Abstract
Context: It has recently been proposed that the increase in bone resorption after the menopause may not be due principally to estrogen deficiency but rather to the concomitant increase in circulating FSH levels.
Objective: The objective of the study was to test whether suppression of FSH secretion in postmenopausal women reduces levels of bone resorption markers.
Design: This was a prospective study.
Setting: The study was conducted at a clinical research unit.
Participants and Interventions: Postmenopausal women were treated with a GnRH agonist (leuprolide acetate, 7.5 mg im every 28 d; n = 21) or placebo injections (control; n = 20). Both groups received the aromatase inhibitor, letrozole, 2.5 mg/d, to eliminate variations in endogenous estrogen levels as a confounder.
Main Outcome Measures: Serum FSH and bone resorption markers [serum C-terminal telopeptide of type I collagen (CTX) and tartrate-resistant acid phosphatase 5b (TRAP5b)] at d 105 (3.5 months) of treatment as compared with baseline.
Results: Compared with baseline, serum FSH levels did not change significantly in controls (+6%) but were reduced (−86%, into the premenopausal range) in the GnRH group. Due to the aromatase inhibitor-induced reduction in estrogen production, serum CTX and TRAP5b levels increased significantly in controls (+20 and +10%, respectively). In the GnRH group, suppression of FSH secretion did not reduce serum CTX or TRAP5b levels; rather, both markers also increased in these women (+34 and +15%, respectively; P = 0.161 and 0.266 for comparison of percent changes between groups).
Conclusions: This direct interventional study demonstrates that FSH does not regulate bone resorption in postmenopausal women.
Suppression of follicle-stimulating hormone secretion in postmenopausal women has no effect on bone resorption markers.
Whereas bone loss after the menopause has generally been attributed to effects of estrogen deficiency (1), there are data indicating that other factors that change during the menopausal transition may contribute to increased bone turnover and bone loss. The observation that early menopausal bone loss begins even when serum estradiol (E2) levels are normal led to the hypothesis a number of years ago by Prior (2) that luteal-phase defects and reductions in progesterone levels during perimenopause contribute to bone loss during this period. In addition to progesterone, androgen levels also decrease during the menopausal transition (3) and could contribute to bone loss. Recently Perrien et al. (4) demonstrated marked reductions in serum inhibins (A and B) during the menopause in women and found that the decreases in inhibin levels were associated with increases in bone turnover markers.
Of all the candidate factors (other than estrogen) for mediating bone loss during the menopause, perhaps the one that has received the greatest attention has been FSH. Ebeling et al. (5) initially demonstrated that, in perimenopausal women, increases in bone resorption markers were best correlated with serum FSH rather than E2 levels. Subsequent data from the Study of Women’s Health Across the Nation (SWAN) showed that spine and hip bone mineral density (BMD) losses during the menopause transition were most strongly related to the interaction between initial FSH levels and longitudinal FSH changes and not to E2 or androgen levels (6). These findings raised the possibility that FSH may have direct effects on bone; however, as noted by the authors of the SWAN study (6), FSH could also be a better predictor of BMD changes during the perimenopause than E2 because it may serve as a more robust proxy measure of ovarian dynamics involving E2 than single E2 measurements.
The issue of the possible direct effects of FSH on bone has generated considerable controversy over the past several years after the publication of two apparently contradictory mouse studies (7,8). Thus, Sun et al. (7) studied FSH receptor null (FORKO) mice and found that despite being hypogonadal, these mice had normal bone mass. These investigators also found that osteoclasts and their precursors possessed FSH receptors and that FSH (but not LH) increased osteoclast formation and function in vitro. Based on these findings, the authors of the study concluded that high circulating FSH levels caused hypogonadal bone loss (7). By contrast, Gao et al. (8) subsequently found that the FORKO mice did have reduced bone mass; moreover, bilateral ovariectomy reduced the elevated circulating testosterone (T) levels in the FORKO mice and decreased bone mass to levels indistinguishable from those in ovariectomized controls. These investigators came to the opposite conclusions from those of Sun et al. (7), namely that sex steroids regulated bone turnover in the FORKO mice independently of any bone-resorptive action of FSH.
Given the correlative human data and the conflicting mouse data for a role for FSH in regulating bone resorption, we sought to test the hypothesis, in a direct interventional human study, that suppression of FSH secretion in postmenopausal women using a GnRH agonist reduced levels of bone resorption markers. Because, as noted above, the peri- and early menopausal years are associated with changes in not only FSH but also in progesterone, androgen, and inhibin levels, we attempted to isolate the possible effects of FSH on bone by studying women who were clearly past the menopausal transition, in whom levels of these other hormones would be stable and low (2,4). Furthermore, we eliminated variations in endogenous estrogen levels as a confounder by using an aromatase blocker in both our control and experimental groups. As such, we attempted, as much as is possible in a human clinical-investigative study, to isolate changes in FSH as the only significant variable that differed between our control and experimental groups, thereby rigorously testing whether FSH had any biological effects in regulating bone resorption in humans.
Subjects and Methods
Study subjects and experimental protocol
Forty-six postmenopausal women were randomized into a double-blind, placebo-controlled study to receive either a GnRH agonist (leuprolide acetate, Lupron Depot; TAP Pharmaceuticals, Deerfield, IL; 7.5 mg im every 28 d) or placebo injections (control group). Both groups were concomitantly treated with the aromatase inhibitor (AI), letrozole (Femara; Novartis, East Hanover, NJ; 2.5 mg/d). Five women (three in the placebo group and two in the GnRH group) dropped out of the study at various times after randomization due to side effects (four due principally to musculoskeletal symptoms from the AI and one due to a forearm fracture after a fall during the course of the study); thus, we report data on 21 subjects in the GnRH group and 20 subjects in the control group who completed all study visits.
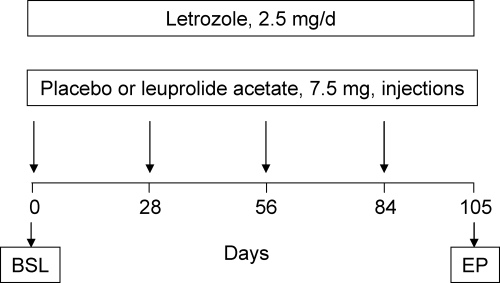
Figure 1 provides a schematic overview of the study protocol. After fasting (0800 h) baseline blood draws on 2 consecutive days, all subjects were placed on letrozole and received either the GnRH agonist or placebo injections, which were then repeated every 28 d (total of four injections). The last injections were on d 84, and 21 d later (to ensure that we concluded the study before the 28 d duration of the GnRH dose), subjects had blood drawn again on 2 consecutive days. Interim blood draws were obtained at d 28, 56, and 84 for measurement of FSH levels only, to define the time course of changes in FSH. Baseline and 28, 56, 84, and 105 d samples were analyzed for FSH levels. For the bone turnover markers, we used the average of the two values obtained from the consecutive blood draws at baseline and 105 d (end point). For serum LH, E2, estrone (E1), and T, we used a single baseline and end point blood sample.
Figure 1.
Overview of the study design. Please see text for details. BSL, Baseline; EP, end point.
We defined menopause as no menses for 1 yr or more (or documented ovariectomy) and a serum FSH level above 30 IU/liter. All of the study subjects were at least 5 yr postmenopausal, in good health, with no clinically significant abnormalities in any of the screening laboratory serum studies (calcium, phosphorus, alkaline phosphatase, creatinine, TSH, and hemoglobin). Subjects were not included if they had been maintained on corticosteroids, anticonvulsant therapy, pharmacological thyroid hormone therapy, bisphosphonates, calcitonin, or estrogen therapy within the past 12 months. Subjects with a clinical history of an osteoporotic fracture (vertebral, hip, or distal forearm) were also excluded. A screening 25-hydroxyvitamin D (25-OHD) level was also obtained, and if this was less than 25 ng/ml, the subjects were treated with 1000 IU/d of vitamin D3 for 8 wk. A 25-OHD level was then rechecked, and the subject enrolled only if this level was 25 ng/ml or greater. Three subjects (two in the GnRH and one in the control group) required treatment with vitamin D3 before enrollment in the study. All subjects provided written informed consent, and the study was approved by the Mayo Clinic Institutional Review Board.
Serum assays
Serum calcium and phosphorus were measured by an automated photometric assay (interassay coefficient of variation (CV) <10%; Roche Diagnostics, Indianapolis, IN). Serum creatinine was measured using an automated enzymatic colorimetric assay (interassay CV <10%; Roche Diagnostics). Serum 25-OHD was measured using liquid chromatography-tandem mass spectroscopy (interassay CV <7%; API 5000; Applied Biosystems-MDS Sciex, Foster City, CA). Serum C-terminal telopeptide of type I collagen (CTX) and serum osteocalcin were measured by automated immunometric assays on the Roche Cobas e411 (interassay CVs <10%; Roche), and serum tartrate-resistant acid phosphatase isoform type 5b (TRAP5b) was measured by ELISA (interassay CV <10%; Immunodiagnostic Systems, Fountain Hills, AZ). Serum amino-terminal propeptide of type I collagen (PINP) was measured by RIA (interassay CV <10%; Immunodiagnostic Systems). Serum FSH and LH were measured using automated immunoenzymatic assays on the Beckman Coulter Unicel DXI 800 (interassay CV <10%; Beckman Coulter, Fullerton, CA). Serum E2, E1, and T were measured using liquid chromatography-tandem mass spectroscopy (API 5000; Applied Biosystems-MDS Sciex), as previously described (9). Values as low as 1.25 pg/ml for E2, 2.5 pg/ml for E1, and 1 ng/dl for T were detectable by this method, with interassay CVs of 7.5, 12.8, and 6.3% for E2, E1, and T, respectively.
Statistical analyses
All data are reported as the mean ± sem. Within-group pairwise comparisons between the baseline and end point values were done using paired t tests. Changes in each variable (end point minus baseline) and percent changes (change/baseline) were compared between the control and GnRH groups using two-sample t tests. We also analyzed changes in the bone turnover markers using nonparametric statistics [signed rank test (for pairwise comparisons) or rank sum test (for nonpaired comparisons)]. Because this analysis provided very similar results to those using t tests, all results are presented using parametric statistics. P < 0.05 was considered significant.
Results
Table 1 shows the baseline anthropometric and biochemical variables in the study subjects. As is evident, the subjects in the two groups were well matched for all of the variables, except for serum creatinine, which was slightly (but significantly) higher in the women randomized to GnRH treatment, although still well within the normal range.
Table 1.
Baseline anthropometric and biochemical variables in the study subjects
| Control | GnRH | P value | |
|---|---|---|---|
| n | 20 | 21 | |
| Age (yr) | 66.1 ± 1.3 | 67.4 ± 1.2 | 0.482 |
| Height (m) | 1.61 ± 0.01 | 1.65 ± 0.02 | 0.070 |
| Weight (kg) | 73.3 ± 2.4 | 77.2 ± 3.6 | 0.385 |
| Body mass index (kg/m2) | 28.4 ± 1.0 | 28.5 ± 1.4 | 0.913 |
| Serum parameters | |||
| Calcium (mg/dl) | 9.7 ± 0.1 | 9.6 ± 0.1 | 0.397 |
| Phosphorus (mg/dl) | 3.7 ± 0.1 | 3.6 ± 0.1 | 0.688 |
| Creatinine (mg/dl) | 0.76 ± 0.02 | 0.85 ± 0.03 | 0.007 |
| 25-OHD (ng/ml) | 35.0 ± 1.5 | 36.2 ± 1.5 | 0.577 |
Data are mean ± sem.
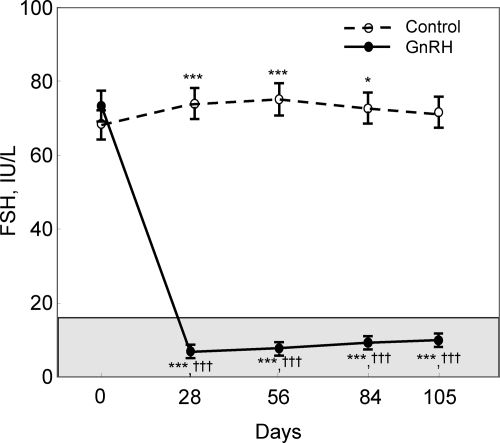
Figure 2 shows the changes in serum FSH levels over time in the two groups. Serum FSH levels increased transiently in the control group, likely due to the AI-induced suppression of estrogen levels (see below). However, at the final study point, serum FSH levels were not statistically different from baseline. By contrast, serum FSH levels decreased markedly in the GnRH group (by 91% at d 28 and 86% at d 105), into the premenopausal range for this assay. As expected, serum LH levels also decreased markedly in the GnRH group but remained unchanged in the control group (Table 2). Both groups had near complete suppression of endogenous E2 and E1 levels (below the detection limit of the E2 and E1 assays in all subjects). Due to the suppression of LH secretion, serum T levels decreased (by 21%) from the already low T levels present in these postmenopausal women in the GnRH group but remained unchanged in the control group (Table 2).
Figure 2.
Changes in serum FSH over time in the control and GnRH groups. The shaded region represents the premenopausal reference range. *, P < 0.05, ***, P < 0.001 vs. d 0; †††, P < 0.001 for comparison with the control group at the specific time point.
Table 2.
Baseline and end point LH and sex steroid values in the study subjects
| Control
|
GnRH
|
P value | |||
|---|---|---|---|---|---|
| Baseline | End point | Baseline | End point | ||
| LH (IU/liter) | 33.1 ± 4.3 | 31.1 ± 4.5 | 28.3 ± 2.4 | 0.3 ± 0.03a | <0.001 |
| E2 (pg/ml) | 4.9 ± 0.8 | N.D. | 6.0 ± 0.7 | N.D. | |
| E1 (pg/ml) | 24.6 ± 3.3 | N.D. | 28.1 ± 2.2 | N.D. | |
| T (ng/dl) | 19.2 ± 2.3 | 20.1 ± 2.3 | 19.7 ± 1.7 | 14.0 ± 1.2b | 0.002 |
Data are mean ± sem and the P value is for comparison of the change in the control vs. GnRH groups. N.D., Not detected (below the detection limit of the assays).
P < 0.001 vs. baseline.
P < 0.01 vs. baseline.
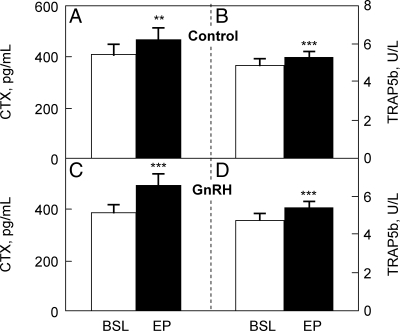
Serum CTX (Fig. 3A) and TRAP5b (Fig. 3B) increased significantly in the control group, again likely due to the AI-induced reduction in estrogen levels. Suppression of FSH secretion in the GnRH group women did not reduce either CTX or TRAP5b levels; instead, both markers also increased significantly in these women (Fig. 3, C and D). In fact, the absolute increase in serum CTX in the GnRH group (+117 ± 26 pg/ml) was somewhat greater (P = 0.063) than in the control group (+57 ± 18 pg/ml); absolute increases in serum TRAP5b did not differ between groups (GnRH, +0.66 ± 0.15 U/liter; control, +0.46 ± 0.11 U/liter; P = 0.424 for comparison between groups). The percent changes in serum CTX and TRAP5b in the control group (+20 ± 7 and +10 ± 3%, respectively) were not significantly different from the percent changes in these markers in the GnRH group (+34 ± 7 and +15 ± 3%, respectively; P = 0.161 and 0.266, respectively, for comparison of percent changes between groups).
Figure 3.
Serum CTX (A) and serum TRAP5b (B) in the control women; serum CTX (C) and serum TRAP5b (D) in the GnRH-treated women. BSL, Baseline; EP, end point. **, P < 0.01, ***, P < 0.001 for comparison with baseline.
Table 3 shows the changes in the bone formation markers in the two groups. Neither serum osteocalcin nor serum PINP changed significantly in the control group. Serum osteocalcin did not change, but serum PINP did increase significantly in the GnRH group, and changes in PINP levels were significantly different between groups (Table 3). Taken together, these findings demonstrate that rather than leading to a reduction in bone resorption (and bone turnover), the suppression of FSH secretion associated with GnRH treatment tended to have the opposite effect, namely a borderline greater increase in serum CTX and a significantly greater increase in serum PINP levels compared with the control group.
Table 3.
Baseline and end point values for bone formation markers in the study subjects
| Control
|
GnRH
|
P value | |||
|---|---|---|---|---|---|
| Baseline | End point | Baseline | End point | ||
| Osteocalcin (ng/ml) | 23.3 ± 2.1 | 23.3 ± 1.8 | 23.0 ± 1.5 | 24.2 ± 1.9 | 0.238 |
| PINP (μg/liter) | 61.9 ± 6.9 | 63.5 ± 5.9 | 56.6 ± 4.2 | 64.8 ± 4.8a | 0.036 |
Data are mean ± sem, and the P value is for comparison of the change in the control vs. GnRH groups.
P < 0.01 vs. baseline.
Discussion
The major finding of our study was that GnRH agonist-induced suppression of serum FSH levels in postmenopausal women into the premenopausal range did not reduce markers of bone resorption. In fact, not only did the bone resorption markers fail to decrease in the GnRH group, but rather serum CTX tended to increase to a greater extent in the GnRH compared with the control group over the course of the study. Our study was also of sufficient duration to allow ample time for changes in bone resorption markers to become evident. These findings thus demonstrate that FSH does not regulate bone resorption in humans. As such, the use of pharmacological antagonists of FSH secretion and/or action is unlikely to be a viable approach for limiting postmenopausal bone loss.
As noted earlier, we studied women who were clearly past the menopausal transition (rather than peri- or early postmenopausal women) because we wanted to minimize confounding by changes in other hormonal variables known to occur during and early after menopause. Thus, although we did not measure progesterone or inhibin (A or B) levels in our study subjects, both hormones have previously been shown to be low and stable in women of the age range in our study (2,4). We accounted for possible variations between groups in estrogen levels by placing the women in both groups on an AI; indeed, as has previously been demonstrated for effects of AIs on bone resorption markers in postmenopausal women (10), the further suppression of the already low estrogen levels in these women likely accounts for the modest increases in bone resorption markers we observed in both groups. This finding also helps place the lack of FSH effects on bone resorption in further biological context, in that the suppression of FSH secretion was not even able to offset the observed increase in bone resorption markers after elimination of the already very low estrogen levels in these postmenopausal women.
Whereas bone resorption markers increased significantly after treatment with the AI in both the control and GnRH groups, changes in bone formation markers were less consistent. Thus, serum osteocalcin levels did not change significantly in either group, and serum PINP levels increased significantly in the GnRH, but not control, groups. These findings may reflect the fact that suppression of residual estrogen levels in postmenopausal women leads initially to an increase in bone resorption with a later increase in bone formation due to the coupling of bone resorption and bone formation (11). Thus, at the 3.5-month time point used in this study, bone resorption markers clearly increased, whereas bone formation markers may have just started to increase, leading to some variability between groups.
We recognize that, in our experimental model, we could not control for the observed differences in LH levels between groups due to the effects of the GnRH agonist in suppressing not only FSH but also LH production. LH itself does not appear to have direct skeletal effects (7); however, due to the suppression of LH production, the women in the GnRH group did have slight (21%) reductions in circulating T levels compared with the control women. This modest reduction in T production, even in the absence of aromatization to estrogens, could potentially be responsible for the trend toward greater increases in bone turnover markers (specifically serum CTX and PINP) we observed in the GnRH compared with the control women. We would reiterate, however, that if FSH had clinically meaningful effects on bone resorption, we should have observed a decrease (or at the least a smaller increase) in bone resorption markers in the GnRH compared with the control women, the opposite of the trend for changes in serum CTX we observed.
Based on the previously demonstrated associations of FSH levels with bone turnover markers (5) as well as with rates of bone loss (6) during the perimenopausal years, it could be argued that despite our negative findings, FSH may be having effects on bone resorption during this early menopausal period that are no longer evident in women who are past the menopausal transition, as was the case for our study subjects. We believe this possibility is unlikely and would be unprecedented based on what is known regarding other hormonal regulators of bone metabolism. For example, sustained increases in PTH secretion, as in primary hyperparathyroidism, lead to increases in bone resorption and reductions in BMD, particularly at cortical sites, in both pre- and postmenopausal women (12,13,14,15). Conversely, estrogen reduces bone resorption in early as well as late postmenopausal women (1). Thus, the notion that FSH is a major driver of bone resorption during the early menopause and yet has no effects whatsoever on bone resorption later in menopause seems relatively implausible.
Based on our findings, we would agree with one of the original postulates of the SWAN investigators, namely that FSH could be a better predictor of BMD changes during the perimenopause than E2 because it may serve as a more robust proxy measure of changes in ovarian function than single E2 measurements (6). In addition, changes in other hormones in the perimenopausal years, such as progesterone (2) or inhibins (4), could contribute to bone loss during this period, even in the setting of relatively normal estrogen levels (albeit generally only measured at a single time point). Indeed, as noted earlier, Perrien et al. (4) previously found that decreases in inhibin levels across the menopause correlated better with markers of bone turnover than did increases in FSH levels. Because inhibins are the major determinants of FSH secretion (16), our findings would suggest that the observed associations between FSH levels and bone turnover or bone loss during the menopause may largely be explained by the further association of FSH with inhibin or sex steroid levels. Clearly, further studies are needed to address this possibility.
Our studies in postmenopausal women are also consistent with the recent data of Ritter et al. (17), who found that either daily injections or continuous infusion of FSH in male mice for 1 month had no effect on femoral BMD by peripheral quantitative computed tomography or bone structural parameters by microcomputed tomography. When combined with the data of Gao et al. (8) demonstrating reduced bone mass in FORKO mice, these studies in mice and now our direct interventional study in humans provide fairly compelling evidence that FSH does not directly regulate bone resorption. Further studies are needed, however, to evaluate the extent to which other hormonal factors besides estrogen modulate bone loss in women during and after the menopause.
Acknowledgments
We thank James Peterson for help with preparation of the figures and tables and Dr. B. Lawrence Riggs for helpful advice and suggestions.
Footnotes
This work was supported by National Institutes of Health Grants AG004875 and UL1-RR24150 (Center for Translational Science Activities), U.S. Public Health Service.
Disclosure Summary: None of the authors have a conflict to disclose.
First Published Online July 7, 2010
For editorial see page 4864
Abbreviations: AI, Aromatase inhibitor; BMD, bone mineral density; CTX, C-terminal telopeptide of type I collagen; CV, coefficient of variation; E1, estrone; E2, estradiol; FORKO, FSH receptor null; 25-OHD, 25-hydroxyvitamin D; PINP, amino-terminal propeptide of type I collagen; SWAN, Study of Women’s Health Across the Nation; T, testosterone; TRAP5b, tartrate-resistant acid phosphatase isoform type 5b.
References
- Riggs BL, Khosla S, Melton 3rd LJ 2002 Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302 [DOI] [PubMed] [Google Scholar]
- Prior JC 1990 Progesterone as a bone-trophic hormone. Endocr Rev 11:386–398 [DOI] [PubMed] [Google Scholar]
- Wierman ME, Basson R, Davis SR, Khosla S, Miller KK, Rosner W, Santoro N 2006 Androgen therapy in women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 91:3697–3710 [DOI] [PubMed] [Google Scholar]
- Perrien DS, Achenbach SJ, Bledsoe SE, Walser B, Suva LJ, Khosla DG, Gaddy D 2006 Bone turnover across the menopause transition: correlations with inhibins and follicle-stimulating hormone. J Clin Endocrinol Metab 91:1848–1854 [DOI] [PubMed] [Google Scholar]
- Ebeling PR, Atley LM, Guthrie JR, Burger HG, Dennerstein L, Hopper JL, Wark JD 1996 Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab 81:3366–3371 [DOI] [PubMed] [Google Scholar]
- Sowers MR, Jannausch M, McConnell D, Little R, Greendale GA, Finkelstein JS, Neer RM, Johnston J, Ettinger B 2006 Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab 91:1261–1267 [DOI] [PubMed] [Google Scholar]
- Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun JJ, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M 2006 FSH directly regulates bone mass. Cell 125:247–260 [DOI] [PubMed] [Google Scholar]
- Gao J, Tiwari-Pandey R, Samadfam R, Yang Y, Miao D, Karaplis AC, Sairam MR, Goltzman D 2007 Altered ovarian function affects skeletal homeostasis independent of the action of follicle-stimulating hormone (FSH). Endocrinology 148:2613–2621 [DOI] [PubMed] [Google Scholar]
- Khosla S, Amin S, Singh RJ, Atkinson EJ, Melton 3rd LJ, Riggs BL 2008 Comparison of sex steroid measurements in men by immunoassay versus mass spectroscopy and relationships with cortical and trabecular volumetric bone mineral density. Osteoporos Int 19:1465–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heshmati HM, Khosla S, Robins SP, O'Fallon WM, Melton 3rd LJ, Riggs BL 2002 Role of low levels of endogenous estrogen in regulation of bone resorption in late postmenopausal women. J Bone Miner Res 17:172–178 [DOI] [PubMed] [Google Scholar]
- Khosla S 2008 Estrogen and bone: insights from estrogen-resistant, aromatase-deficient, and normal men. Bone 43:414–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappard C, Roux C, Laugier P, Paillard M, Houillier P 2006 Bone status in primary hyperparathyroidism assessed by regional bone mineral density from the whole body scan and QUS imaging at calcaneus. Joint Bone Spine 73:86–94 [DOI] [PubMed] [Google Scholar]
- Guo CY, Thomas WE, al-Dehaimi AW, Assiri AM, Eastell R 1996 Longitudinal changes in bone mineral density and bone turnover in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab 81:3487–3491 [DOI] [PubMed] [Google Scholar]
- Silverberg SJ, Shane E, de la Cruz L, Dempster DW, Feldman F, Seldin D, Jacobs TP, Siris ES, Cafferty M, Parisien MV 1989 Skeletal disease in primary hyperparathyroidism. J Bone Miner Res 4:283–291 [DOI] [PubMed] [Google Scholar]
- Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP 1999 A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med 341:1249–1255 [DOI] [PubMed] [Google Scholar]
- Vale W, Bilezikjian LM, Rivier C 1994 Reproductive and other roles of inhibins and activins. In: Knobil E, Neil JD, eds. The physiology of reproduction. New York: Raven Press; 1861–1878 [Google Scholar]
- Ritter V, Thuering B, Saint Mezard P, Luong-Nguyen NH, Seltenmeyer Y, Junker U, Fournier B, Susa M, Morvan F 2008 Follicle-stimulating hormone does not impact male bone mass in vivo or human male osteoclasts in vitro. Calcif Tissue Int 82:383–391 [DOI] [PubMed] [Google Scholar]