Abstract
Approximately 2,500 adults (ages 18–97) completed multiple study-test trials of a list of unrelated words. Consistent with past research, females outperformed males in the recall task. To assess whether sex differences in recall performance were attributable to differences in acquiring and/or retaining information, the data were analyzed at the individual item level to distinguish gains (i.e., items not recalled on Trial n that were recalled on Trial n+1) and losses (i.e., items recalled on Trial n that were not recalled on Trial n+1). Being a male, increased age, lower verbal episodic memory ability, and lower vocabulary ability were associated with smaller gains and greater losses. Even when controlling for the influence of other individual difference variables, being a male was still associated with fewer gains across the majority of trials. These results suggest that one factor contributing to sex differences in recall performance are differences in acquiring new items rather than differences in retaining information across trials.
Keywords: Sex Differences, Verbal Learning, Memory
Introduction
Many researchers have demonstrated that females tend to outperform males on verbal memory tasks, while males tend to show superior performance to females on visuospatial tasks (e.g., de Frias, Nilsson, & Herlitz, 2006; Herlitz, Lovén; Thilers, & Rehnman, 2010; Herlitz, Nilsson, & Bäckman, 1997; Herlitz & Rehnman, 2008; Lewin, Wolgers & Herlitz, 2001). However, the female advantage on verbal materials is dependent on the type of memory assessment. Specifically, females tend to recall more items on verbal episodic memory tests, but sex differences are not evident on other forms of memory such as priming and semantic memory (Herlitz et al., 1997). Multitrial verbal learning tasks, in which participants receive multiple study-test trials of the same items, are a common method for assessing sex differences in verbal episodic memory, and females recall more items than males in these types of tasks (e.g., Bleecker, Bolla-Wilson, Agnew, & Meyers, 1988; Bolla-Wilson & Bleecker, 1986; Geffen, Moar, O’Hanlon, Clark, & Geffen, 1990; Kramer, Delis, & Daniel, 1988; Van Der Elst, Van Boxtel, Van Breukelen, & Jolles, 2005). While previous research on sex differences in multitrial verbal learning has focused on the mean level of recall performance, the rationale for the current project was that it may be informative to examine changes at the level of individual items to determine whether sex differences in overall performance are primarily associated with differences in acquiring new items, or with differences in retaining previously recalled information. For example, two individuals could show the same overall mean increase in performance across successive trials, but in one case it is because there are large gains but also some losses, whereas in another case it is because there are modest gains but no failures to remember previously recalled items. Decomposition of performance into gains (i.e., items not recalled on Trial n that were recalled on Trial n+1) and losses (i.e., items recalled on Trial n that were not recalled on Trial n+1) therefore has the potential to provide a more precise depiction of the underlying mechanisms of the sex differences in multitrial verbal learning while providing a characterization of changes in performance across successive trials.
Several studies have investigated age-related effects on multitrial verbal learning with this type of decomposition of multitrial memory performance. For example, Davis et al. (2003) and Dunlosky and Salthouse (1996) found that increased age was associated with fewer gains and greater losses across successive trials, and a similar pattern of differences has been reported in comparisons of healthy adults with Alzheimer’s patients (e.g., Moulin, James, Freeman, & Jones, 2004; Woodard, Dunlosky, & Salthouse,1999). These studies therefore indicate that poorer recall performance associated with normal aging and with some types of pathological aging is associated with deficits in acquiring new information as well as declines in maintaining previously recalled information. To our knowledge, however, no studies have decomposed sex differences in multitrial verbal learning performance into gains and losses to assess the nature of the female advantage in verbal memory tasks.
Because females often show superior memory performance on verbal episodic memory tests (e.g., Herlitz & Rehnman, 2008), we were also interested in determining the extent to which any observed sex differences in gains and losses might be partially mediated through sex differences in a more general verbal episodic memory ability. All of the research participants performed the Wechsler Memory Scale III (WMS-III; Wechsler, 1997b) Logical Memory test and a paired-associates test (Salthouse, Fristoe, & Rhee, 1996) to provide measures of verbal episodic memory. Four vocabulary tests were also administered and combined to use as an additional predictor of performance because lower vocabulary scores have been associated with poorer verbal recall performance (Bolla-Wilson & Bleecker, 1986).
Method
Participants
Table 1 contains the demographics of the participants classified by gender and divided into three age groups. The data set included 1,630 females and 867 males ranging from 18 to 97 years of age from nine studies. Participants were recruited from the community through newspaper advertisements, flyers and referrals from other participants, and had to have completed at least a high school level of education to be eligible to participate. Increased age was associated with greater amount of education (r = .17, p < .01), and males (coded as 0 with females coded as 1) had significantly more education than females (r = −.08, p < .01). A 2 (Sex: Male vs. Female) × 3 (Age Group: Young, Middle, Older) ANOVA produced similar results, with main effects of sex, F (1, 2482)1= 13.60, and age group, F (2, 2482) = 30.06, p’s <.01. LSD post hoc tests revealed significant differences between all three age groups. However the Age × Sex interaction on years of education was not significant, F (2, 2482) = 2.19. Increased age was associated with somewhat lower ratings of subjective health on a scale from 1 (excellent”) to 5 (poor), r = .16, p < .01, but there were no sex differences in the health ratings, r = −.04, p >.05. This same pattern of results was obtained when conducting a 2 (Sex: Male vs. Female) × 3 (Age Group: Young, Middle, Older) ANOVA on health scores, with the only significant finding being a main effect of age group, F (2, 2491) = 18.82, p <.01. The age-adjusted scaled scores from the WMS-III Logical Memory test (1997b) and the WAIS-III vocabulary test (1997a) are also included in the table to indicate the representativeness of the sample. Because the scaled scores have a mean of 10, and a standard deviation of 3, the sample can be inferred to consist of high functioning participants, and this was evident both in the entire sample and in each of the three different age groups.
Table 1.
Number of participants, years education, and health scores by sex and age.
| All | 18–39 years | 40–59 years | 60–97 years | |||||
|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | Males | Females | |
| N | 867 | 1630 | 267 | 465 | 265 | 683 | 335 | 482 |
| Educ | 16.0 (2.7) | 15.6 (2.6) | 15.3 (2.4) | 15.1 (2.4) | 16.0 (2.8) | 15.7 (2.5) | 16.6 (2.8) | 15.9 (2.9) |
| Health | 2.0 (.89) | 1.9 (.87) | 1.8 (.85) | 1.8 (.77) | 2.0 (.86) | 1.9 (.89) | 2.0 (.92) | 2.1 (.91) |
| Vocab | 13.2 (2.9) | 12.8 (3.0) | 14.0 (2.9) | 12.6 (3.3) | 12.5 (2.8) | 12.4 (2.9) | 13.2 (2.8) | 13.5 (2.7) |
| LM | 11.7 (2.9) | 12.2 (2.7) | 11.6 (2.8) | 11.9 (2.6) | 11.5 (2.9) | 11.9 (2.7) | 12.0 (2.9) | 12.7 (2.8) |
Note: Numbers represent the mean and parentheses contain the standard deviation; Educ = years education; Vocab = WAIS-III Vocabulary scaled scores; LM = Logical Memory (WMS-III) scaled scores
Procedure
The participants completed the Word List Recall test of the WMS-III (Wechsler, 1997b) along with other cognitive ability tests in a two hour session. Vocabulary ability was assessed with the WAIS Vocabulary (Wechsler, 1997a), Picture Vocabulary (Woodcock & Johnson, 1990), Antonym Vocabulary (Salthouse, 1993), and Synonym Vocabulary (Salthouse, 1993) tests. Verbal episodic memory ability was assessed with a story recall task (WMS-III Logical Memory, Wechsler, 1997b) and a paired associates task involving unrelated words (Salthouse et al., 1996). Z-scores were computed for the Paired-Associates and Logical Memory tests, and these scores were averaged to form a composite verbal episodic memory ability variable. In addition, Z-scores were computed for the four vocabulary tasks and were averaged to form a composite vocabulary ability variable.
The Word List Recall task consisted of two lists, List 1 and List B, each containing twelve words read at a rate of approximately 1 second per word. The words in the list were presented in the same order across trials. Immediately after hearing each list the participants recalled as many words as they could remember in any order. Following the fourth recall trial of List 1, the experimenter read the distracter list, List B, which the participants immediately attempted to recall. After the participants recalled words from List B, they were asked to recall as many words from List 1 that they could remember. Unlike Trials 1 through 4 of List 1, the experimenter did not read the words before participants attempted to recall the words on Trial 5.
Results
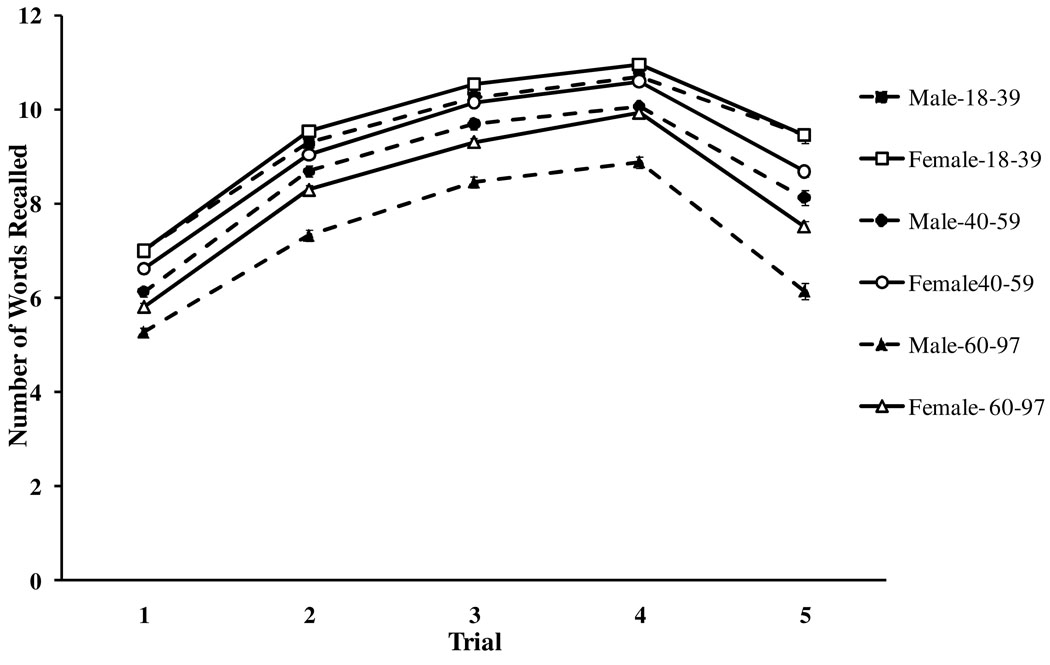
Figure 1 displays the mean number of words recalled across trials by sex and age, arbitrarily divided into three age groups. Consistent with previous multitrial verbal learning studies the average number of words recalled increased across trials (Trials 1–4), and it decreased following the intervening distracter list (Trial 5). Furthermore, 3 (Age Group: Young, Middle, Older) × 2 (Sex: Male vs. Female) ANOVAs on mean recall performance revealed that increased age and being a male was associated with fewer items recalled across all trials, F’s > 19.87, p’s <.01. It can be seen that the functions were nearly parallel, although the age × sex interactions were significant, in the direction of larger female advantage at older ages.
Figure 1.
Number of words recalled across trials by sex and age, arbitrarily divided into three groups, with standard error bars.
To investigate correlates of recall performance, we first computed simple correlations between recall performance and the individual difference variables of sex, age, verbal episodic memory ability based on the paired associates and story recall composite score, and vocabulary ability. The simple correlations represent the relationship between one of the individual difference variables and recall performance while ignoring the influence of the other individual difference variables, and these values are shown in the top left half of Table 2. As expected, females (coded as 1) outperformed males (coded as 0) on every recall trial, as indicated by the positive sex-mean recall correlations on each trial. In addition, increased age, lower verbal episodic memory ability, and lower vocabulary ability were associated with fewer words recalled.
Table 2.
Simple and semi-partial correlations between the target variables (i.e., mean recall, gains, and losses) and sex, age, verbal episodic memory ability, and vocabulary ability.
| Number or | Simple | Semi-Partial | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Proportion of Items | Sex | Age | EM | Voc | Sex | Age | EM | Voc | |
|
Mean Number of Words Recalled(N = 2,497) |
|||||||||
| Trial 1 | 6.35 (1.89) | .11* | −.34* | .54* | .19* | .04 | −.16* | .34* | .06* |
| Trial 2 | 8.75 (2.16) | .14* | −.33* | .51* | .17* | .07* | −.17* | .32* | .06* |
| Trial 3 | 9.80 (2.04) | .15* | −.33* | .49* | .13* | .09* | −.17* | .30* | .04 |
| Trial 4 | 10.27 (1.93) | .17* | −.33* | .48* | .14* | .13* | −.19* | .28* | .06* |
| Trial B | 6.14 (2.12) | .12* | −.29* | .50* | .21* | .07* | −.14* | .31* | .09* |
| Trial 5 | 8.29 (2.80) | .13* | −.40* | .53* | .14* | .07* | −.22* | .32* | .05* |
| Proportion of Gains(N = 2,497) | |||||||||
| Trial 1–2 | .63 (.25) | .13* | −.31* | .45* | .09* | .06* | −.13* | .31* | −.01 |
| Trial 2–3 | .68 (.30) | .15* | −.29* | .37* | .06* | .09* | −.16* | .23* | .01 |
| Trial 3–4 | .68 (.34) | .16* | −.23* | .27* | .04 | .12* | −.14* | .16* | .01 |
| Trial 4–5 | .33 (.39) | .07* | −.11* | .22* | .06* | .03 | −.05 | .15* | .01 |
| Proportion of Losses(N= 2,497) | |||||||||
| Trial 1–2 | .14 (.17) | −.05 | .13* | −.22* | −.15* | −.04 | .09* | −.10* | −.11* |
| Trial 2–3 | .12 (.14) | −.06* | .21* | −.31* | −.08* | −.03 | .10* | −.19* | −0.2 |
| Trial 3–4 | .09 (.12) | −.09* | .22* | −.37* | −.15* | −.06* | .10* | −.22* | −.07* |
| Trial 4–5 | .26 (.21) | −.10* | .38* | −.47* | −.10* | −.03 | .21* | −.28* | −0.3 |
| Number of Gains (N = 923) | |||||||||
| Trial 1–2 | 3.28 (1.89) | .09* | −.06 | .05 | −.06 | .07 | −.03 | .05 | −.06 |
| Trial 2–3 | 1.93 (1.31) | −.01 | .03 | −.07 | −.08* | −.02 | .01 | −.04 | −.07 |
| Trial 3–4 | 1.29 (1.23) | .15* | −.12* | .11* | .00 | .12* | −.10* | .05 | .02 |
| Trial 4–5 | .45 (.71) | .00 | .05 | −.04 | .02 | .00 | .03 | −.03 | .02 |
| Number of Losses (N = 923) | |||||||||
| Trial 1–2 | .86 (1.00) | −.02 | −.07 | .07 | −.04 | −.05 | −.04 | .07 | −.05 |
| Trial 2–3 | .89 (.96) | .08 | −.08* | .10* | .02 | .05 | −.05 | .07 | .01 |
| Trial 3–4 | .83 (.97) | −.03 | .04 | −.12* | −.09* | −.03 | .02 | −.08 | −.05 |
| Trial 4–5 | 2.43 (1.89) | −.02 | .19* | −.17* | .01 | .02 | .11* | −.11* | .01 |
p < .01
Note: EM = Verbal Episodic Memory, Voc = Vocabulary; Parentheses contain the standard deviation. Females were coded as 1, males as 0. Simple = bivariate correlations; Semi-partial = standardized beta coefficients.
N = 2,497 represents the entire sample. A sub-sample of the participants who recalled 9 items or fewer on Trial 3 (N = 923) were included in the absolute number analyses of gains and losses.
Because there were significant correlations among sex, age, vocabulary, and verbal episodic memory, we were interested in examining the influences of sex and age after controlling for vocabulary and verbal episodic memory ability. The right half of Table 2 contains the semi-partial correlations representing the unique influence of the individual difference variable (e.g., sex) on the target variable (e.g., Mean Recall Trial 1) after controlling for the influence of the other individual difference variables (e.g., age, verbal episodic memory ability, and vocabulary ability). Sex-related effects remained on the majority of the trials after controlling for the influence of age, verbal episodic memory ability, and vocabulary ability.
Of greatest interest were the gains and losses across successive trials computed for individual items. Gains were measured as the proportion of items not recalled on Trial n that were recalled on Trial n+1. For example, an individual who recalled 4 out of 12 items on Trial 1 would have 8 items that could be acquired on Trial 2. If 2 of the 8 items were recalled on Trial 2, the individual would have a gain proportion of 0.25 (i.e., 2/8). Losses were calculated as the proportion of items recalled on Trial n that were not recalled on Trial n+1. For example, if a person failed to recall 2 of the 4 previously recalled items, the loss proportion would be 0.5 (i.e., 2/4). Proportional analyses were selected as our primary measure of gains and losses instead of absolute analyses (i.e., number of items gained or lost) because absolute analyses were subject to misleading ceiling effects in the gain measure. Specifically, since the word list was relatively short, individuals who recalled many items on the early trials (e.g., Trial 1 and 2) had few items that could be gained on later trials (e.g., Trials 3–5).
The left half of Table 2 displays the simple correlations among gains and losses and the individual difference variables. Being a male was associated with a smaller proportion of intertrial gains and greater losses across trials, with the exception of losses between Trial 1 and Trial 2. The proportional analyses also revealed that increased age, lower verbal episodic memory ability, and lower vocabulary ability were associated with smaller gains and greater losses across trials.
Similar to the mean recall analyses, simultaneous regression analyses were conducted to examine the unique associations between the individual difference variables and gains and losses. Statistically independent relations of sex were observed for gains, but the majority of the sex differences on losses were no longer significant after controlling for verbal episodic memory ability, vocabulary ability, and age. Interestingly, even when verbal episodic memory ability was controlled, there were still unique age-related effects on both gains and losses.
In order to examine the robustness of the results, the analyses on the gains and loss variables were repeated using absolute numbers instead of proportions. To minimize artifacts attributable to potential ceiling effects in the gains measure, absolute analyses were restricted to participants who recalled nine items or fewer on the third recall trial (N = 923). The results of these analyses are reported in the bottom of Table 2. Notice that although the magnitudes of some of the relations were smaller with the absolute measures, the qualitative pattern was similar to that with the proportion measures and there was still a significant female advantage in the absolute number of gains from Trial 3 to 4 and no significant sex differences in the absolute number of losses across any trials. These results are therefore consistent with the conclusion that sex differences appear to be largely manifested through differences in gaining new items across trials.
A final analysis was conducted in order to assess whether sex differences are more pronounced among individuals at different levels of performance. The procedure consisted of computing Z-scores for the sum of Trials 1–4, partialling the influence of age from the z-scores, and then determining the values of these age-partialled residuals at successive percentiles separately for males and females. Figure 2 portrays the relation between performance at each percentile for females as a function of performance for the corresponding percentile for males. The interesting information in this type of plot are the parameters of the regression equation, as the intercept indicates the baseline difference between the two groups, and a slope different from 1.0 indicates that the group differences vary as a function of the level of performance. As displayed in the figure, the intercept was .27, indicating an overall advantage for females. However, the slope of .90 was significantly less than 1, which means that the female advantage was smaller among the highest performing individuals. That is, application of the regression equation leads to a predicted advantage (i.e., deviation above the positive diagonal) of .41 for females among individuals performing at the 10th percentile of their respective distributions, but to a predicted advantage of .18 for individuals performing at the 90th percentile of their respective distributions. Thus, as depicted in the figure, at higher percentiles there was less deviation from the diagonal, which indicates that sex differences are smaller among the highest performing individuals. Follow up correlational analyses indicated that individuals performing at higher percentiles were more educated, r = .16, and had higher self-reported health, r = −.10, p’s < .01.
Figure 2.
Percentile plot for recall residuals by sex.
Discussion
The results reported above are consistent with previous research documenting that males recall fewer words than females in verbal memory tests (e.g., Bleecker et al., 1988; Bolla-Wilson & Bleecker, 1986; Geffen et al., 1990). Sex differences were still apparent on most of the recall trials after controlling for the influence of age and vocabulary ability. A novel finding in the current study was that unique sex differences were apparent even when controlling for a broad episodic verbal memory ability based on the average Z-scores of paired associates and story recall tests. Overall, these results suggest that sex differences in verbal recall performance are not simply attributable to differences in broad verbal episodic memory or level of vocabulary abilities. Furthermore, another novel aspect was that the observed sex differences in recall performance were smaller among the highest performing individuals. This finding is intriguing because it implies that whatever is responsible for the sex difference may interact with the level of ability of the individual.
The primary goal of the current study was to decompose the sex differences in multitrial recall into gains and losses, which to our knowledge has not previously been done. Our results replicated those of prior studies which have found adult age differences in both gains and losses (Davis et al., 2003; Dunlosky & Salthouse, 1996). With the exception of losses from Trial 1 to Trial 2, males were found to have smaller across-trial gains and larger across-trial losses than females.
Higher vocabulary ability has been associated with better recall performance (Bolla-Wilson & Bleecker, 1986), and our results are consistent with this finding. The decomposition of the word recall performance indicated that higher vocabulary was primarily associated with an advantage in retention of information, such that higher-vocabulary individuals lost fewer items across trials.
Since sex differences in verbal learning, particularly in across-trial gains, were independent of other individual difference variables, the question arises as to what is responsible for these differences? One possibility is that sex differences in recall performance are due to differences in organization of the to-be-remembered information. For example, females have been reported to be more likely to use semantic clustering in multitrial verbal learning tests with categorized word lists (Kramer et al., 1988). However, when controlling for verbal intelligence these same researchers did not find sex differences in clustering by item position, which is the most plausible type of organization with the repeated lists of unrelated words used in the current study.
Another potential explanation for the sex differences may involve differences in item-specific processing because intertrial gains have been attributed to this type of processing (See Mulligan & Lozito, 2004, for a review). It is therefore possible that females, to a greater extent than males, may attend to more attributes of specific items (e.g., rhymes or semantic associates of the words), which may facilitate across trials gains. Further, in tests of verbal learning females tend to show more cerebral blood flow in the left temporal pole, an associative cortex region postulated to be involved in sensory integration and knowledge representations (Ragland, Coleman, Gur, Glahn, & Gur, 2000). Other neuroimaging evidence also indicates sex differences in regional activation, with increased relative glucose metabolic rate (rGMR) in BA 12 being associated with better verbal memory performance in females in a multitrial learning task, while males showed an increase in BA 24 (Hazlett et al., 2010). Nyberg, Habib, and Herlitz (2000) have noted sex differences near BA 44/45 in verbal episodic memory tests. It is therefore possible that differences in activation across the frontal lobe in varying BA regions may help to potentially explain the sex differences in multitrial verbal learning tasks.
In addition to sex differences in regional activation, researchers have also noted differences in brain structure (e.g., Chen, Sachdev, Wen, Anstey, 2007; Leonard et al., 2008; Sullivan, Rosenbloom, Desmond, & Pfefferbaum, 2001), and there appears to be a link between brain matter volume and multitrial verbal learning performance. Specifically, Gur et al. (1999) demonstrated that females had a higher percentage of gray matter volume while males had a higher percentage of white matter volume, but interestingly a comparison of the slope of the regression line on white matter volume indicated that increased white matter in females was associated with better verbal learning performance. The authors suggested that more somatodendritic tissue in the gray matter allowed for greater connectivity among neurons, with females more efficiently using their available white matter, possibly contributing to better verbal task performance. Likewise, Luders et al. (2004) have also demonstrated anatomical sex differences in brain structure, with females displaying more complexity in frontal and parietal regions of the brain compared to males. In light of the studies indicating sex differences in regional activation within the frontal lobe (e.g., Hazlett et al., 2010; Nyberg et al., 2000), the finding that sex variations in complexity within the frontal regions of the brain may warrant further investigation of whether these differences are correlated with verbal episodic memory performance.
Overall, while the underlying mechanisms for why sex differences in multitrial verbal learning are unclear, the results from the current study suggest that the sex differences in recall performance are primarily manifested in the efficiency of gaining new items across trials rather than differences in retaining information. Furthermore, the results suggest that the sex differences are smaller among the highest performing individuals.
It is important to note that males may encode information to the same extent as females but fail to retrieve the information at the time of test, or males may encode fewer items when studying. Although it is not possible to determine whether the sex differences are primarily attributable to encoding or to retrieval differences in this study, our results suggest that sex differences in multitrial learning studies are not due to males failing to retain information across trials but rather are manifested in the gain of fewer new items. The discovery that sex differences are larger among lower performing individuals is also intriguing and worth pursuing in future research.
Acknowledgements
This research was supported by NIA Grants RO1AG019627 and R37AG024270 to TAS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note the degrees of freedom do not equal 2491 because not all participants reported their years of education.
Contributor Information
Lacy E. Krueger, Email: Lacy_Krueger@tamu-commerce.edu.
Timothy A. Salthouse, Email: salthouse@virginia.edu.
References
- Bleecker ML, Bolla-Wilson K, Agnew J, Meyers DA. Age-related sex differences in verbal memory. Journal of Clinical Psychology. 1988;44:403–411. doi: 10.1002/1097-4679(198805)44:3<403::aid-jclp2270440315>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Bolla-Wilson K, Bleecker ML. Influence of verbal intelligence, sex, age, and education on the Rey Auditory Verbal Learning Test. Developmental Neuropsychology. 1986;2:203–211. [Google Scholar]
- Chen X, Sachdev PS, Wen W, Anstey KJ. Sex differences in regional gray matter in healthy individuals aged 44–48 years: A voxel-based morphometric study. NeuroImage. 2007;36:691–699. doi: 10.1016/j.neuroimage.2007.03.063. [DOI] [PubMed] [Google Scholar]
- Davis HP, Small SA, Stern Y, Mayeux R, Feldstein SN, Keller FR. Acquisition, recall, and forgetting of verbal information in long-term memory by young, middle-aged, and elderly individuals. Cortex. 2003;39:1063–1091. doi: 10.1016/s0010-9452(08)70878-5. [DOI] [PubMed] [Google Scholar]
- de Frias C, Nilsson L-G, Herlitz A. Sex differences in cognition are stable over a 10-year period in adulthood and old age. Aging, Neuropsychology, and Cognition. 2006;13:574–587. doi: 10.1080/13825580600678418. [DOI] [PubMed] [Google Scholar]
- Dunlosky J, Salthouse TA. A decomposition of age-related differences in multitrial free recall. Aging, Neuropsychology, & Cognition. 1996;3:2–14. [Google Scholar]
- Geffen G, Moar KJ, O’Hanlon AP, Clark CR, Geffen LB. Performance measures of 16- to 86- year-old males and females on the Auditory Verbal Learning Test. The Clinical Neuropsychologist. 1990;4:45–63. doi: 10.1080/13854049008401496. [DOI] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, et al. Sex differences in brain gray and white matter in healthy young adults: Correlations with cognitive performance. The Journal of Neuroscience. 1999;19:4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett EA, Byne W, Brickman AM, Mitsis EM, Newmark R, Haznedar MM, et al. Effects of sex and normal aging on regional brain activation during verbal memory performance. Neurobiology of Aging. 2010;31:826–838. doi: 10.1016/j.neurobiolaging.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitz A, Lovén J, Thilers P, Rehnman J. Sex differences in episodic memory: The where but not the why. In: Bäckman L, Nyberg L, editors. Memory, aging and the brain: A Festschrift in honour of Lars-Göran Nilsson. New York, NY, US: Psychology Press; 2010. pp. 132–143. [Google Scholar]
- Herlitz A, Nilsson L-G, Bäckman L. Gender differences in episodic memory. Memory & Cognition. 1997;25:801–811. doi: 10.3758/bf03211324. [DOI] [PubMed] [Google Scholar]
- Herlitz A, Rehnman J. Sex differences in episodic memory. Current Directions in Psychological Science. 2008;17:52–56. [Google Scholar]
- Kramer JH, Delis DC, Daniel M. Sex differences in verbal learning. Journal of Clinical Psychology. 1988;44:907–915. [Google Scholar]
- Leonard CM, Towler S, Welcome S, Halderman LK, Otto R, Eckert MA, et al. Size matters: Cerebral volume influences sex differences in neuroanatomy. Cerebral Cortex. 2008;18:2920–2931. doi: 10.1093/cercor/bhn052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin C, Wolgers G, Herlitz A. Sex differences favoring women in verbal but not in visuospatial episodic memory. Neurospsychology. 2001;15:165–173. doi: 10.1037//0894-4105.15.2.165. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Jancke L, Steinmetz H, et al. Gender differences in cortical complexity. Nature Neuroscience. 2004;7:799–800. doi: 10.1038/nn1277. [DOI] [PubMed] [Google Scholar]
- Moulin CJA, James N, Freeman JE, Jones RW. Deficient acquisition and consolidation: Intertrial free recall performance in Alzheimer's disease and mild cognitive impairment. Journal of Clinical and Experimental Neuropsychology. 2004;26:1–10. doi: 10.1076/jcen.26.1.1.23940. [DOI] [PubMed] [Google Scholar]
- Mulligan NW, Lozito JP. Self-generation and memory. The Psychology of Learning and Motivation. 2004;45:175–214. [Google Scholar]
- Nyberg L, Habib R, Herlitz A. Brain activation during episodic memory retrieval: Sex differences. Acta Psychologica. 2000;105:181–194. doi: 10.1016/s0001-6918(00)00060-3. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Coleman AR, Gur RC, Glahn DC, Gur RE. Sex differences in brain-behavior relationships between verbal episodic memory and resting regional cerebral blood flow. Neuropsychologia. 2000;38:451–461. doi: 10.1016/s0028-3932(99)00086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Speed and knowledge as determinants of adult age differences in verbal tasks. Journal of Gerontology: Psychological Sciences. 1993;48:29–36. doi: 10.1093/geronj/48.1.p29. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Fristoe N, Rhee SH. How localized are age-related effects on neuropsychological measure? Neuropsychology. 1996;10:272–285. [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Desmond JE, Pfefferbaum A. Sex differences in corpus callosum size: Relationship to age and intracranial size. Neurobiology of Aging. 2001;22:603–611. doi: 10.1016/s0197-4580(01)00232-9. [DOI] [PubMed] [Google Scholar]
- Van Der Elst W, Van Boxtel MPJ, Van Breukelen GJP, Jolles J. Rey’s verbal learning test: Normative data for 1855 healthy participants aged 24–81 years and the influence of age, sex, education, and mode of presentation. Journal of the International Neuropsychological Society. 2005;11:290–302. doi: 10.1017/S1355617705050344. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale – Third Edition. San Antonio, TX: The Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale – Third Edition. San Antonio, TX: The Psychological Corporation; 1997b. [Google Scholar]
- Woodard JL, Dunlosky J, Salthouse TA. Task decomposition analysis of intertrial free recall performance on the Rey Auditory Verbal Learning Test in normal aging and Alzheimer’s disease. Journal of Clinical and Experimental Neuropsychology. 1999;21:666–676. doi: 10.1076/jcen.21.5.666.872. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, Johnson MB. Woodcock-Johnson Psycho-Educational Battery – Revised. Allen, TX: DLM; 1990. [Google Scholar]