Abstract
Context: Circulating fibroblast growth factor (FGF)-23 is variably elevated in individuals with X-linked hypophosphatemia (XLH), and klotho has recently been shown to effect renal phosphate handling, yet limited data are available on circulating FGF23 and klotho in XLH.
Objective: The objective of the study was to characterize circulating FGF23 and klotho in XLH.
Design: Children and adults with XLH withheld medication for 14 d. Fasting serum FGF23, PTH, klotho, phosphate, and 1,25 dihydroxyvitamin D were obtained. Treated adults were also sampled, and circadian sampling was performed in selected individuals.
Setting: The study was conducted at a hospital research unit at an academic medical center.
Patients and Other Participants: Participants included 23 individuals with XLH and eight controls.
Interventions: There were no interventions.
Main Outcome Measures: Serum klotho and FGF23 were measured.
Results: FGF23 was greater in XLH than in controls and greater in treated XLH subjects compared with XLH subjects not receiving medical therapy. Children had higher klotho levels than adults, but values in XLH were similar to controls. A strong positive correlation between FGF23 and PTH was found in XLH subjects, whereas there was no relationship between these variables in controls. Circulating klotho, but not FGF23, has a diurnal pattern.
Conclusions: Serum klotho declines with age and demonstrates circadian variation but is normal in XLH. Serum FGF23 is similar in children and adults, is elevated in XLH, further increases with therapy, and demonstrates no diurnal variation. The direct relationship between FGF23 and PTH in subjects with XLH suggests that FGF23 regulation of PTH secretion is aberrant in this disorder.
Serum klotho levels are normal in X-linked hypophosphatemia; serum FGF23 is elevated, and greater in treated patients compared to those not receiving therapy.
The biochemical hallmarks of X-linked hypophosphatemia (XLH) are hypophosphatemia due to renal phosphate wasting and inappropriately low circulating levels of 1,25-dihydroxyvitamin D [1,25(OH)2D] (1). Affected individuals have a propensity to develop parathyroid hyperplasia (2). More recently, elevated circulating fibroblast growth factor (FGF)-23 levels have been reported in XLH (3), tumor-induced osteomalacia (4), and autosomal dominant hypophosphatemia (5). Another recently described cause of hypophosphatemia is an increase in circulating klotho due to a chromosomal break upstream of the klotho coding region, resulting in an increase in expression of the klotho gene (6). Conversely, hyperphosphatemia and increased renal phosphate retention has been reported in an individual with a loss-of-function mutation in klotho (7). Klotho, a type II membrane protein, is critical for FGF23 signaling by forming a ternary ligand binding complex with FGF receptors (8,9).
Using newly developed assays to measure circulating klotho and intact FGF23, we sought to characterize circulating FGF23 and klotho in children and adults with XLH and in healthy control subjects. We examined the relationships of these factors to serum levels of PTH and phosphorus (P), looked for diurnal variation in their levels, and assessed changes in response to treatment.
Subjects and Methods
Subjects and study design
Twenty-three subjects (16 adults and seven children/adolescents; five males and 18 females) with a clinical diagnosis of XLH were recruited during clinic visits to the Yale Bone Center or via referring physicians. Twenty had a family history consistent with XLH, and three did not. Children ranged from 9 to 15 yr of age and adults from 36 to 70 yr of age. Eight unaffected control subjects (four adults, 43–60 yr of age, and four children/adolescents, 9–20 yr of age; five males and three females) were recruited locally. Exclusion criteria included concomitant renal failure (serum creatinine <1.5 mg/dl), low serum 25-hydroxyvitamin D (<20 ng/ml), or treatment with medications potentially affecting skeletal metabolism (such as glucocorticoids and anticonvulsants). Mean serum creatinine in XLH subjects (0.7 ± 0.18 mg/dl; 62 ± 15.9 μmol/liter) was no different from that in unaffected control subjects (0.8 ± 0.12 mg/dl; 71 ± 10.6 μmol/liter) in unaffected control subjects; other details of renal calcium handling and demographics are provided in Supplemental Table 1, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org. Specifically, there were no differences between groups with respect to creatinine clearance, urinary calcium to creatinine ratios, or calcium clearance to creatine clearance ratios. Subjects with XLH discontinued therapy 14 d before the study. Serum P, creatinine, PTH, 1,25(OH)2D, FGF23, and klotho were measured in a fasting morning blood sample, obtained at the midpoint of a 2-h urine collection, which was used to measure urine P and creatinine; these data were used to calculate the tubular maximum for phosphate reabsorption expressed per unit glomerular filtration rate (TMP/GFR) (10). To determine whether there was a diurnal pattern to FGF23 levels, we additionally sampled other subjects to obtain a total of 44 affected subjects and eight unaffected controls. This group was sampled every 4 h for a 26-h period. Twenty-six-hour profiles of serum klotho were performed in eight individuals (five XLH and three control subjects). To assess effects of concurrent therapy, another group of four adults with XLH were similarly sampled while receiving standard therapy (calcitriol and phosphate). This study was approved by the Yale University Human Investigation Committee and written informed consent was obtained.
Data collection and analytical methods
Serum and urinary P and creatinine were measured using autoanalyzer technology in the Core Laboratory of the Yale Center for Clinical Investigation. Serum 1,25(OH)2D was measured using an RIA kit from DiaSorin, Inc. (Stillwater, MN). Serum PTH was measured using a midregion assay as described (4). Serum FGF23 was measured using the Kainos intact ELISA kit and soluble klotho was assayed using an ELISA kit (both kindly provided by Kyowa Hakko Kirin Co., Ltd., Tokyo, Japan). Data were plotted as observed means and se of the observed means of the respective groups. Comparisons were performed between adults and children, between XLH and control subjects, between treated and untreated XLH subjects, and between XLH subjects with normal circulating PTH levels and those with elevated circulating PTH levels with an analysis of covariance model using SAS software (SAS Institute, Cary, NC). Correlations between biochemical variables were examined using Pearson correlations.
Results
Effect of age group and disease status
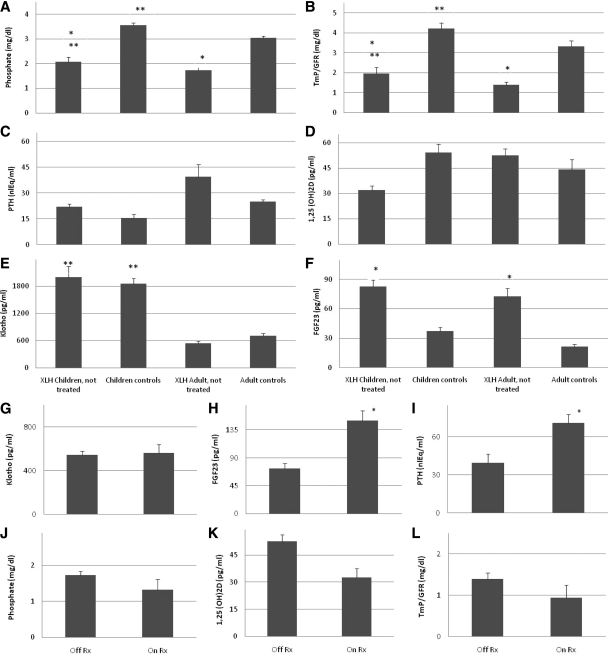
Serum P and TMP/GFR differed as expected by age group (Fig. 1, A and B); younger subjects had greater serum P concentrations and higher TMP/GFR values than adult subjects (P < 0.05). As also expected, these parameters differed by disease status (P < 0.0001). Neither PTH nor 1,25(OH)2D levels differed by age or disease status (Fig. 1, C and D). Mean circulating levels of FGF23 were significantly higher in subjects with XLH compared with controls (P < 0.0001) (Fig. 1F), but no effect of age was observed (P = 0.23). In contrast, children had greater circulating levels of klotho than adults (P < 0.0001) (Fig. 1E), but there was no effect of disease status (P = 0.80).
Figure 1.
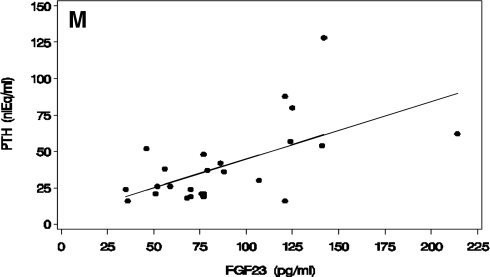
A–F, Fasting morning values (mean and sem) for serum P (A), TMP/GFR (B), serum PTH (C), serum 1,25(OH)2D (D), serum klotho (E), and serum FGF23 (F) in children with XLH (first column of each panel), unaffected control children (second column), adults (third column), and unaffected adult controls (fourth column). Affected subjects were untreated at the time of sample collection. Mean and sem are shown. A single asterisk above a column representing values for XLH individuals indicates a significant difference from controls of the same age group (significance levels reported in text). A double asterisk above a column representing children indicates a significant difference from adults of the same disease/control group. G–L, Serum klotho (G), FGF23 (H), PTH (I), P (J), 1,25(OH)2D (K), and TMP/GFR (L) in adult subjects off therapy (left column) compared with on therapy (right column). Mean and sem are shown. A single asterisk above a column representing values for treated XLH individuals indicates a significant difference from untreated XLH individuals. M, The relationship between serum PTH and FGF23 values in XLH subjects are shown. There is a positive correlation observed in subjects with XLH (PTH = 5.3 + 0.40 × FGF23). To convert serum phosphate concentration and TMP/GFR from mass units (milligrams per deciliter) to standard international units (millimoles per liter), divide by 3.1. To convert 1,25(OH)2D units from mass units (picograms per milliliter) to standard international units (picomoles per liter), multiply by 2.4. Normal ranges include: serum P (A and J) in 9–20 yr olds: 3.1–6.2 mg/dl, and in adults, 2.7–4.4 mg/dl; TMP/GFR (B and L) in 9–20 yr olds: 2.2–5.3 mg/dl, and in adults, 2.1–4.8 mg/dl; PTH (C, I, and M), 10–25 nanoliter-equivalents/ml (nlEq/ml); 1,25(OH)2D (D and K), 25–66 pg/ml; FGF23 less than 50 pg/ml (F, H, and M); klotho (E and G) does not have an established reference range apart from the data presented in the control subject data above.
Effect of treatment on circulating FGF23 and klotho levels
Treated and untreated adult XLH subjects had similar values of circulating klotho (Fig. 1G, P = 0.65), whereas circulating FGF23 levels were substantially greater in the treated group (Fig. 1H, P < 0.001). We wondered whether this difference correlated with differences in circulating PTH levels, which were also greater in the treated group (Fig 1I, P = 0.034). To identify whether treatment status or circulating levels of PTH correlated more closely with FGF23, we examined the effect of therapy on FGF23 levels in subjects of similar PTH status. In XLH subjects with elevated PTH (>25 nanoliter-equivalents/ml), mean serum FGF23 was greater in treated compared with untreated subjects (159 ± 16.9 vs. 89 ± 10.3 pg/ml, respectively; P < 0.001). We also examined the relationship of circulating PTH to FGF23 in all untreated subjects. Within this group, mean FGF23 was not significantly different in hyperparathyroid and euparathyroid subjects (89 ± 10.3 vs. 68 ± 9.6 pg/ml, respectively, P = 0.1). Thus, FGF23 levels appear to be affected by treatment independent of PTH status, but a contribution of PTH cannot be completely excluded. We observed slightly lower fasting values for serum P, 1,25(OH)2D, and TMP/GFR in treated subjects compared with untreated subjects (Fig. 1, J–L), although these differences did not achieve statistical significance [P = 0.14, 0.07, and 0.17, respectively, for P, 1,25(OH)2D, and TMP/GFR].
Correlation analysis
Correlation analysis was performed on data from the 23 XLH subjects and eight control individuals to identify potential determinants of circulating FGF23 and klotho. In subjects with XLH, there is a strong direct correlation between FGF23 and PTH (Pearson coefficient 0.602, P = 0.002) (Fig. 1M) but no significant correlation between these variables in control subjects. PTH was not significantly correlated with serum P or TMP/GFR in either XLH or control subjects.
Circadian patterns of circulating FGF23 and klotho
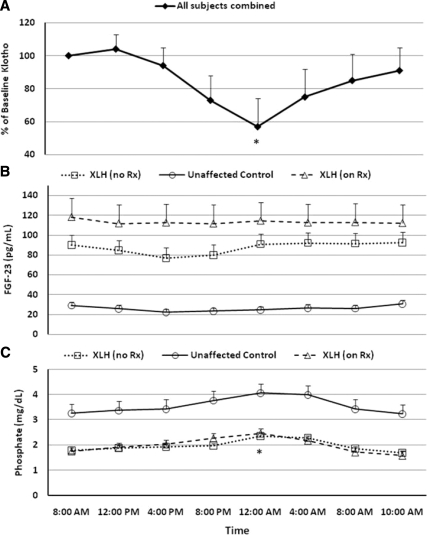
Despite the differences observed in circulating klotho levels between children and adults, both groups demonstrated a similar diurnal change in circulating levels, with a nadir at midnight. Levels then increased through the early morning to return to or near baseline values (Fig. 2A). There was no significant diurnal variation in circulating FGF23 levels (Fig. 2B). A moderate increase in serum P occurred through the evening hours with a peak at midnight, and a subsequent return to baseline through the early morning hours (Fig. 2C). PTH levels were significantly greater in untreated XLH subjects than in unaffected controls (P < 0.0001, Supplemental Fig. 1).
Figure 2.
Circadian pattern of circulating klotho (A), FGF23 (B), and P (C). The observed mean and sem are plotted at each time point. For klotho, values from all subjects are presented as the percent of the 0800 h value on d 1. For FGF23 and P, data from XLH subjects off therapy are shown in the dotted lines with open squares, data from XLH subjects receiving standard therapy are shown in dashed lines with open triangles, and data from unaffected control subjects are shown in solid lines with open circles. At midnight, there is an approximately 40% reduction in circulating klotho with a gradual return to near baseline values in the early morning hours. *, Value significantly different from baseline (P < 0.05). This correlates with the midnight increase in serum P observed in treated and untreated patients with XLH. (Midnight values were less than baseline for the treated and untreated XLH subjects, but the difference did not achieve statistical significance for unaffected controls). There is no significant diurnal change in serum FGF23 levels, but values are higher in patients with XLH receiving treatment than in untreated patients (P = 0.0004). Irrespective of treatment, FGF23 levels are much higher in patients with XLH than in normal individuals. To convert serum phosphate concentration from mass units (milligrams per deciliter) to standard international units (millimoles per liter), divide by 3.1. Normal ranges are: serum FGF23 less than 50 pg/ml (B); serum P (C): 2.7–6.2 mg/dl; serum klotho (A) does not have an established reference range, apart from data presented in the control subjects here and in Fig. 1.
Discussion
The discovery of FGF23 and its pathogenic role in XLH, tumor-induced osteomalacia, and autosomal-dominant hypophosphatemic rickets has provided important new insights into the hormonal control of phosphate homeostasis. The recent identification of klotho as an essential cofactor for FGF23 actions has added a novel dimension to this unique regulatory system. The development of assays capable of measuring circulating concentrations of FGF23 and soluble klotho has provided the opportunity to assess the relationship of blood levels of these factors to other measures of mineral homeostasis and the PTH-1α-hydroxylase axis in XLH.
We confirmed the expected findings of low serum P levels and a reduced renal tubular phosphate threshold (TMP/GFR) in subjects with XLH. Also as expected, both serum P and TMP/GFR were lower in adults with XLH than in children with the disease. Circulating levels of FGF23 were greater in subjects with XLH, compared with controls, but there was no effect of age on these levels in either group. In contrast, circulating klotho did not differ between control subjects and those with XLH, although younger subjects (9–20 yr of age) demonstrated greater circulating klotho than older subjects.
A major clinical problem that often complicates the course of XLH is the propensity of patients to develop secondary hyperparathyroidism, usually in association with therapy. Interestingly, it has been reported that FGF23 inhibits PTH secretion in vivo in rodents and in parathyroid cell primary cultures (11). We therefore explored the relationships between PTH, klotho, and FGF23. In subjects with XLH, there was a significant positive correlation between PTH and FGF23. This finding is similar to that described in chronic kidney disease, in which levels of both PTH and FGF23 increase with declining renal function (12,13). However, our XLH population had normal renal function (estimated GFR). No such relationship was evident in healthy control subjects. These data suggest that the propensity of XLH individuals to develop secondary and even tertiary hyperparathyroidism may be due to a resistance to FGF23 at the level of the parathyroid cell, limiting FGF23’s ability to inhibit PTH secretion.
We identified a diurnal variation in circulating klotho but not FGF23. Because FGF23 is thought to serve as a chronic regulator of phosphorus homeostasis, the absence of diurnal variation is not surprising. Blood levels of klotho decrease to their circadian nadir at midnight and gradually return to baseline levels thereafter. The physiological significance of this pattern is not known; however, it is of interest to note that the diurnal variance of serum phosphate occurs in the converse manner, reaching a peak at midnight, raising the possibility that the overnight reduction in circulating klotho may play a role in mediating the midnight peak observed in serum P by reducing FGF23-mediated signaling.
Finally, we confirm the previously reported effect of treatment with calcitriol/phosphate on serum FGF23 levels in XLH subjects (14). Our treatment population was enriched for hyperparathyroid individuals, and the question arises as to whether PTH itself may contribute to this effect. Our analysis of treated and untreated hyperparathyroid subjects suggests that treatment is the dominant effect but does not exclude the possibility that PTH may also be playing a role. Treatment was associated with modest reductions in P, TMP/GFR and 1,25(OH)2D levels in the fasting state, suggesting that the increased FGF23 levels seen with treatment may result in counterproductive effects on mineral homeostasis.
In summary, we report an age-dependent decrease in circulating klotho and that levels are normal in XLH. In contrast, FGF23 levels are elevated in XLH, and treatment further increases these levels, suggesting that this may limit the effectiveness of conventional therapy. In addition, FGF23 and PTH are positively correlated in XLH, raising the possibility that an aberrant parathyroid cell response to FGF23 or cellular resistance to the hormone may be part of the pathogenesis of secondary hyperparathyroidism in this disease. There is no diurnal pattern of circulating FGF23 levels, but soluble klotho levels reach a nadir at midnight, which corresponds to a rise in serum P.
Acknowledgments
We are most grateful to Dr. Hisashi Hasegawa (Kyowa Hakko Kirin Co., Ltd.) for providing the immunoassay materials for serum klotho and FGF23 and to the staff of the Hospital Research Unit facilities of the Yale Center for Clinical Investigation. We are also deeply indebted to the subjects and their families who participated in the study.
Footnotes
This work was supported by National Institutes of Health (NIH) Center for Research Translation Award P50-AR054086 to the Yale Center for X-Linked Hypophosphatemia and was made possible by the Yale Center for Clinical Investigation (supported by CTSA UL1 RR024139 from the National Center for Research Resources of the NIH).
Disclosure Summary: T.O.C. and K.L.I. perform consulting duties for Kyowa Hakko Kirin. J.H.Z., B.E., S.N., C.S., E.O., and C.M.G. have nothing to declare.
First Published Online August 4, 2010
Abbreviations: FGF, Fibroblast growth factor; GFR, glomerular filtration rate; 1,25(OH)2D, 1,25-dihydroxyvitamin D; P, phosphorus; TMP, tubular maximum for phosphate reabsorption; XLH, X-linked hypophosphatemia.
References
- Carpenter TO, Drezner M 2007 Primary disorders of phosphate metabolism. In: Arnold A, section ed. (www.endotext.org, version of September 10, 2007). South Dartmouth, MA: mdtext.com, inc. [Google Scholar]
- Carpenter TO, Mitnick MA, Ellison A, Smith C, Insogna KL 1994 Nocturnal hyperparathyroidism: a frequent feature of X-linked hypophosphatemia. J Clin Endocrinol Metab 78:1378–1383 [DOI] [PubMed] [Google Scholar]
- Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Jüppner H 2003 Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 348:1656–1663 [DOI] [PubMed] [Google Scholar]
- Carpenter TO 2003 Oncogenic osteomalacia—a complex dance of factors. N Engl J Med 348:1705–1708 [DOI] [PubMed] [Google Scholar]
- White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ 2001 Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int 60:2079–2086 [DOI] [PubMed] [Google Scholar]
- Brownstein CA, Adler F, Nelson-Williams C, Iijima J, Li P, Imura A, Nabeshima Y, Reyes-Mugica M, Carpenter TO, Lifton RP 2008 A translocation causing increased α-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci USA 105:3455–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, Goetz R, Mohammadi M, White KE, Econs MJ 2007 A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest 117:2684–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque MS 2009 The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol 5:611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T 2006 Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444:770–774 [Google Scholar]
- Ardeshirpour L, Cole DE, Carpenter TO 2007 Evaluation of bone and mineral disorders. Pediatr Endocrinol Rev 5(Suppl 1):584–598 [PubMed] [Google Scholar]
- Galitzer H, Ben-Dov I, Lavi-Moshayoff V, Naveh-Many T, Silver J 2008 Fibroblast growth factor 23 acts on the parathyroid to decrease parathyroid hormone secretion. Curr Opin Nephrol Hypertens 17:363–367 [DOI] [PubMed] [Google Scholar]
- Galitzer H, Ben-Dov IZ, Silver J, Naveh-Many T 2010 Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int 77:211–218 [DOI] [PubMed] [Google Scholar]
- Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M 2005 Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16:2205–2215 [DOI] [PubMed] [Google Scholar]
- Imel EA, DiMeglio LA, Hui SL, Carpenter TO, Econs MJ 2010 Treatment of XLH with calcitriol and phosphate increases circulating FGF23 concentrations. J Clin Endocrinol Metab 95:1846–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]