Abstract
Context: Dehydroepiandrosterone sulfate (DHEA-S), a major circulating sex steroid prohormone, declines with age. Low levels have been associated with increased cardiovascular disease (CVD) risk and all-cause mortality, although these results have not been consistently replicated, particularly in women.
Objective: Our objective was to examine the association of circulating DHEA-S levels, CVD, and mortality risk among postmenopausal women with suspected myocardial ischemia.
Design: In the Women’s Ischemia Syndrome Evaluation, 270 postmenopausal women underwent coronary angiography and blood hormone levels for suspected ischemia and were followed annually. The primary outcome of interest was CVD mortality; secondary analyses included all-cause mortality and nonfatal CVD events (myocardial infarction, stroke, and congestive heart failure) and angiographic obstructive coronary artery disease (CAD).
Results: Women in the lowest DHEA-S tertile had higher CVD mortality (17% 6-yr mortality rate vs. 8%; log-rank P = 0.011), and all-cause mortality (21 vs. 10%; P = 0.011) compared with women with higher DHEA-S levels. The increased CVD mortality risk [hazard ratio (HR) = 2.55; 95% confidence interval (CI) = 1.19–5.45] remained unchanged after adjustment for multiple CVD risk factors (HR = 2.43; 95% CI = 1.06–5.56) but became nonsignificant when further adjusting for the presence or severity of angiographic obstructive CAD (HR = 1.99; 95% CI = 0.87–4.59). Results were similar for all-cause mortality. Lower DHEA-S levels were only marginally but not independently associated with obstructive CAD.
Conclusions: Among postmenopausal women with coronary risk factors undergoing coronary angiography for suspected myocardial ischemia, lower DHEA-S levels were linked with higher CVD mortality and all-cause mortality. Our study provides valuable feasibility data useful for future investigations and possible mechanistic pathways.
In postmenopausal women with coronary risk factors undergoing coronary angiography for ischemia, lower DHEA-S levels are linked with higher cardiovascular disease mortality and all-cause mortality.
Dehydroepiandrosterone sulfate (DHEA-S) is a prohormone that is secreted predominantly by the adrenal glands and is the most abundant endogenous steroid hormone in women and men. Because DHEA-S levels decline progressively with age (1,2), it has been hypothesized that its diminution over time may be related to increasing risk of cardiovascular disease (CVD). Studies have suggested an association of low DHEA-S levels with the risk of CVD and all-cause mortality (3,4,5). However, these results have not been consistently replicated in all reported cohorts, particularly women (6,7,8,9).
Among men, low DHEA-S levels have been consistently associated with an increased risk of all-cause mortality, CVD, and ischemic heart disease (3,5,10,11). Prospective studies in both men and women have shown an association between lower DHEA-S levels and greater atherosclerotic progression as assessed by coronary angiography (12) as well as carotid wall thickness (13). Other studies have failed to find a relationship between DHEA-S levels and CVD outcomes in women (5,10,11,14,15). The recent Women’s Health and Aging Study has demonstrated a U-shaped relationship between DHEA-S level and all-cause mortality, possibly due to higher cancer mortality at the highest levels and higher CVD mortality at the lowest levels (16). The authors suggest that by not accounting for such biological relationships, the association between DHEA-S levels and mortality may be obscured in many studies, resulting in negative or nonsignificant findings.
Given the controversial role of DHEA-S in CVD risk in women, we have evaluated the association of DHEA-S and CVD outcomes in the Women’s Ischemia Syndrome Evaluation (WISE) study, a cohort of women referred for coronary angiography for suspected myocardial ischemia. Our primary outcome measure was death due to CVD. We also considered all-cause mortality and nonfatal CVD events as well as the degree and severity of obstructive coronary artery disease (CAD) at angiography in an effort to evaluate the potential mechanistic links between DHEA-S and CVD outcomes in women.
Subjects and Methods
Patient population
The WISE is a four-center study designed to improve diagnostic testing for ischemic heart disease in women with signs and symptoms of myocardial ischemia referred for coronary angiography, and to investigate prognosis and pathophysiological mechanisms, including the role of reproductive hormones. Participating centers included the University of Alabama at Birmingham; the University of Florida, Gainesville; the University of Pittsburgh, and the Allegheny General Hospital in Pittsburgh. The protocol was approved by the institutional review committees for human protection at each center, and all women provided written informed consent. Women undergoing clinically indicated coronary angiography for suspected ischemia, but without unstable myocardial ischemia, were recruited into WISE. Postmenopausal status was classified using a previously described reproductive status algorithm (17) based on age, the presence of regular menses, time since cessation of menses, and reproductive surgery as well as reproductive hormone profiles. All classifications were reviewed by the WISE Hormone Committee, which included two reproductive endocrinologists who were blinded to coronary angiography findings, CVD outcomes, and DHEA-S levels. The present analysis includes 270 postmenopausal women with DHEA-S assays who were not currently taking exogenous hormones.
Data collection and covariates of interest
All WISE patients underwent a physical examination that included heart rate, blood pressure, height, weight, waist and hip circumference, body mass index (BMI) (kilograms per square meter) and detailed past medical history [previous diagnosis of CAD, congestive heart failure (CHF), diabetes mellitus, hypertension, dyslipidemia, smoking, and reproductive history]. More detailed information on the WISE study design has been published (18). Smoking history was reported as current or ever smoking and quantified by pack-years (number of cigarettes daily multiplied by years of smoking and divided by 20). Polycystic ovary syndrome (PCOS) was defined previously (19). WISE core laboratory assays included fasting cholesterol measures, reproductive sex hormones, and androgen levels, including DHEA-S. Insulin resistance was measured using the homeostasis model assessment (HOMA) (20).
Measurement of DHEA-S
Serum DHEA-S levels were assayed at the WISE reproductive hormone core laboratory from stored samples and were quantified in duplicate by a solid-phase, competitive chemiluminescent enzyme immunoassay on the Immulite analyzer (Siemens Medical Solutions Diagnostics, Los Angeles, CA) (21). The assay sensitivity in this laboratory is 3 μg/dl and within-assay coefficients of variation are 9.5, 8.1, and 6.8% at 189, 421, and 783 μg/dl DHEA-S, respectively. The between-assay coefficients are 15.0, 13.0, and 8.1% at 162, 552, and 899 μg/dl DHEA-S, respectively. Reproductive hormones have exhibited stability with repeat measurements after prolonged frozen storage as well as repeat freeze-thaw cycles (22,23). Consistent methodology was maintained for the duration of the study.
Angiographic assessment of CAD
Quantitative analysis of coronary angiography was performed by the WISE Angiographic Core Laboratory using previously described methodology (24). Measurements included presence and severity of obstructive coronary artery stenosis. Presence of obstructive CAD was defined ≥50% luminal diameter stenosis in one or more epicardial coronary artery. In addition, as described previously (24), each participant was assigned a continuous WISE CAD severity score based on percent stenosis adjusted for any complete collaterals. The final scores were then weighted according to lesion location, with more proximal lesions receiving a higher weighting factor. The possible range of the score is 5 (no detectable stenosis) to 100 (multiple severe lesions), and the actual range in the WISE is 5–78. Because of a skewed distribution, the CAD severity scores were natural log transformed for analysis.
Follow-up methods and outcomes
Patients were followed for up to 9 yr by an experienced study nurse or physician following a prescribed questionnaire, beginning 6 wk after enrollment and yearly thereafter. For women who died, a death certificate and/or physician report on the circumstances of death was obtained. All deaths were adjudicated for cardiovascular causes by the WISE events committee masked to clinical and angiographic data. For the present analysis, primary outcomes were deaths due to CVD. Secondary analyses examined all-cause mortality, a composite outcome of CVD events (CVD mortality, or hospitalization for nonfatal myocardial infarction, heart failure, or stroke) as well as angiographic evidence of CAD presence and severity.
Statistical methods
Because of a skewed distribution, DHEA-S levels were natural log transformed to approximate a normal distribution when used as a continuous variable in correlations. In addition, we specified DHEA-S as a binary variable, with the cut-point for CVD mortality set at the lowest tertile, as determined by receiver operator curve analysis, using the trapezoidal rule in logistic regression in SAS version 9.2 (SAS Institute, Cary, NC).
When comparing differences in clinical and CVD risk factors by DHEA-S tertile category (lowest tertile vs. all others) in Table 1, we used frequencies (percent) for categorical measures and means ± sd for continuous measures. Because age is strongly correlated with DHEA-S as well as many CAD risk factors, all P values were adjusted for age using logistic regression. For variables that showed a skewed distribution (e.g. the coronary severity score), we used nonparametric Wilcoxon statistics to compare scores by DHEA-S tertile category and the natural log transformation to estimate age-adjusted P values.
Table 1.
Baseline characteristics by DHEA-S tertile category: frequencies (percent), means ± sd, and medians (interquartile range)
| Baseline variable | Lowest DHEA-S tertile (0.02–24.7 μg/dl; n = 87) | Middle and highest DHEA-S tertiles (24.8–244.0 μg/dl; n = 183) | Age-adjusted P value |
|---|---|---|---|
| African-American (%) | 15 | 21 | 0.30 |
| Age (yr) (mean ± sd) | 67 ± 9 | 63 ± 10 | 0.0008 |
| Smoking history | |||
| Current smoker (%) | 11 | 21 | 0.46 |
| Ever smoked (%) | 55 | 54 | 0.34 |
| Pack-years of smokinga | |||
| Mean ± sd | 36 ± 36 | 25 ± 19 | 0.25 |
| Median (IQR) | 22 (9–56) | 20 (11–40) | 0.66 |
| Cardiac risk factors | |||
| History of hypertension (%) | 68 | 63 | 0.70 |
| Systolic BP (mean ± sd) | 144 ± 22 | 139 ± 20 | 0.27 |
| Diastolic BP (mean ± sd) | 76 ± 11 | 76 ± 9 | 0.98 |
| Diabetes mellitus (%) | 31 | 33 | 0.45 |
| History of dyslipidemia (%) | 54 | 59 | 0.37 |
| Laboratory values | |||
| Total cholesterol (mg/dl) | 200 ± 51 | 197 ± 45 | 0.36 |
| >200 mg/dl (%) | 46 | 41 | 0.32 |
| LDL cholesterol (mg/dl) (mean ± sd) | 118 ± 45 | 116 ± 40 | 0.43 |
| >130 mg/dl (%) | 35 | 31 | 0.39 |
| HDL cholesterol (mg/dl) | 55 ± 13 | 52 ± 11 | 0.045 |
| <50 mg/dl (%) | 38 | 49 | 0.11 |
| Triglycerides (mg/dl) | 149 ± 93 | 147 ± 90 | 0.68 |
| >150 mg/dl (%) | 40 | 39 | 0.99 |
| High-sensitivity C-reactive protein (mg/dl) | |||
| Mean ± sd | 8.3 ± 21.8 | 7.9 ± 12.6 | 0.87 |
| Median (IQR) | 3.0 (1.3–6.9) | 3.8 (1.8–8.0) | 0.14 |
| HOMA (mean ± sd) | 5.7 ± 7.7 | 5.1 ± 8.3 | 0.64 |
| Median (IQR) | 2.9 (1.2–5.8) | 2.3 (1.4–4.6) | 0.62 |
| Body mass | |||
| BMI (kg/m2) (mean ± sd) | 29.6 ± 6.0 | 30.2 ± 7.3 | 0.99 |
| ≥30 kg/m2 (%) | 38 | 41 | 0.74 |
| Waist to hip ratio (mean ± sd) | 0.89 ± 0.13 | 0.88 ± 0.11 | 0.30 |
| Waist circumference (in.) (mean ± sd) | 38.1 ± 7.0 | 38.4 ± 7.9 | 0.92 |
| >35 in. (%) | 65 | 60 | 0.33 |
| PCOS (%) | 4 | 9 | 0.24 |
| Obstructive CAD | |||
| Presence of angiographic CAD (%) | 56 | 42 | 0.17 |
| CAD severity score (mean ± sd) | 19.2 ± 16.3 | 15.9 ± 15.1 | 0.40 |
BP, Blood pressure; IQR, interquartile range.
Among current smokers only.
We used multivariate logistic regression when evaluating the association between DHEA-S and the presence of obstructive CAD and multivariate linear regression when assessing the association of DHEA-S with the log-transformed CAD severity score. Covariates included age, ethnicity, and known CVD risk factors (e.g. hypertension, diabetes, and smoking).
For survival and event outcome analyses, we present raw as well as Kaplan-Meier calculated event rates. Since age is consistently not a significant predictor of CVD events or mortality in WISE or the present subsample, P values for these rate differences were not adjusted for age. We used Kaplan-Meier survival curves to plot time to event by DHEA-S tertile category and the unadjusted log-rank test to compare survival curves. We then constructed univariate and multivariate Cox proportional hazard models for DHEA-S tertile category and generated hazard ratios (HR) and 95% confidence intervals (CI). Covariates included known CVD risk factors as well as factors shown in our analysis to be associated with DHEA-S levels [age and high-density lipoprotein (HDL)]. All analyses were performed with SAS software (version 9.2). Statistical significance was defined as P = 0.05.
Results
Study population
Of the 936 women in the WISE cohort, a total of 381 WISE women were postmenopausal and did not use exogenous hormones. Of these, 270 had sufficient sample volume and were assayed for DHEA-S concentration. Ages ranged from 40–86 yr (mean 64 ± 10), 19% were African-American, 46% had obstructive CAD (≥50% stenosis in any epicardial artery), 54% had a hysterectomy, and 29% had bilateral oophorectomy. Mean DHEA-S levels were 48.1 ± 39.5 μg/dl (range 0.02–244). These 270 women did not differ from the 111 eligible postmenopausal WISE women without DHEA-S assays on any of the baseline characteristics listed in Table 1; the only exception was that they had larger waist circumference and waist to hip ratios (P = 0.0003 and 0.002, respectively) (data not shown).
Initial receiver operator curve analysis of DHEA-S levels demonstrated a threshold of increased risk for CVD mortality at the lowest tertile (0.02–24.7 vs. 24.8–244 μg/dl); we therefore compared the lowest tertile to all other DHEA-S levels. Table 1 describes baseline characteristics by DHEA-S tertile category. The main difference was age. Women in the lowest DHEA-S tertile were significantly older than those with higher levels (P = 0.0008). Additionally, women in the lower tertiles tended to have higher HDL levels, although this difference was not statistically significant when adjusting for multiple testing (e.g. Bonferroni adjustment). There were no significant differences between the two groups in CAD presence or severity, high-sensitivity C-reactive protein, insulin resistance, presence of PCOS, or other CVD risk factors.
DHEA-S levels were not significantly different between African-American and White women; the mean level was 50.5 ± 36.6 μg/dl among African-Americans and 47.7 ± 40.5 μg/dl among Whites (P = 0.65). There was a significant negative correlation between age and log DHEA-S levels (Pearson r = −0.24; P < 0.0001), but no significant association was found between log DHEA-S levels and BMI, waist to hip ratio, smoking status, diabetes, insulin levels, HOMA, or other CVD risk factors (data not shown).
Relationship between DHEA-S levels and angiographic obstructive CAD
Among women in the lowest DHEA-S tertile, 49 (56%) had obstructive CAD (≥50% stenosis) compared with 76 (42%) in the upper tertiles [unadjusted odds ratio (95% CI) = 1.82 (1.08–3.04); P = 0.023]. When adjusted for age, ethnicity, and multiple established CAD risk factors (Table 2), the odds ratio was attenuated [odds ratio (95% CI) = 1.45 (0.83–2.54); P = 0.20]. Likewise, the log-transformed CAD severity score was somewhat higher among women in the lowest DHEA-S tertile than in the upper tertiles with means ± sd of 2.6 ± 0.8 vs. 2.4 ± 0.8, respectively [unadjusted β (se) = 0.21 (0.10); P = 0.049], but this difference became nonsignificant when adjusting for the same covariates [β (se) = 0.13 (0.10); P = 0.21] (data not shown). The Pearson correlations between presence or log severity of CAD and either log DHEA-S or lowest DHEA-S tertile ranged from −0.08 to −0.16.
Table 2.
DHEA-S Prediction of Obstructive CAD Multivariate Risk Factor Adjusted Logistic Regression Model
| Predictor | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Age | 1.06 | 1.03–1.09 | 0.0002 |
| Ethnicity | 1.27 | 0.63–2.55 | 0.50 |
| History of diabetes | 2.54 | 1.41–4.57 | 0.002 |
| History of hypertension | 1.17 | 0.66–2.06 | 0.59 |
| Ever smoked (yes/no) | 1.23 | 0.72–2.09 | 0.45 |
| BMI (kg/m2) | 0.98 | 0.94–1.02 | 0.28 |
| DHEA-S lowest tertilea | 1.45 | 0.83–2.54 | 0.20 |
Lowest tertile vs. all others.
Relationship between DHEA-S levels and longitudinal outcomes
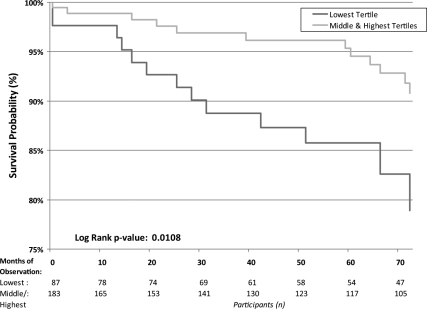
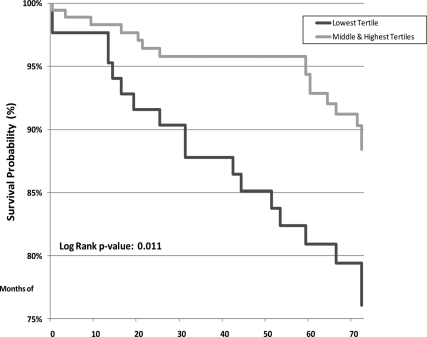
After a median of 6 yr of follow-up, 34 (12.6%) women died of all causes and 27 (10.0%) died of CVD causes. Additionally, 13 (4.8%) were hospitalized for myocardial infarction (MI), 16 (5.9%) for stroke, and 22 (8.2%) for heart failure (CHF). Over half of the deaths occurred in the lowest DHEA-S tertile (53% of deaths from all causes and 55% of CVD deaths), and there were no differences between the middle and highest tertile for either CVD death (P = 0.14) or all-cause mortality (P = 0.14). These differential risks are shown in Kaplan-Meier life-table analysis (Fig. 1). Kaplan-Meier calculated 6-yr CVD mortality rates were 17.4% in women in the lowest DHEA-S tertile vs. 8.2% in all others (unadjusted log-rank P = 0.011; age-adjusted P = 0.014). Similarly, when considering all-cause mortality (Fig. 2), women in the lowest tertile had higher Kaplan-Meier calculated mortality rates (20.6 vs. 9.7%; unadjusted P = 0.011; age-adjusted P = 0.014). There were no differences in Kaplan-Meier calculated outcomes between the groups for MI (P = 0.70; age-adjusted P = 0.45), stroke (P = 0.77; age-adjusted P = 0.76), or CHF (P = 0.96; age-adjusted P = 0.67). For composite CVD outcomes (MI, CHF, stroke, or CVD death), women in the lowest DHEA-S tertile had higher Kaplan-Meier calculated event rates (37%) than did those in the upper tertiles (27%), although this finding was not statistically significant (log-rank P = 0.07; age-adjusted P = 0.045). Inspection of the data with respect to a J-shaped curve for mortality had insufficient statistical power for evaluation.
Figure 1.
Kaplan-Meier estimated survival from CVD mortality by DHEA-S tertile category.
Figure 2.
Kaplan-Meier estimated survival from all-cause mortality by DHEA-S tertile category.
Multivariate modeling of the relationship between DHEA-S levels and CVD mortality
We further evaluated the relationship between lower DHEA-S levels and higher CVD mortality using multivariate Cox proportional hazard regression modeling. An unadjusted model showed a 2.5-fold elevated risk of CVD mortality among women in the lowest DHEA-S tertile compared with the upper tertiles [HR (95% CI) = 2.55 (1.19–5.45); P = 0.016]. Adjusting for age, ethnicity, and established CVD risk factors (Table 3, model 1) did not attenuate the relationship [HR (95% CI) = 2.43 (1.06–5.56); P = 0.036]. A race × DHEA-S interaction term was not statistically significant (P = 0.30), but this may be due to low power caused by a limited number of African-American women (Table 1). However, when adjusting the model for the severity of angiographic CAD (Table 3, model 2), the relationship between DHEA-S and CVD mortality became nonsignificant [HR (95% CI) = 1.99 (0.87–4.59); P = 0.10]. Likewise, when adjusting for the presence of obstructive CAD, the HR was attenuated to 2.06 (0.89–4.79); P = 0.09 (data not shown). A CAD × DHEA-S or CAD severity × DHEA-S interaction was not statistically significant (P = 0.54 and 0.80, respectively).
Table 3.
DHEA-S prediction of time to CVD mortality: multivariate risk factor-adjusted Cox proportional hazard models
| Predictor | Model 1
|
Model 2
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | 1.02 | 0.98–1.07 | 0.30 | 1.01 | 0.97–1.06 | 0.52 |
| Ethnicity | 0.63 | 0.25–1.62 | 0.34 | 0.64 | 0.24–1.68 | 0.37 |
| History of diabetes | 2.72 | 1.14–6.50 | 0.024 | 3.02 | 1.22–7.47 | 0.016 |
| History of hypertension | 1.20 | 0.42–3.41 | 0.73 | 0.98 | 0.33–2.88 | 0.97 |
| Ever smoked (yes/no) | 2.74 | 1.15–6.51 | 0.023 | 3.25 | 1.29–8.18 | 0.012 |
| BMI (kg/m2) | 1.02 | 0.95–1.09 | 0.61 | 1.02 | 0.95–1.09 | 0.67 |
| CAD severity score (log) | 1.60 | 0.96–2.66 | 0.07 | |||
| DHEA-S lowest tertilea | 2.43 | 1.06–5.56 | 0.036 | 1.99 | 0.87–4.59 | 0.10 |
Lowest tertile vs. all others.
The results were very similar when modeling all-cause mortality (data not shown). Low DHEA-S was an independent predictor of all-cause mortality in the adjusted risk factor model [HR (95% CI) = 2.26 (1.08–4.75); P = 0.031]. However, when adjusting for CAD presence or severity, this effect became statistically nonsignificant [HR (95% CI) = 2.02 (0.96–4.28) (P = 0.06) and 1.84 (0.86–3.93) (P = 0.12), respectively].
Is DHEA-S in the causal pathway between CAD and CVD mortality?
We further explored the potential modulating effect of CAD on the relationship between DHEA-S and CVD mortality by reversing the modeling order (data not shown). Specifically, unadjusted, both log CAD severity and DHEA-S lowest tertile were significant predictors of CVD death [HR (95% CI) = 1.88 (1.18–2.99) (P = 0.007) and 2.55 (1.19- 5.45) (P = 0.016), respectively]. When both variables were entered simultaneously, the effect of both was attenuated, but log DHEA-S became statistically nonsignificant [HR (95% CI) = 1.76 (1.10–2.82) (P = 0.018) and 2.07 (0.95–4.52) (P = 0.07), respectively].
Discussion
Among postmenopausal women undergoing coronary angiography for suspected myocardial ischemia, lower circulating DHEA-S levels were predictive of higher CVD mortality and all-cause mortality. This relationship was independent of other major CVD risk factors but became attenuated when adjusting for the presence or severity of angiographic CAD. Although lower DHEA-S levels were marginally associated with angiographic obstructive CAD, this may suggest that the association between DHEA-S and CVD mortality could be mechanistically linked to atherosclerosis.
The current results advance our understanding from prior studies that were unable to adjust for the variety of confounders in the relationship between DHEA-S levels and CVD outcomes. Specifically, the WISE study includes a wide range of demographic variables as well as established and novel CVD risk markers in addition to core laboratory measured angiographic CAD (18). Previous studies using univariate models have shown weight, age, and smoking status to be significantly associated with DHEA-S change (2). Models in our investigation were adjusted for these covariates as well as chronic conditions (e.g. hypertension and diabetes mellitus) known to influence CVD mortality and inflammatory markers commonly used in clinical practice. In multivariate proportional hazard modeling adjusted for the multiple risk modifiers noted above, we continued to observe statistically significant lower CVD-related and all-cause survival among women with lower DHEA-S levels.
DHEA-S, an endogenous hormone secreted by the adrenal gland, is the major precursor of androgens and estrogens. Blood DHEA-S levels begin to decrease after age 30 and are also reduced in anorexia, renal disease, non-insulin-dependent diabetes, AIDS, adrenal insufficiency, and critical illness (23). DHEA-S levels may also be decreased by drugs, including insulin, corticosteroids, opiates, and danazol. Our understanding of the role of DHEA-S and its physiological decline with aging, certain disease states, and medication use is incomplete, and it is likely that the relationship between DHEA-S and adverse outcomes may reflect a variety of other, as yet unaccounted for, disease states that are independent of age. If this is so, low DHEA-S would be an important marker in women signaling the need for further diagnostic workup.
Although DHEA-S may also play a direct role in the increased mortality rates found in the present as well as previous studies, the precise mechanisms linking DHEA-S to CVD mortality risk are unclear. Animal studies in primates have also demonstrated the protective role of DHEA-S against atherogenesis. DHEA-S may have an antiinflammatory and/or antioxidant role (24) or may exert action via conversion to estrogen in a paracrine mechanism. There is evidence that DHEA-S increases nitric oxide synthesis in endothelial cells independent of its conversion to estrogen (25). More recently, DHEA-S has been implicated in cell-signaling pathways that result in endothelial cell proliferation and angiogenesis (26) and protection against apoptosis (27). The specific receptor for DHEA-S involved in these pathways has yet to be identified (28). Given these possible roles for DHEA-S, its absence may promote inflammation and endothelial damage that would contribute to CVD. Although the exact mechanism of DHEA-S is unknown, this still is an area of ongoing research that underscores the complex mechanism of sex hormone metabolism in the pathophysiology of diseases.
The finding that the relationship between DHEA-S and CVD mortality became attenuated when statistically adjusting for angiographic CAD, but not when adjusting for other major predictors such as diabetes and smoking, cannot be easily explained. Given the low correlation between DHEA-S and CAD, we cannot conclude that DHEA-S is a major marker or proxy for CAD. On the other hand, the slight reduction in the strength of association of prevalent CAD with CVD mortality when adjusting for DHEA-S provides evidence that DHEA-S may be in the causal pathway between CAD and CVD death. The most likely explanation may be that obstructive CAD and DHEA-S share certain causal pathways in women, such as nitric oxide synthesis and endothelial cell damage.
While this current investigation does indicate a significant association between adverse outcomes with low DHEA-S levels, some limitations in the study design warrant comment. First, DHEA-S levels were obtained from a single blood collection and so diurnal variations in hormone level may limit the observed association. However, the circadian variation of DHEA-S has not been shown to be significant (25). Second, DHEA-S levels decline gradually after puberty and a single specimen does not allow differentiation between a low baseline level vs. a rapid rate of decline. However, previous data demonstrate DHEA-S levels to be relatively stable within individuals, over 1–5 yr of prospective observation (26,27,28,29,30). Finally, our inability to find relationships between DHEA-S levels and BMI and/or insulin levels, similar to that found in women without PCOS in cross-sectional observation (31) and in a small study of normal-weight and obese healthy controls (32) as well as in the HERITAGE Family Study (33) may be due to relatively low statistical power related to sample size. Although we did not find a relationship with inflammatory markers as suggested by other studies (34), our findings are consistent with previous studies with regard to a negative correlation between age and DHEA-S levels (22,35,36). These results may not be generalizable to the general population and cannot be interpreted to prove causality.
In conclusion, among postmenopausal women with coronary risk factors undergoing coronary angiography for suspected myocardial ischemia, lower circulating DHEA-S levels are linked with higher CVD mortality and all-cause mortality. Although it is unclear whether or not DHEA-S exerts direct physiological effects or is simply a marker of health status, further studies assessing the effects of DHEA-S alteration should be pursued to clarify its role in cardiovascular health. Our study results, combined with additional mechanistic studies, provide valuable feasibility data useful for planning future investigations.
Footnotes
This work was supported by contracts from the National Heart, Lung, and Blood Institute, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, a GCRC Grant MO1-RR00425 from the National Center for Research Resources, and grants from the Gustavus and Louis Pfeiffer Research Foundation (Denville, NJ), The Women’s Guild of Cedars-Sinai Medical Center (Los Angeles, CA), The Ladies Hospital Aid Society of Western Pennsylvania (Pittsburgh, PA), the Edythe L. Broad Endowment, Cedars-Sinai Medical Center (Los Angeles, CA), and the Barbra Streisand Women’s Cardiovascular Disease Research and Education Program, Cedars-Sinai Medical Center.
Disclosure Summary: C.N.B.M. discloses lecture honorarium, stock ownership, and consultant/advisory board membership. Other authors have nothing to disclose.
First Published Online August 25, 2010
Abbreviations: BMI, Body mass index; CAD, coronary artery disease; CI, confidence interval; CHF, congestive heart failure; CVD, cardiovascular disease; DHEA-S, dehydroepiandrosterone sulfate; HDL, high-density lipoprotein; HOMA, homeostasis model assessment; HR, hazard ratio; MI, myocardial infarction; PCOS, polycystic ovary syndrome; WISE, Women’s Ischemia Syndrome Evaluation.
References
- Labrie F, Bélanger A, Cusan L, Gomez JL, Candas B 1997 Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab 82:2396–2402 [DOI] [PubMed] [Google Scholar]
- Tannenbaum C, Barrett-Connor E, Laughlin GA, Platt RW 2004 A longitudinal study of dehydroepiandrosterone sulphate (DHEAS) change in older men and women: the Rancho Bernardo Study. Eur J Endocrinol 151:717–725 [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Khaw KT, Yen SS 1986 A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N Engl J Med 315:1519–1524 [DOI] [PubMed] [Google Scholar]
- LaCroix AZ, Yano K, Reed DM 1992 Dehydroepiandrosterone sulfate, incidence of myocardial infarction, and extent of atherosclerosis in men. Circulation 86:1529–1535 [DOI] [PubMed] [Google Scholar]
- Trivedi DP, Khaw KT 2001 Dehydroepiandrosterone sulfate and mortality in elderly men and women. J Clin Endocrinol Metab 86:4171–4177 [DOI] [PubMed] [Google Scholar]
- Golden SH, Maguire A, Ding J, Crouse JR, Cauley JA, Zacur H, Szklo M 2002 Endogenous postmenopausal hormones and carotid atherosclerosis: a case-control study of the atherosclerosis risk in communities cohort. Am J Epidemiol 155:437–445 [DOI] [PubMed] [Google Scholar]
- Johannes CB, Stellato RK, Feldman HA, Longcope C, McKinlay JB 1999 Relation of dehydroepiandrosterone and dehydroepiandrosterone sulfate with cardiovascular disease risk factors in women: longitudinal results from the Massachusetts Women’s Health Study. J Clin Epidemiol 52:95–103 [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Goodman-Gruen D 1995 Dehydroepiandrosterone sulfate does not predict cardiovascular death in postmenopausal women. The Rancho Bernardo Study. Circulation 91:1757–1760 [DOI] [PubMed] [Google Scholar]
- Thijs L, Fagard R, Forette F, Nawrot T, Staessen JA 2003 Are low dehydroepiandrosterone sulphate levels predictive for cardiovascular diseases? A review of prospective and retrospective studies. Acta Cardiol 58:403–410 [DOI] [PubMed] [Google Scholar]
- Bernini GP, Moretti A, Sgró M, Argenio GF, Barlascini CO, Cristofani R, Salvetti A 2001 Influence of endogenous androgens on carotid wall in postmenopausal women. Menopause 8:43–50 [DOI] [PubMed] [Google Scholar]
- Herrington DM 1995 Dehydroepiandrosterone and coronary atherosclerosis. Ann NY Acad Sci 774:271–280 [DOI] [PubMed] [Google Scholar]
- Herrington DM, Gordon GB, Achuff SC, Trejo JF, Weisman HF, Kwiterovich Jr PO, Pearson TA 1990 Plasma dehydroepiandrosterone and dehydroepiandrosterone sulfate in patients undergoing diagnostic coronary angiography. J Am Coll Cardiol 16:862–870 [DOI] [PubMed] [Google Scholar]
- Bernini GP, Sgro' M, Moretti A, Argenio GF, Barlascini CO, Cristofani R, Salvetti A 1999 Endogenous androgens and carotid intimal-medial thickness in women. J Clin Endocrinol Metab 84:2008–2012 [DOI] [PubMed] [Google Scholar]
- Legrain S, Berr C, Frenoy N, Gourlet V, Debuire B, Baulieu EE 1995 Dehydroepiandrosterone sulfate in a long-term care aged population. Gerontology 41:343–351 [DOI] [PubMed] [Google Scholar]
- Tilvis RS, Kahonen M, Harkonen M 1999 Dehydroepiandrosterone sulfate, diseases and mortality in a general aged population. Aging (Milano) 11:30–34 [PubMed] [Google Scholar]
- Cappola AR, Xue QL, Walston JD, Leng SX, Ferrucci L, Guralnik J, Fried LP 2006 DHEAS levels and mortality in disabled older women: the Women’s Health and Aging Study I. J Gerontol A Biol Sci Med Sci 61:957–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Merz CN, Braunstein GD, Berga SL, Bittner V, Hodgson TK, Gierach GL, Reis SE, Vido DA, Sharaf BL, Smith KM, Sopko G, Kelsey SF 2004 Determination of menopausal status in women: the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. J Womens Health (Larchmt) 13:872–887 [DOI] [PubMed] [Google Scholar]
- Merz CN, Kelsey SF, Pepine CJ, Reichek N, Reis SE, Rogers WJ, Sharaf BL, Sopko G 1999 The Women’s Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol 33:1453–1461 [DOI] [PubMed] [Google Scholar]
- Shaw LJ, Bairey Merz CN, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, Kelsey SF, Kip KE, Cooper-Dehoff RM, Johnson BD, Vaccarino V, Reis SE, Bittner V, Hodgson TK, Rogers W, Pepine CJ 2008 Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health—National Heart, Lung, and Blood Institute sponsored Women’s Ischemia Syndrome Evaluation. J Clin Endocrinol Metab 93:1276–1284 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Stanczyk FZ, Slater CC, Ramos DE, Azen C, Cherala G, Hakala C, Abraham G, Roy S 2009 Pharmacokinetics of dehydroepiandrosterone and its metabolites after long-term oral dehydroepiandrosterone treatment in postmenopausal women. Menopause 16:272–278 [DOI] [PubMed] [Google Scholar]
- Orentreich N, Brind JL, Rizer RL, Vogelman JH 1984 Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab 59:551–555 [DOI] [PubMed] [Google Scholar]
- Reyna R, Traynor KD, Hines G, Boots LR, Azziz R 2001 Repeated freezing and thawing does not generally alter assay results for several commonly studied reproductive hormones. Fertil Steril 76:823–825 [DOI] [PubMed] [Google Scholar]
- Sharaf BL, Pepine CJ, Kerensky RA, Reis SE, Reichek N, Rogers WJ, Sopko G, Kelsey SF, Holubkov R, Olson M, Miele NJ, Williams DO, Merz CN 2001 Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory). Am J Cardiol 87:937–941; A3 [DOI] [PubMed] [Google Scholar]
- Ostrowska Z, Zwirska-Korczala K, Pardela M, Drozdz M, Kos-Kudla B, Buntner B 1998 Circadian variations of androstenedione, dehydroepiandrosterone sulfate and free testosterone in obese women with menstrual disturbances. Endocr Regul 32:169–176 [PubMed] [Google Scholar]
- Azziz R, Bradley Jr E, Huth J, Boots LR, Parker Jr CR, Zacur HA 1990 Acute adrenocorticotropin-(1-24) (ACTH) adrenal stimulation in eumenorrheic women: reproducibility and effect of ACTH dose, subject weight, and sampling time. J Clin Endocrinol Metab 70:1273–1279 [DOI] [PubMed] [Google Scholar]
- Ghadir S, Azziz R 2006 Reproducibility of the adrenal androgen response to adrenocorticotropic hormone stimulation. Fertil Steril 86:484–486 [DOI] [PubMed] [Google Scholar]
- Thomas G, Frenoy N, Legrain S, Sebag-Lanoe R, Baulieu EE, Debuire B 1994 Serum dehydroepiandrosterone sulfate levels as an individual marker. J Clin Endocrinol Metab 79:1273–1276 [DOI] [PubMed] [Google Scholar]
- Yildiz BO, Woods KS, Stanczyk F, Bartolucci A, Azziz R 2004 Stability of adrenocortical steroidogenesis over time in healthy women and women with polycystic ovary syndrome. J Clin Endocrinol Metab 89:5558–5562 [DOI] [PubMed] [Google Scholar]
- Cappola AR, O'Meara ES, Guo W, Bartz TM, Fried LP, Newman AB 2009 Trajectories of dehydroepiandrosterone sulfate predict mortality in older adults: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci 64:1268–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidus L, Lindstedt G, Lundberg PA, Bengtsson C, Gredmark T 1986 Concentrations of sex-hormone binding globulin and corticosteroid binding globulin in serum in relation to cardiovascular risk factors and to 12-year incidence of cardiovascular disease and overall mortality in postmenopausal women. Clin Chem 32:146–152 [PubMed] [Google Scholar]
- Azziz R, Zacur HA, Parker Jr CR, Bradley Jr EL, Boots LR 1991 Effect of obesity on the response to acute adrenocorticotropin stimulation in eumenorrheic women. Fertil Steril 56:427–433 [PubMed] [Google Scholar]
- Ukkola O, Gagnon J, Rankinen T, Thompson PA, Hong Y, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C 2001 Age, body mass index, race and other determinants of steroid hormone variability: the HERITAGE Family Study. Eur J Endocrinol 145:1–9 [DOI] [PubMed] [Google Scholar]
- Iwasaki Y, Asai M, Yoshida M, Nigawara T, Kambayashi M, Nakashima N 2004 Dehydroepiandrosterone-sulfate inhibits nuclear factor-κB-dependent transcription in hepatocytes, possibly through antioxidant effect. J Clin Endocrinol Metab 89:3449–3454 [DOI] [PubMed] [Google Scholar]
- Nafziger AN, Bowlin SJ, Jenkins PL, Pearson TA 1998 Longitudinal changes in dehydroepiandrosterone concentrations in men and women. J Lab Clin Med 131:316–323 [DOI] [PubMed] [Google Scholar]
- Kumar A, Woods KS, Bartolucci AA, Azziz R 2005 Prevalence of adrenal androgen excess in patients with the polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf) 62:644–649 [DOI] [PubMed] [Google Scholar]