition, we excluded all women with chronic medical illnesses, such as diabetes and chronic hypertension. These diseases have been associated with increased risk for preeclampsia. If women with these chronic medical conditions differentially limited vitamin D intake or sun exposure, inclusion of these women could confound the observed association.
In summary, the results of our nested case-control study support an association between maternal vitamin D deficiency and severe preeclampsia. The well-established role of vitamin D deficiency in other health problems (30), coupled with our data, supports further investigation into the role of vitamin D in the development of preeclampsia. Supplementing vitamin D among deficient women either in the preconception period or in early pregnancy should be tested as a potentially effective intervention to prevent severe preeclampsia.
Footnotes
This work was supported by the National Institutes of Health Grant 5K12HD050113-04 (to A.M.S.) and the Massachusetts General Hospital Center for D-Receptor Activation Research (to C.A.C.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online August 18, 2010
Abbreviations: CI, Confidence interval; 25(OH)D, 25-hydroxyvitamin D; OR, odds ratio.
References
- Saftlas AF, Olson DR, Franks AL, Atrash HK, Pokras R 1990 Epidemiology of preeclampsia and eclampsia in the United States, 1979–1986. Am J Obstet Gynecol 163:460–465 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention 1998 Maternal mortality–United States, 1982–1996. MMWR Morb Mortal Wkly Rep 47:705–707 [PubMed] [Google Scholar]
- Sibai BM, Gordon T, Thom E, Caritis SN, Klebanoff M, McNellis D, Paul RH 1995 Risk factors for preeclampsia in healthy nulliparous women: a prospective multicenter study. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol 172:642–648 [DOI] [PubMed] [Google Scholar]
- Sibai BM 2004 Magnesium sulfate prophylaxis in preeclampsia: lessons learned from recent trials. Am J Obstet Gynecol 190:1520–1526 [DOI] [PubMed] [Google Scholar]
- Benedetti TJ, Kates R, Williams V 1985 Hemodynamic observations in severe preeclampsia complicated by pulmonary edema. Am J Obstet Gynecol 152:330–334 [DOI] [PubMed] [Google Scholar]
- Drislane FW, Wang AM 1997 Multifocal cerebral hemorrhage in eclampsia and severe pre-eclampsia. J Neurol 244:194–198 [DOI] [PubMed] [Google Scholar]
- Morriss MC, Twickler DM, Hatab MR, Clarke GD, Peshock RM, Cunningham FG 1997 Cerebral blood flow and cranial magnetic resonance imaging in eclampsia and severe preeclampsia. Obstet Gynecol 89:561–568 [DOI] [PubMed] [Google Scholar]
- Cunningham FG, Fernandez CO, Hernandez C 1995 Blindness associated with preeclampsia and eclampsia. Am J Obstet Gynecol 172:1291–1298 [DOI] [PubMed] [Google Scholar]
- Xiao R, Sorensen TK, Williams MA, Luthy DA 2003 Influence of pre-eclampsia on fetal growth. J Matern Fetal Neonatal Med 13:157–162 [DOI] [PubMed] [Google Scholar]
- Friedman SA, Schiff E, Kao L, Sibai BM 1995 Neonatal outcome after preterm delivery for preeclampsia. Am J Obstet Gynecol 172:1785–1788; discussion 1788–1792 [DOI] [PubMed] [Google Scholar]
- Smith GC, Pell JP, Walsh D 2001 Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet 357:2002–2006 [DOI] [PubMed] [Google Scholar]
- Irgens HU, Reisaeter L, Irgens LM, Lie RT 2001 Long term mortality of mothers and fathers after pre-eclampsia: population-based cohort study. BMJ 323:1213–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibai B, Dekker G, Kupferminc M 2005 Pre-eclampsia. Lancet 365:785–799 [DOI] [PubMed] [Google Scholar]
- Díaz L, Noyola-Martínez N, Barrera D, Hernández G, Avila E, Halhali A, Larrea F 2009 Calcitriol inhibits TNF-α-induced inflammatory cytokines in human trophoblasts. J Reprod Immunol 81:17–24 [DOI] [PubMed] [Google Scholar]
- Hyppönen E 2005 Vitamin D for the prevention of preeclampsia? A hypothesis. Nutr Rev 63:225–232 [DOI] [PubMed] [Google Scholar]
- Fischer D, Schroer A, Lüdders D, Cordes T, Bücker B, Reichrath J, Friedrich M 2007 Metabolism of vitamin D3 in the placental tissue of normal and preeclampsia complicated pregnancies and premature births. Clin Exp Obstet Gynecol 34:80–84 [PubMed] [Google Scholar]
- Cruikshank DP, Chan GM, Doerrfeld D 1993 Alterations in vitamin D and calcium metabolism with magnesium sulfate treatment of preeclampsia. Am J Obstet Gynecol 168:1170–1176; discussion 1176–1177 [DOI] [PubMed] [Google Scholar]
- Seely EW, Wood RJ, Brown EM, Graves SW 1992 Lower serum ionized calcium and abnormal calciotropic hormones levels in preeclampsia. J Clin Endocrinol Metab 74:1436–1440 [DOI] [PubMed] [Google Scholar]
- Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM 2007 Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab 92:3517–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibai BM 2003 Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol 102:181–192 [DOI] [PubMed] [Google Scholar]
- Sibai BM, Mercer B, Sarinoglu C 1991 Severe preeclampsia in second trimester: recurrence risk and long term prognosis. Am J Obstet Gynecol 165:1408–1412 [DOI] [PubMed] [Google Scholar]
- ACOG Committee on Practice Bulletins 2002 Diagnosis and management of preeclampsia and eclampsia. No. 33. Obstet Gynecol 99:159–167 [DOI] [PubMed] [Google Scholar]
- Holick MF 2007 Vitamin D deficiency. N Engl J Med 357:266–281 [DOI] [PubMed] [Google Scholar]
- Canadian Paediatric Society 2007 Vitamin D supplementation: recommendations for Canadian mothers and infants. Paediatr Child Health 12:583–598 [PMC free article] [PubMed] [Google Scholar]
- Roth HJ, Schmidt-Gayk H, Weber H, Niederau C 2008 Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography-tandem mass spectrometry as a reference. Ann Clin Biochem 45:153–159 [DOI] [PubMed] [Google Scholar]
- Singh RJ, Taylor RL, Reddy GS, Grebe SKG 2006 C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab 91:3055–3061 [DOI] [PubMed] [Google Scholar]
- Haugen M, Brantsaeter AL, Trogstad L, Alexander J, Roth C, Magnus P, Meltzer HM 2009 Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology 20:720–726 [DOI] [PubMed] [Google Scholar]
- Marya RK, Rathee S, Manrow M 1987 Effect of calcium and vitamin D supplementation on toxaemia of pregnancy. Gynecol Obstet Invest 24:38–42 [DOI] [PubMed] [Google Scholar]
- Olsen SF, Secher NJ 1990 A possible preventive effect of low-dose fish oil on early delivery and pre-eclampsia: indications from a 50-year-old controlled trial. Br J Nutr 64:599–609 [DOI] [PubMed] [Google Scholar]
- Adams JS, Hewison M 2010 Update in vitamin D. J Clin Endocrinol Metab 95:471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]