Abstract
Recent studies demonstrate that most cyanobacteria produce the neurotoxin beta-N-methylamino-L-alanine (BMAA) and that it can biomagnify in at least one terrestrial food chain. BMAA has been implicated as a significant environmental risk in the development of neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and Amyotrophic Lateral Sclerosis (ALS). We examined several blooms of cyanobacteria in South Florida, and the BMAA content of resident animals, including species used as human food. A wide range of BMAA concentrations were found, ranging from below assay detection limits to approximately 7000 μg/g, a concentration associated with a potential long-term human health hazard.
Keywords: BMAA, cyanobacteria, Florida, harmful algal blooms, neurodegenerative disease, toxin
1. INTRODUCTION
There is a general consensus that Harmful Algal Blooms (HABs) are increasing worldwide (Smayda, 1990, Hallegraeff, 1993, Anderson et al., 2002, Glibert et al., 2005). This is primarily the result of an increasing human population generating increasing nutrient runoff from fertilizer, animal waste, sewage, and soil erosion (Nixon, 1995; Richardson and Jorgensen, 1996; Moffat, 1998; Heisler et al., 2008; Anderson et al., 2002, 2008). The increased mobilization of nutrients has led to the eutrophication, first of small water bodies such as ponds, lakes and rivers (Vollenweider, 1992a); then of estuaries such as Chesapeake Bay (Cooper and Brush, 1991; Harding and Perry, 1997); and more recently of large seas such as the Black Sea (Bodeanu, 1992; Mee, 1992; Cociasu et al., 1996), Baltic Sea (Larsson et al., 1985; Nehring, 1992), and Adriatic Sea (Vollenweider et al., 1992b; Justic et al., 1995); and of continental shelf areas such as the Mississippi River delta (Turner and Rabalais, 1991, 1994; Justic et al., 1995).
Blooms of cyanobacteria appear to also be increasing worldwide in response to increased nutrient inputs (Chorus and Bartram, 1999; Paerl, 2008). Cyanobacteria have the ability to produce a wide array of secondary metabolites (Moore, 1996; Welker and von Dohren, 2006; Sivonen and Borner, 2008), many of which are noxious or toxic to animals and/or humans (Carmichael and Falconer, 1993; Chorus and Bartram, 1999; Carmichael, 2001; Carmichael et al., 2001; Ibelings et al., 2008; Falconer, 2008; Pilotto, 2008; Stewart et al., 2008). Many of these toxins are dermatoxins, hepatotoxins, or neurotoxins, but only a few species of cyanobacteria are known to produce each of these toxins. Recently Cox et al. (2005) have produced laboratory results that suggest that virtually all cyanobacteria species produce the neurotoxin beta-N-methylamino-L-alanine (BMAA). The data demonstrate a 500-fold variation in the amount of BMAA produced among the cyanobacteria species examined, but how much of this variation is genetic and how much is environmental-physiological is not known at the present time. Metcalf et al. (2008) and Esterling and Downing (2008) have also presented data suggesting that most cyanobacteria produce BMAA in the environment.
In vitro studies have shown that BMAA is toxic to neurons at concentrations as low as 10-30 nM (Weiss and Choi, 1988; Weiss et al., 1989; Rao et al, 2006; Lobner et al., 2007). BMAA is structurally similar to glutamate and binds to glutamate receptors (Richter and Mena, 1989; Copani et al., 1990; Smith and Meldrum, 1990; Allen et al, 1995; Mash, 2008). Abnormal stimulation of these receptors may play a role in neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and Amyotrophic Lateral Sclerosis (Takahashi et al., 1997). BMAA has also been shown to be neurotoxic in a variety of animal models Spencer et al, 1987; Karamyan and Speth, 2008).
BMAA, as an amino acid, is water soluble, not lipid soluble, and this has a low octanol-water partition coefficient (Yunger and Cramer, 1981). It therefore would not be expected to biomagnify up the food chain (Mackay, 1982; Connolly and Pedersen, 1988; Arnot and Gobar, 2006; Kelly et al., 2007). However, unlike the more moderate bioaccumulation observed with most water soluble cyanobacteria toxins (Xie et al., 2005; Ibelings and Chorus, 2007; Ibelings and Havens, 2008; Funari and Testai, 2008), Cox et al. (2003), Banack and Cox (2003), Murch et al. (2004), and Banack et al. (2006) have demonstrated a 10,000-fold biomagnification of free BMAA and 50-fold biomagnification of total BMAA in a food chain in Guam from symbiotic cyanobacteria to cycads to fruit bats (Pteropids, also known as “flying foxes”). Cox and Sacks (2002) hypothesized that the increased consumption of these fruit bats by one ethnic group of people in Guam, the Chamorros, in the 1940s led to a 100-fold increase in the development of Amyotrophic Lateral Sclerosis (ALS)-Parkinsonism dementia complex in the Chamorros living in Guam. The observation of high concentrations of BMAA in the autopsied brains of Chamorros who died of these neurodegenerative diseases and the absence of BMAA in the age-matched non-neurological control brains of Canadians who died of other causes suggested a possible link between BMAA and neurodegenerative diseases (Murch et al., 2004). Recently Pablo et al. (2009) have demonstrated high concentrations of BMAA in Americans who died of Alzheimer’s disease or ALS, but little or no BMAA in the brains of age-matched non-neurological controls, or in cases of Huntington’s disease, a genetic disorder. These data suggest that the unusual Guam situation is not unique, and that BMAA may biomagnify in other food chains, enter the human diet, and potentially trigger neurodegenerative disease.
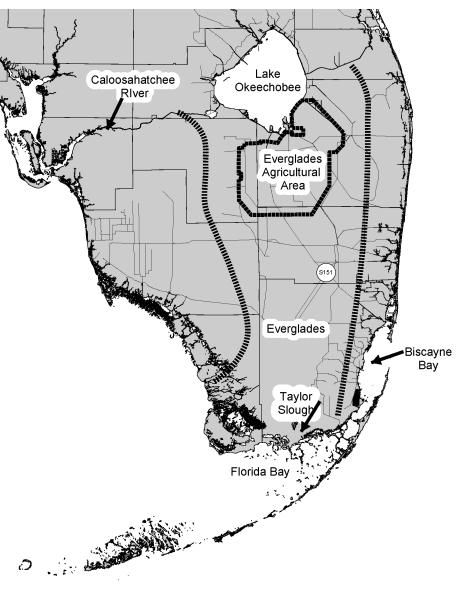
Together, these recent studies suggest that BMAA from cyanobacteria could be involved in neurodegenerative diseases. As BMAA is not lipophilic, it would not be expected to biomagnify, yet the indirect evidence suggests that it can biomagnify in at least certain circumstances. To determine if BMAA is present and can biomagnify in aquatic food chains, BMAA concentrations were analyzed in animals collected from water bodies in South Florida (Fig. 1) known to have blooms of cyanobacteria.
Figure 1.
Map of major water bodies in South Florida. Lines of parallel bars indicate general watershed boundaries of the Everglades.
2. METHODS
2.1 Sampling methods and sites
Water samples were collected on a roughly monthly basis at numerous stations in Florida Bay (Fig. 2), Biscayne Bay (Fig. 3), and the Caloosahatchee River (Fig. 4). Sample tissue to be analyzed for BMAA content were collected from various frozen animals that had been collected for a variety of other research projects in these water bodies.
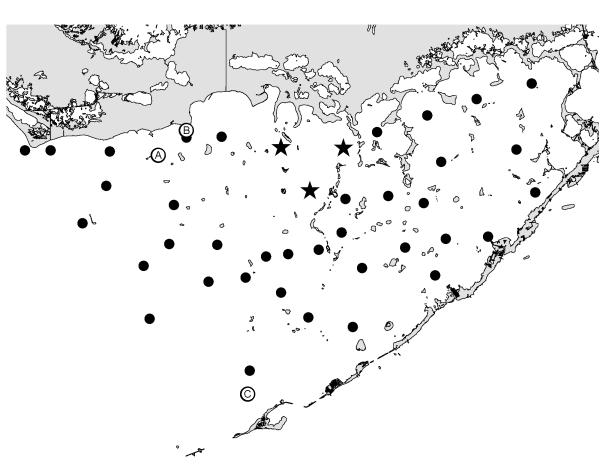
Figure 2.
Location of sampling stations in Florida Bay. Dots are water samples. Stars are water samples used in Figure 13. Letters are where animals were collected.
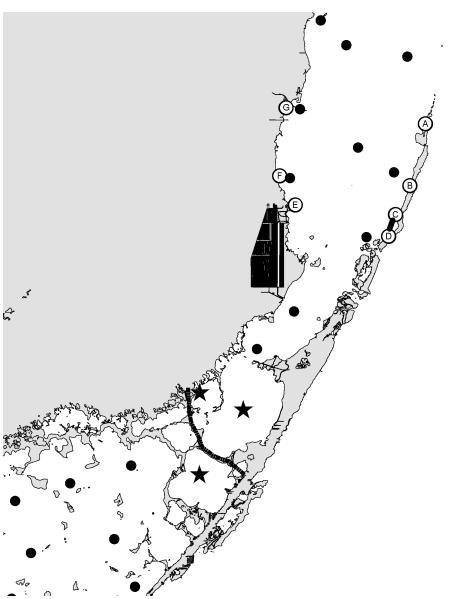
Figure 3.
Location of sampling stations in eastern Florida Bay-south Biscayne Bay. Dots are water samples. Stars are water samples used in Figure 15. Letters are where animals were collected. Parallel bars show area where mangrove trees were destroyed.
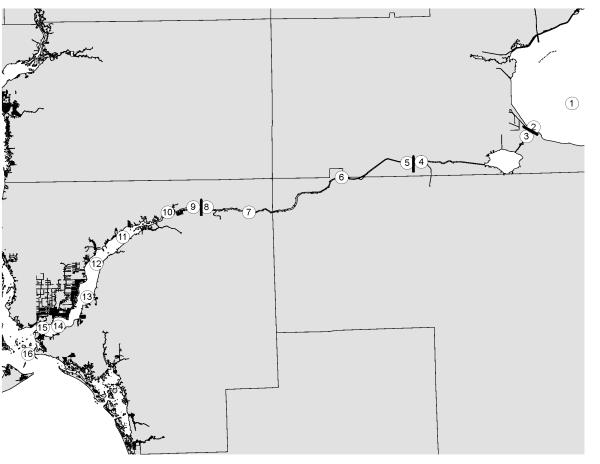
Figure 4.
Location of sampling stations along the Caloosahatchee River. Circles are water sampling stations. Bars are dams on the river.
2.2 Cyanobacteria abundance
Cyanobacteria abundance in water samples was estimated by measuring concentrations of phycocyanin. Water samples were filtered through GF/F glass fiber filters and the filters were frozen at −20°C until extracted. These filters were extracted overnight with a phosphate buffer (0.05M H2KPO4,0.05M HK2PO4; pH 6.5; with 0.01%(v/v) mercaptoethanol added) at 5°C and analyzed for phycocyanin with a SPEX Fluorolog-3 spectrofluorometer, using 580 nm excitation and 640 nm emission. The spectrofluorometer was calibrated using pure phycocyanin.
2.3 Water flow and nutrients
Water flow and nutrient data were obtained from the hydrometeorological and water quality databases of the South Florida Water Management District and the Lee County Environmental Laboratory.
2.4 Extraction and measurement of BMAA
Quantitation of BMAA was performed on frozen, unfixed tissues by a modification of previously validated methods (Cox et al., 2003; Banack and Cox, 2003). All tissue samples were analyzed and assayed in duplicate. Briefly, frozen-pulverized liquid nitrogen submerged (Model# 36903-10, Cole-Parmer, Quebec, Canada) tissues (1:10 w/v) were hydrolyzed overnight in 6 M HCl (1:10 w/v) at 110°C. Particulate matter was removed in 500 μl aliquots by ultrafiltration (Ultrafree-MC, Millipore) at 15,800 × g. Two hundred microliters of the extract was vacuum centrifuged in a speed-vac (Thermo-Savant SC250DDA Speed Vac Plus with a Savant refrigerator trap RVT 4104). The lyophilized residue was resuspended in 200 μl 0.1 M trichloroacetic acid (TCA) then washed with 100 μl of chloroform for extraction of any residual lipids. The aqueous layer was then transferred to a fresh tube and the chloroform layer was discarded. Samples (5 μl) and standards were derivatized with 6-aminoquinolyl-N-hydrosuccinimidyl carbamate (AQC) using the AccQ-Fluor reagent (Waters Corp, Millford, MA; WAT052880). BMAA was separated from the protein amino acids by reverse-phase elution (Waters Nova-Pak C18 column, 3.9 × 300 mm using 140 mM sodium acetate, 5.6 mM triethylamine, pH 5.7 (mobile phase A), and 58% acetonitrile in water (mobile phase B) at 1 ml per min at 37°C. The elution gradient (30 min) was as follows: time 0 = 75% A; 2 min = 75% A curve 6; 17 min = 63% A curve 7; 18.5 min = 100% B curve 6; 23.5 min = 100% B curve 6; 25 min = 75% A curve 6; 30 min = 75% A. Samples were run in duplicate followed by an AQC blank (containing borate buffer and AQC tag) to ensure that there was no carryover between samples. BMAA was quantified by detection of the AQC fluorescent tag (Waters 2475 Multi λ-Fluorescence Detector) with excitation at 250 nm and emission at 395 nm. Experimental samples were compared to standard spiked similar matrix negative for endogenous BMAA, containing commercial (Sigma B-107; >95% purity) or authentic standards of BMAA (99% purity). The percentage of recovery of BMAA was >94%.
Identification of a BMAA peak detected by reverse phase HPLC was verified by LC/MS/MS using product ion mode in a triple quadrupole system. The frozen (unfixed) sample was hydrolyzed for 18 hours in 6N HCL at 110°C (Fountoulakis et al., 1998) and then dried to remove HCl in a Thermo-Savant SC250DDA Speed Vac Plus (Waltham, MA). The sample was reconstituted in dilute HCl (20 mM) and derivatized with AQC, which increased the molecular weight of the BMAA analyte from 118 to 458. Individual compounds were eluted from the column with a gradient elution of 140 mM sodium acetate buffer, 5.6 mM triethylamine, pH 5.7 (mobile phase A) and 52% acetonitrile (mobile phase B) with a flow rate of 1.0 ml/min. Separation was achieved by liquid chromatography (Thermo model Surveyor LC, San Jose, CA) using a Thermo Hypersil GOLD 100 × 2.1 mm, 3 μm particle column at 0.28 ml/min using the eluents of 0.1% formic acid (v/v) in water (eluent A) and 0.1% formic acid in acetonitrile (eluent B) with the following gradient: time 0 = 100% A, 2 min = 100% A, 16 min = 80% A, 20 min = 2% A, 22 min = 2% A, 24 min = 81% B, 26 min = 81% A, 27 min = 100% A, 31 min = 100% A. Nitrogen gas was supplied to the H-ESI probe (heated electrospray ionization) with a nebulizing pressure of 25 psi and a vaporizing temperature of 300°C. The mass spectrometer was operated in the positive electrospray ionization (ESI) mode under the following conditions: nebulizing pressure of 25 psi, vaporizing temperature of 300°C, capillary temperature set at 280°C, capillary offset of 35, tube lens offset of 94, source collision energy of 5v. The protonated molecular ion of double derivatized BMAA (m/z 459) was used as the precursor ion for SRM (single reaction monitoring) analysis. Three transitions were monitored: 459 to 119 (collision energy of 19v), 459 to 171(collision energy of 32v), 459 to 289 (collision energy of 16v). The ratios of these three product ions were compared to the ratios of the product ions created by injection of AQC-derivatized pure BMAA standard (synthesized and then triple crystallized by Peter Nunn, University of Portsmouth, U.K.) into the triple quadrupole LC/MS/MS under the same conditions.
2.5 Measurement of Glutamate
The effects of BMAA are purported to affect glutamate-associated mechanisms (Rao et al., 2006; Lobner et al, 2007). To validate the methods used for amino acid hydrolysis/extraction, we quantified the area ratio of glutamate (rt = 2.47 min) compared to an internal standard (istd) norleucine (rt = 18.91 min.) using standard curves of the specific matrix taken from various species. Briefly, increasing amounts (0-250 ng/injection)of glutamate along with one concentration (50ng/injection) of istd were spiked into 0.1 mg tissue/injection and analyzed by HPLC as per methods similarly described for BMAA.
3. RESULTS
3.1 BMAA Extraction-Quantitation Method Validation
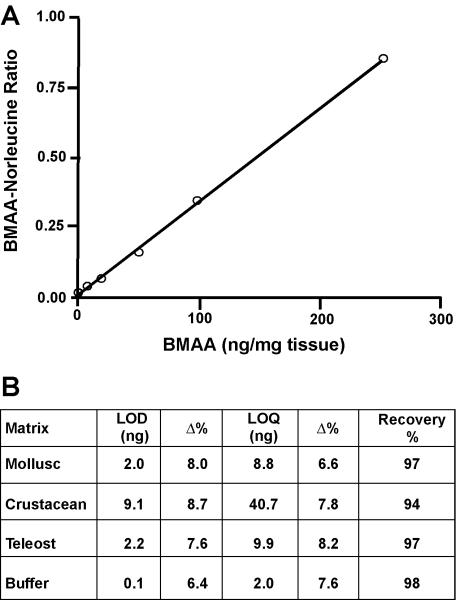
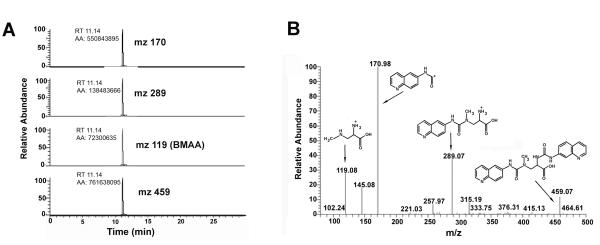
Using HPLC we were able to detect significant concentrations of BMAA in various marine samples taken from various locations of the South Florida marine ecosystems (see Fig. 5 for a representative chromatogram). Initial assays using BMAA-free matrix spiked with increasing amounts of L-BMAA were conducted to determine if there were any differences in the lower limits of detection (LOD) and lower limits of quantitation (LOQ) for the extraction/instrumental method on the various matrices assayed. Results from these assays demonstrated an increasing threshold for detection in matrix dependent on origin (buffer<mollusc<teleost fish<<crustacean), and a recovery range of 94-98% (Fig. 6). The average linear dynamic range (1-250 ng) for BMAA spiked standard curves in matrix (Grey snapper, Lutjanus griseus, muscle) gave an R2 value of 0.998 (P < 0.0001). The presence of BMAA from marine samples found to have positive HPLC results were confirmed using triple quadrupole LC/MS/MS (Fig. 7). The application of triple quadrupole LC/MS/MS has four checks to verify the identity of the BMAA peak: (i) a specific single parent mass [molecular weight (MW): 459] is selected in the first quadrupole with all other masses excluded from the second quadrupole; (ii) the column retention time of the peak is unique; (iii) collision-induced dissociation of product ions in the second quadrupole must be all detected in the third quadrupole; (iv) the product ion ratios must match repetitive runs of the standard injected at a similar concentration within 5% variation. All four of these LC/MS/MS checks were verified with ratios of the product ions (MW: 289, 171, 119) of BMAA matching the daughter ion ratios of the derivatized standard (Fig. 7).
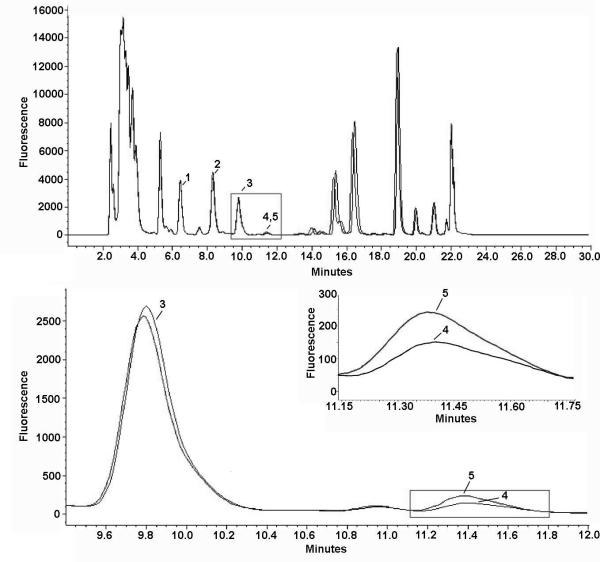
Figure 5.
HPLC identification of beta-N-methylamino-L-alanine (L-BMAA) extracted from blue crab (Callinectes sapidus). A) Representative chromatogram of 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) tagged amino acids in protein bound extract of muscle from a blue crab (blue trace) taken from Biscayne Bay. Peaks indicated by numbers are the amino acids closest to BMAA: L-tyrosine (1), L-valine (2), and L-methionine(3). The sample chromatographic peak of ACQ-BMAA (4) was confirmed by comparison (black trace, inset) to a co-run identical sample spiked with a known quantity of L-BMAA (100 ng). B) Matrix spiked with L-BMAA and 2,4 DAB synthetic standards. Although DAB is a structural isomer of BMAA, these results demonstrate two distinct chromatographic peaks separated by a later retention time for AQC-tagged DAB.
Figure 6.
Calibration of the HPLC assay of beta-N-methylamino-L-alanine (BMAA) A. Hydrolyzed protein matrix from a control (BMAA-negative) specimen of Grey snapper (Lutjanus griseus) was spiked with increasing amounts of L-BMAA and compared to spiked norleucine (istd). Neutralized samples derivatized with AQC were analyzed by HPLC without further treatment. Each point represents an average of three runs assayed in duplicate. The response was linear up to 250 ng/mg, r2 = 0.998). B. Validation parameters and results summary from case study of specimens in different phyla. The limit of detection (LOD) and quantitation (LOQ) were validated in HCl extracts of specific matrix protein and compared to buffer blanks to ensure specificity and sensitivity of the quantitation limits. Recovery was determined in control matrix extracts spiked with BMAA prior to acid hydrolysis and compared to the same extract with BMAA added immediately before derivatization with AQC as described in the methods. %CV, Percent Coefficient of variation; %Δ, Percent deviation of the mean from target value.
Figure 7.
LC/MS/MS identification and verification of L-BMAA in blue crab from Biscayne Bay. A) Triple quadrupole LC/MS/MS verification of BMAA in a representative marine sample (blue crab). Ion chromatograms of product ion from collision induced dissociations of m/z 459. The chromatography of the three major ions produced are: (1) protonated AQC derivative fragment (m/z 171) is the quantitation ion, (2) the protonated-BMAA AQC fragment (m/z 289) is the fist qualifier ion with a ratio of 38% and (3) the protonated-BMAA fragment (m/z 119) is the second qualifier ion with a ratio of 27%. B) Full product ion scan of an injection of AQC derivatized BMAA (217 fmole) in LC/MS/MS. Spectrum shows the dissociation of m/z 459 at 20 V.
3.2 Measurement of Glutamate
HPLC analysis of glutamate was conducted on select marine samples (mollusc (1 sample), crustacean (2 samples), and teleost fish (2 samples)) as an internal control and reference value for comparison to BMAA concentrations. To determine the significance of the BMAA concentrations, averages of glutamate and BMAA concentrations were converted to mole units and the calculated ratios gave a range of 18-45 for the measured glutamate to BMAA concentrations (Table 1). Glutamate measured in these samples were in good agreement with previous estimates (Dunn et al., 1949), providing validation for the amino acid extraction methods described here.
Table 1.
Glutamate Concentrations in Aquatic Samples
| Glutamate (MW 146) | BMAA (MW 119) | ||||||
|---|---|---|---|---|---|---|---|
| Common Name (Species) |
μg/g | μmole/g | Glutamate (%Tot Prot)† |
μg/g | μmole/g | BMAA (%Tot Prot) |
Glut:BMAA (molar ratio) |
| Blue crab (Callinectes sapidus) |
52,045 | 356 | 14.9 | 2,286 | 19 | 0.7 | 18.6 |
| Oyster (Pinctada margaritifera) |
14,050 | 96 | 7.6 | 275 | 2 | 0.1 | 41.6 |
| Puffer fish (Sphoeroides parvus) |
60,870 | 417 | 17.8 | 1,907 | 16 | 0.6 | 26.0 |
| Shrimp (Panaeus duorarum) |
64,264 | 440 | 11.8 | 1,156 | 10 | 0.2 | 45.3 |
| White perch (Pomoxis annularis) |
51,878 | 355 | 15.1 | 1,806 | 15 | 0.5 | 23.4 |
Concentrations were determined using a modification of the HPLC-AQC method and compared to an identical sample spiked with a known amount of glutamate. Glutamate/BMAA ratios were determined by conversion of the average concentrations by weight for each analyte to their molar concentrations.
Reference value for average glutamate concentration in Teleost fish muscle is 14.6% of total protein (Dunn et al., 1949)
Dunn MS, Camien MN, Eiduson S, and Malin RB (1949). The nutritive value of canned foods. I Amino acid content of fish and meat products. Journal of Nutrition 39:177-185.
3.3 Florida Bay
A large bloom of cyanobacteria developed in north central Florida Bay in the 1980s, which has persisted ever since (Brand, 2002). Although there are no quantitative data before 1989, many fishers and other boaters who were frequently in Florida Bay were quoted by DeMaria (1996) as observing algal blooms and water “discoloration” beginning in 1981 and increasing thereafter. They stated “In 1981, the water got dirtier, the blooms grew, and the seagrass started dying.” and “From 1981 to 1986, the decline [of the bay] was gradual. In 1987, the bay started to collapse quickly.”
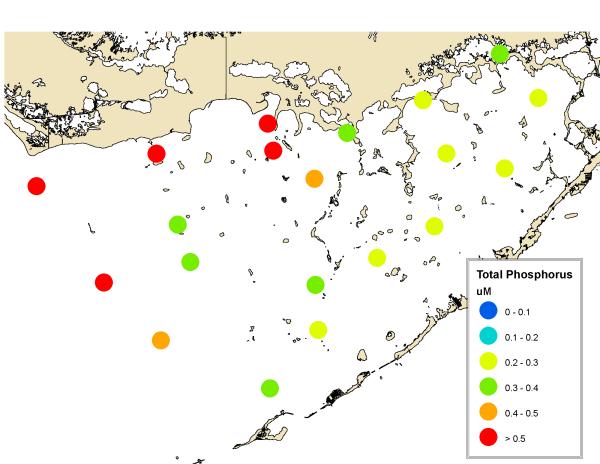
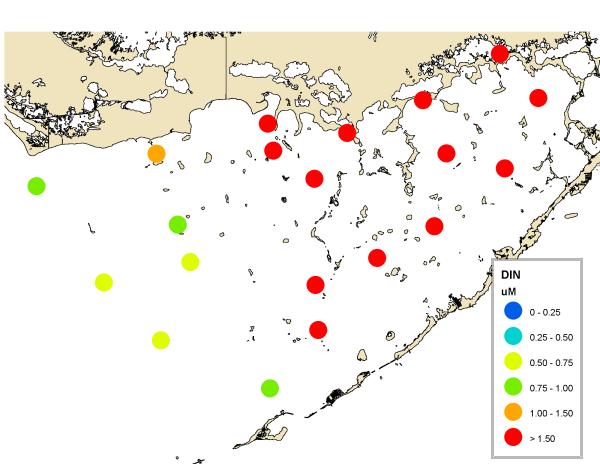
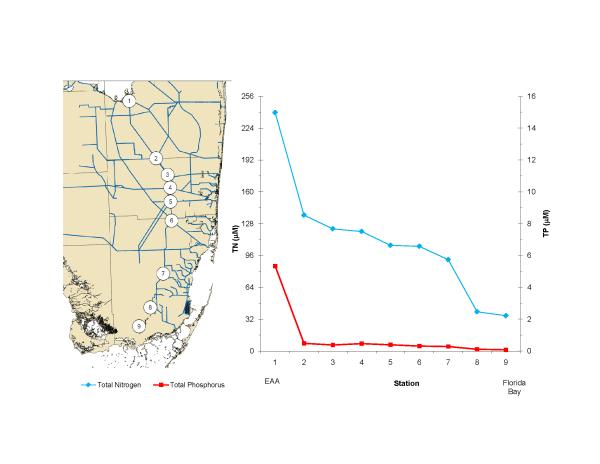
The bloom is located primarily in northcentral Florida Bay and is primarily cyanobacteria (Fig. 8). It occurs in an area where high phosphorus in the west (Fig. 9) mixes with high nitrogen in the east (Fig. 10). It has been hypothesized that the phosphorus in western Florida Bay is from natural phosphorite deposits tens of meters below being transported by geothermal circulation to the surface (Top et al., 2001; Brand, 2002). Phosphorus is low in eastern Florida Bay because there are no phosphorite deposits in that area and the calcium carbonate that is the primary mineral of the Florida peninsula chemically scavenges phosphate from seawater (DeKanel and Morse, 1978; Kitano et al., 1978). This occurs in both groundwater flowing through limestone as well as shallow surface water in which calcareous sediments are resuspended. The nitrogen in eastern Florida Bay, on the other hand, is from the decomposition of nitrogen-rich organic peat in the northern Everglades as a result of the drainage of the Everglades and conversion into agricultural fields (now called the Everglades Agricultural Area), primarily sugar cane (Brand, 2002). Figure 11 shows the high nitrogen and phosphorus in this water in the north and the gradual decline to the south. Plants scavenge phosphorus from the water as it flows to the south through the Everglades, but they cannot scavenge all of the nitrogen because of the initial high N:P ratio (Brand, 2002). Diversion of this nitrogen-rich water in the 1980s into eastern Florida Bay (Fig. 12) led to the blooms of cyanobacteria developing in northcentral Florida Bay where nitrogen from the east meets phosphorus from the west (Brand, 2002). Because of the highly seasonal nature of the rainfall and runoff in South Florida, cyanobacteria abundance is low during the dry season and high during the wet season (Fig. 13). Florida Bay is a major nursery area for shrimp, and critical to the Florida shrimping industry. Samples of pink shrimp in Florida Bay have high concentrations of BMAA (Table 2). Grey snapper collected south of the bloom have either no or only moderate concentrations of BMAA (Table 1).
Figure 8.
Average abundance of cyanobacteria in Florida Bay in 2000-2008 measured monthly as phycocyanin concentration.
Figure 9.
Average distribution of total phosphorus in Florida Bay in 2000-2008. Monthly data from the South Florida Water Management District.
Figure 10.
Average distribution of inorganic nitrogen (nitrate, nitrite, and ammonia) in Florida Bay in 2000-2008. Monthly data from the South Florida Water Management District.
Figure 11.
Transect of average nitrogen and phosphorus concentrations in 2008 from the Everglades Agricultural Area (EAA) through the Everglades into Florida Bay. Note that the scales for nitrogen and phosphorus are adjusted to the Redfield ratio of 16:1. These data show that phosphorus is the limiting nutrient in the Everglades. Monthly data from the South Florida Water Management District.
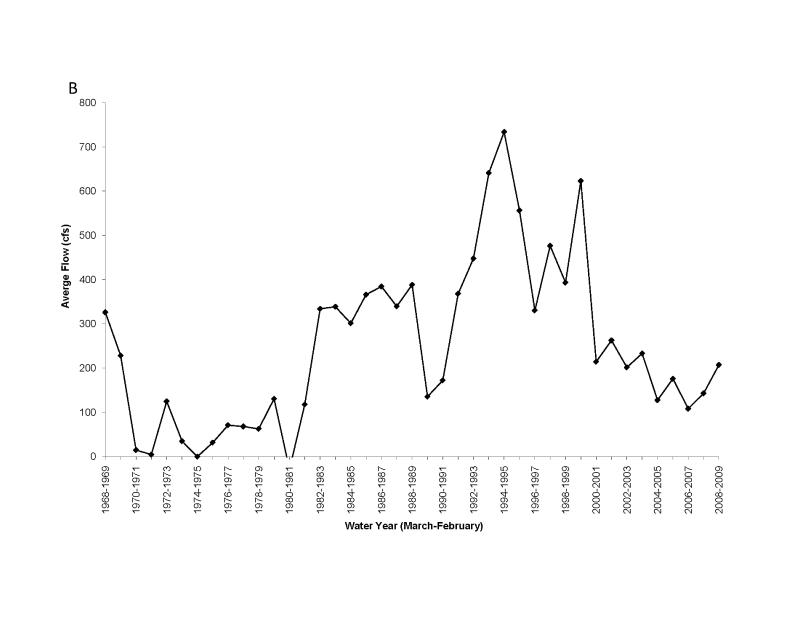
Figure 12.
Long term changes in water flow from the Everglades Agricultural Area to Florida Bay, as measured at S151 (A) and Taylor Slough (B) (locations shown in Fig. 1). Data from the South Florida Water Management District.
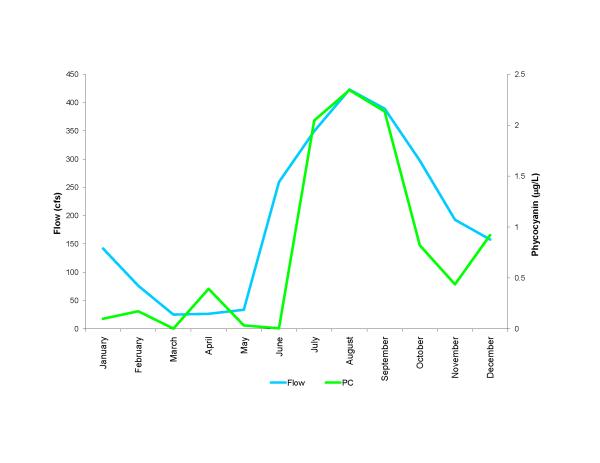
Figure 13.
Average seasonal flow of water into Florida Bay and concentrations of cyanobacteria (measured as phycocyanin) over the 2000 to 2008 time period. Flow data from the South Florida Water Management District.
Table 2.
Concentrations of BMAA in animals in Florida Bay.
| Sample | Species | Scientific name | Station | Location | Date | tissue type | Total BMAA (ug/g) | ||
|---|---|---|---|---|---|---|---|---|---|
| Assay 1 | Assay 2 | Assay 3 | |||||||
| MC1 | pink shrimp | Panaeus duorarum | A | Murray Key | 6/25/2008 | muscle | 1890 | 1247 | 1361 |
| MC2 | pink shrimp | Panaeus duorarum | B | Joe Kemp Key | 8/24/2008 | muscle | 3042 | 2860 | |
| JL2 | grey snapper | Lutjanus griseus | C | 24.8500 N 80.8330 W | 10/12/2008 | muscle | ND | ||
| JL4 | grey snapper | Lutjanus griseus | C | 24.8500 N 80.8330 W | 10/12/2008 | muscle | ND | ||
| JL1 | grey snapper | Lutjanus griseus | C | 24.8500 N 80.8330 W | 10/12/2008 | muscle | 20 | ||
| JL3 | grey snapper | Lutjanus griseus | C | 24.8500 N 80.8330 W | 10/12/2008 | muscle | 53 | ||
| JL5 | grey snapper | Lutjanus griseus | C | 24.8500 N 80.8330 W | 10/12/2008 | muscle | 188 | ||
3.4 South Biscayne Bay-Eastern Florida Bay
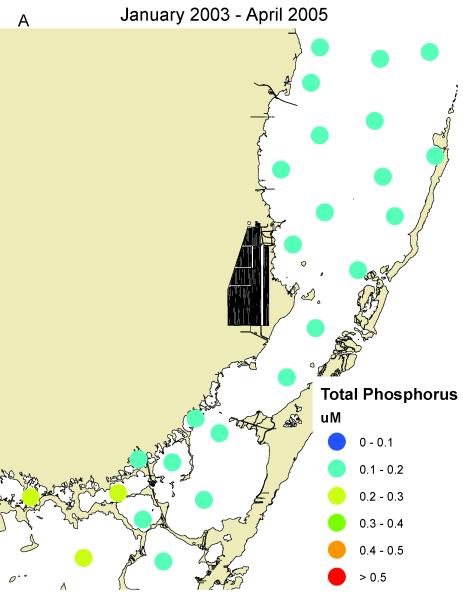
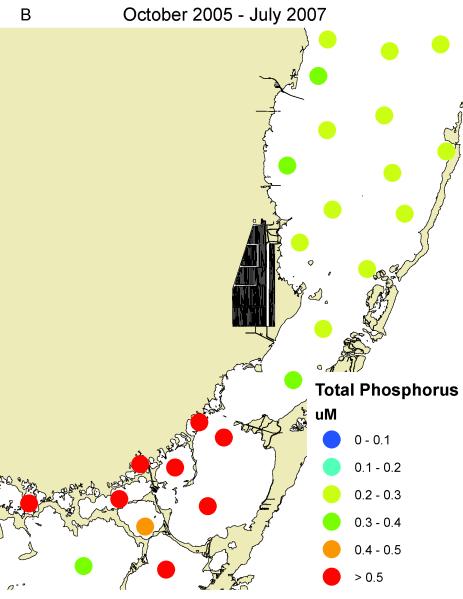
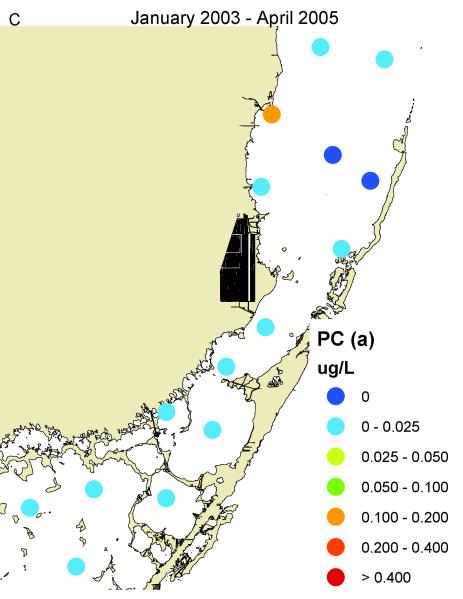
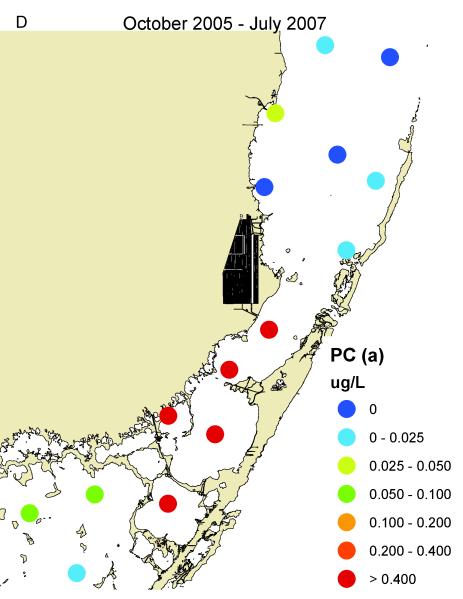
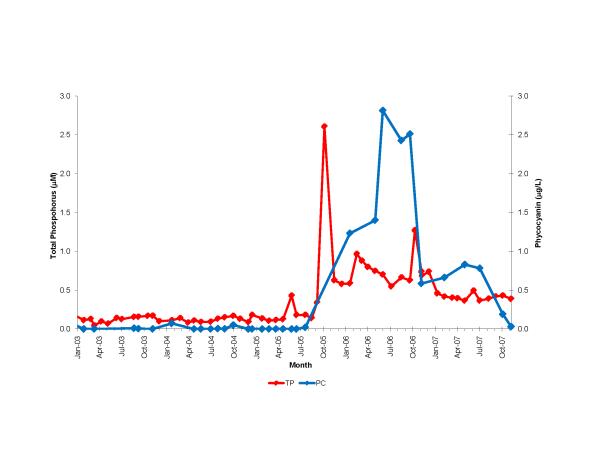
South Biscayne Bay-Eastern Florida Bay normally has extremely low concentrations of phosphorus (Fig. 14A) because of calcium carbonate scavenging (Brand, 2002), and low abundance of cyanobacteria (Fig. 14C). In the summer of 2005, large areas of mangroves in this area were destroyed in a road widening project (Fig. 3), and the organic material was buried in the surrounding sediments of South Biscayne Bay-Eastern Florida Bay. The subsequent anaerobic conditions and acidification of the calcium carbonate sediments released large amounts of phosphorus (Figs. 14B, 15). As phosphorus was the limiting nutrient in this area, the additional phosphorus led to large blooms of cyanobacteria (Figs. 14D, 15) that persisted for approximately two years. Samples of fish and crustaceans collected just north of this area in the spring and summer of 2007 (Fig. 3) as the bloom was on the decline show some species with no BMAA and others with very high concentrations (Table 3).
Figure 14.
Concentrations of total phosphorus and cyanobacteria (measured as phycocyanin) in eastern Florida Bay-south Biscayne Bay. A. Total phosphorus before mangrove destruction. B. Total phosphorus after mangrove destruction. C. Cyanobacteria before mangrove destruction. D. Cyanobacteria after mangrove destruction. Total phosphorus concentrations from South Florida Water Management District.
Figure 15.
Time course of total phosphorus and cyanobacteria (measured as phycocyanin) at locations (stars) shown in Fig. 3 in the area of mangrove destruction. Total phosphorus data from South Florida Water Management District.
Table 3.
Concentrations of BMAA in animals in Biscayne Bay.
| Sample | Species | Scientific name | Station | Location | Date | tissue type | Total BMAA (ug/g) | ||
|---|---|---|---|---|---|---|---|---|---|
| Assay 1 | Assay 2 | Assay 3 | |||||||
| NH34 | pink shrimp | Panaeus duorarum | C-D transect | Elliot Key | summer, 2007 | muscle | 55 | ||
| NH14 | pink shrimp | Panaeus duorarum | A | 25.5150N 80.1795W | spring, 2007 | muscle | 181 | 362 | 457 |
| NH28 | pink shrimp | Panaeus duorarum | F | 25.4653N 80.3359W | 2/12/2007 | muscle | 942 | ||
| NH17 | blue crab | Callinectes sapidus | G | 25.5314N 80.3286W | spring, 2007 | muscle | ND | ||
| NH24 | blue crab | Callinectes sapidus | F | 25.4653N 80.3359W | summer, 2007 | muscle | 303 | 212 | |
| NH31 | blue crab | Callinectes sapidus | C-D transect | Elliot | summer, 2007 | muscle | 357 | 324 | |
| NH4 | blue crab | Callinectes sapidus | A | 25.5150N 80.1795W | spring, 2007 | muscle | 4979 | 3037 | |
| NH16 | blue crab | Callinectes sapidus | E | 25.4369N 80.3193W | spring, 2007 | muscle | 6976 | 6557 | |
| NH20 | spider crab | Clupeoides | C-D transect | Elliot Key | summer, 2007 | muscle | ND | ND | |
| NH33 | anchovy-sardine | Anchoa sp. | C-D transect | Elliot Key | summer, 2007 | muscle | ND | ||
| NH15 | hardhead silverside | Atherinomorus stipes | A | 25.5150N 80.1795W | spring, 2007 | muscle | ND | ND | ND |
| NH27 | hardhead silverside | Atherinomorus stipes | F | 25.4653N 80.3359W | 2/12/2007 | muscle | ND | ND | ND |
| NH38 | hardhead silverside | Atherinomorus stipes | C-D transect | Elliot Key | Summer, 2007 | muscle | ND | ||
| NH9 | pinfish | Lagodon rhomboides | G | 25.5314N 80.3286W | spring, 2007 | muscle | ND | ND | |
| NH36 | pinfish | Lagodon rhomboides | C-D transect | Elliot Key | summer, 2007 | muscle | ND | ||
| NH6 | mojarra | Gerreidae | D | 25.4060N 80.2196W | spring, 2007 | muscle | ND | ||
| NH21 | mojarra | Gerreidae | C-D transect | Elliot Key | Elliot | muscle | ND | ND | |
| NH25 | mojarra | Gerreidae | F | 25.4653N 80.3359W | spring, 2007 | muscle | 154 | ||
| NH3 | mojarra | Gerreidae | A | 25.5150N 80.1795W | spring, 2007 | muscle | 567 | 456 | |
| NH8 | grey snapper | Lutjanus griseus | D | 25.4060N 80.2196W | spring, 2007 | muscle | ND | ||
| NH12 | grey snapper | Lutjanus griseus | A | 25.5150N 80.1795W | spring, 2007 | muscle | ND | ND | |
| NH40 | grey snapper | Lutjanus griseus | C-D transect | Elliot Key | summer, 2007 | muscle | ND | ||
| NH2 | school master snapper | Lutjanus apodus | A | 25.5150N 80.1795W | spring, 2007 | muscle | ND | ND | |
| NH11 | band tail puffer | Sphoeroides spengleri | A | 25.5150N 80.1795W | spring, 2007 | muscle | ND | ND | ND |
| NH29 | band tail puffer | Sphoeroides spengleri | F | 25.4653N 80.3359W | spring, 2007 | muscle | 455 | 417 | |
| NH18 | least puffer | Sphoeroides parvus | G | 25.5314N 80.3286W | spring, 2007 | muscle | 194 | 213 | |
| NH10 | least puffer | Sphoeroides parvus | A | 25.5150N 80.1795W | spring, 2007 | muscle | 7351 | 6523 | |
| HN30 | scrawled cowfish | Acanthostracion quadricornis | A | 25.5150N 80.1795W | 1/20/2007 | muscle | 34 | 47 | 36 |
| NH23 | sea bream | Archosargus rhomboidalis | B | 25.4550N 80.1965W | summer, 2007 | muscle | 484 | 636 | |
| NH39 | sea bream | Archosargus rhomboidalis | C-D transect | Elliot Key | summer, 2007 | muscle | 2349 | 2091 | |
| NH37 | sailors choice grunt | Haemulon parra | C-D transect | Elliot Key | summer, 2007 | muscle | 242 | 203 | |
| NH7 | sailors choice grunt | Haemulon parra | D | 25.4060N 80.2196W | spring, 2007 | muscle | 722 | 674 | |
| NH32 | bluestriped grunt | Haemulon sciurus | C-D transect | Elliot Key | summer, 2007 | muscle | ND | ||
| NH1 | bluestriped grunt | Haemulon sciurus | A | 25.5150N 80.1795W | spring, 2007 | muscle | 1723 | 1568 | |
| NH5 | bluestriped grunt | Haemulon sciurus | D | 25.4060N 80.2196W | spring, 2007 | muscle | 3776 | 2945 | |
| NH19 | redfin needlefish | Strongylura notata | G | 25.5314N 80.3286W | spring, 2007 | muscle | ND | ||
| NH26 | redfin needlefish | Strongylura notata | F | 25.4653N 80.3359W | 2/12/2007 | muscle | ND | ND | |
| NH13 | great barracuda | Sphyraena barracuda | A | 25.5150N 80.1795W | spring, 2007 | muscle | ND | ||
| NH22 | great barracuda | Sphyraena barracuda | B | 25.4550N 80.1965W | summer, 2007 | muscle | ND | ND | |
| NH35 | great barracuda | Sphyraena barracuda | C-D transect | Elliot Key | summer, 2007 | muscle | ND | ||
3.5 Caloosahatchee River
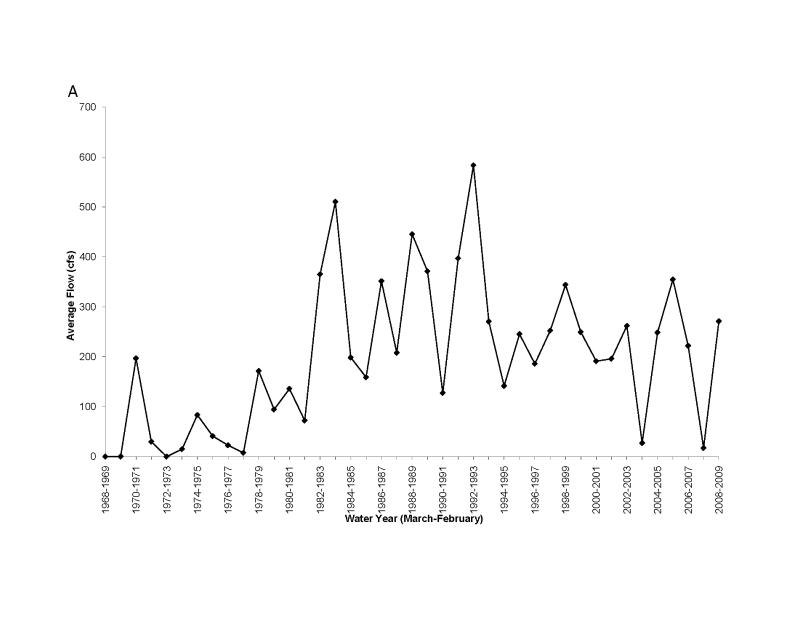
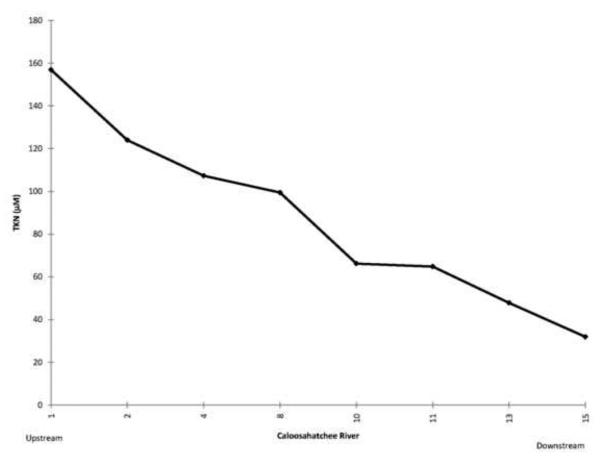
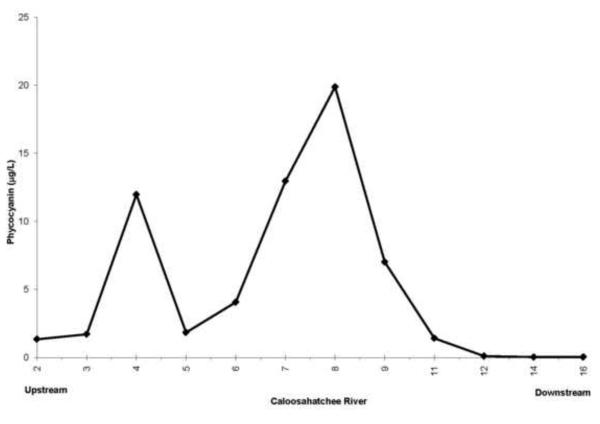
Some of the nutrient rich water from the Everglades Agricultural Area is pumped into Lake Okeechobee and then to the west down the Caloosahatchee River (Fig. 1, Brand, 2002). Because of the phosphorite deposits, waters along the western side of the Florida peninsula tend to be rich in phosphorus and thus nitrogen limited (Brand, 2002). High concentrations of nitrogen flow out of Lake Okeechobee and down the Caloosahatchee River (Fig. 16) and large blooms of cyanobacteria develop (Fig. 17). Moderate amounts of BMAA were found in molluscs and high concentrations were found in fish in the river (Table 4).
Figure 16.
Average concentrations of total nitrogen along the Caloosahatchee River from Lake Okeechobee to Sanibel Island May-December, 2008. Monthly data from South Florida Water Management District.
Figure 17.
Average concentrations of cyanobacteria (measured as phycocyanin) along the Caloosahatchee River from Lake Okeechobee to Sanibel Island May-December, 2008.
Table 4.
Concentrations of BMAA in animals in the Caloosahatchee River.
| Sample | Species | Scientific name | Station | Location | Date | tissue type | Total BMAA (ug/g) | |
|---|---|---|---|---|---|---|---|---|
| Assay 1 | Assay 2 | |||||||
| LB31 | paper pondshell mussel | Utterbackia imbecillis | 2 | 26.8319N 81.0901W | 9/26/2008 | mantle muscle | 261 | 251 |
| LB28 | oyster | Crassostra virginica | 11 | 26.6769N 81.8444W | 8/14/2008 | mantle muscle | 305 | 281 |
| JC4 | bowfin | Amia calva | 8 | 26.7291N 81.7284W | 1/24/2008 | head | 554 | 609 |
| JC5 | bowfin | Amia calva | 8 | 26.7291N 81.7284W | 1/24/2008 | tail | 2559 | 2245 |
| JC7 | alligator gar | Atractosteus spatula | 8 | 26.7291N 81.7284W | 1/24/2008 | head | 2140 | 2290 |
| JC8 | alligator gar | Atractosteus spatula | 8 | 26.7291N 81.7284W | 1/24/2008 | tail | 857 | 797 |
| JC9 | allicator gar | Atractosteus spatula | 8 | 26.7291N 81.7284W | 1/24/2008 | central body | 1207 | 990 |
| JC1 | largemouth bass | Micropterus salmoides | 8 | 26.7291N 81.7284W | 1/24/2008 | head | 1130 | 949 |
| JC2 | largemouth bass | Micropterus salmoides | 8 | 26.7291N 81.7284W | 1/24/2008 | tail | 2388 | 2245 |
4. DISCUSSION
Because the animal samples were collected for other research projects unrelated to our research on cyanobacteria blooms and the BMAA hypothesis, the spatial and temporal patterns of animal samples are not optimal for examining the hypothesized link between cyanobacteria blooms and BMAA accumulation in animals. Nevertheless, these initial results indicate that high concentrations of BMAA can accumulate in some aquatic animals in areas of cyanobacteria blooms. Not enough samples have been analyzed to discern any clear patterns, but some possibilities do appear.
In south Biscayne Bay, there was large variation in the BMAA concentrations between individuals of a species in pink shrimp, blue crabs, mojarra, band tail puffers, least puffers, and bluestriped grunts (Table 3). The replicability of our methods suggest that these differences are real and not the result of methodological problems. The cause of this variation is unknown but several possibilities exist. As these animals were collected from an area north of the bloom, depending on their migration patterns, some animals may have spent considerable time in the bloom (or the organisms in their diet did so) while others may have spent little or no time in the bloom. While our research focussed on planktonic cyanobacteria, benthic cyanobacteria and cyanobacteria epiphytic on seagrass and macroalgal blades are also present. In these shallow waters, microalgal abundance per square meter is typically around 20 times higher on the sediment surface than in the water column above (Brand and Suzuki, 1999). Microhabitat differences in the relative abundance of these different groups of cyanobacteria and their BMAA content might account for the differences observed. Genetic differences between individuals in their ability to accumulate BMAA are also a possibility (discussed below), just as there are genetic differences among humans in their susceptibility to develop neurodegenerative diseases. Whatever the cause of this variation, it must be kept in mind when examining data on only a few individuals of a species.
Many crustaceans appear to have high concentrations of BMAA. Pink shrimp in Florida Bay (Table 2) have high concentrations of BMAA, comparable to those found in the fruit bats of Guam (3556 μg/g; Cox et al., 2003). Pink shrimp and blue crabs in south Biscayne Bay have a wide range of concentrations (Table 3). All three species of fish from the Caloosahatchee River (Table 4) have high concentrations of BMAA similar to that of the fruit bats of Guam. The filter feeding molluscs in the Caloosahatchee River and estuary have only moderate amounts of BMAA (Table 4). Grey snapper in Florida Bay south of its cyanobacteria bloom have moderate amounts of BMAA (Table 2), while grey snapper in Biscayne Bay north of its cyanobacteria bloom have none (Table 3).
In south Biscayne Bay, a wide range of BMAA concentrations was observed among the 17 species examined. A perusal of the data does not reveal a classical biomagnification pattern, as top carnivores such as barracuda and grey snapper show no BMAA, while species near the bottom of the food chain, such as pink shrimp, pufferfish, and sea bream have relatively high concentrations. A pattern that does appear, however, is a tendency for higher concentrations of BMAA in species that feed on the benthos, and lower concentrations in species that feed on the plankton. An exception to this pattern is pinfish, which primarily feed on benthic vegetation, but had no BMAA. No BMAA was found in anchovies, silversides, needlefish, and barracuda that feed in the water column, while high concentrations were found in pink shrimp, blue crabs, pufferfish, and cowfish that feed on the bottom. This could be an indication that benthic cyanobacteria produce more BMAA than planktonic species, but we have no direct information on this. A perusal of the data of Cox et al. (2005) does suggest that such a pattern may exist.
At the present time, we do not know why cyanobacteria produce BMAA and thus, what environmental patterns may exist. Similarly, we do not know the mechanism of bioaccumulation of this amino acid. Because BMAA is water soluble, the standard mechanism for biomagnification of lipophilic compounds is not plausible. One possibility is differential uptake and excretion. Because BMAA is structurally similar to glutamate in the presence of bicarbonate as a carbamate (Weiss et al., 1988), neutral acid uptake enzymes in the gastrointestinal tract and elsewhere may take up BMAA from dietary sources, but enzymes involved in the turnover, degradation, and excretion of amino acids may not be able to recognize BMAA and eliminate it. A differential in uptake vs. excretion enzymes may be one possible mechanism for the large differences observed between individuals and species in this study.
Ultimately there are many sources of variation that could account for the results shown here. The laboratory data of Cox et al. (2005) have shown a 500-fold variation in the amount of BMAA produced by cyanobacteria. Much of this is likely the result of genetic differences between species, but some is likely due to physiological differences in response to environmental differences. As a result, one would expect microhabitat variation among benthic and epiphytic cyanobacteria, and differences between planktonic and benthic communities. As BMAA propagates up the food chain, differential uptake and excretion will cause varying amounts of biomagnification. The age of animals could be important, with older animals accumulating more BMAA. The time course of BMAA exposure over the lifetime of an animal could be important, depending on the ability of an animal to slowly or quickly excrete BMAA after short term exposure. While it is clear that most cyanobacteria produce BMAA, we cannot, at this time, be sure that there are no other sources of BMAA such as heterotrophic bacteria.
Based upon this study and other data, it appears that the situation in Guam is not unique, and that high concentrations of BMAA do occur in parts of aquatic food webs. To enhance the chances of finding BMAA in aquatic animals, this study focussed on areas of unnatural blooms of cyanobacteria caused by nutrient enrichment by human activities. Cyanobacteria are of course a significant part of natural aquatic habitats unaffected by human activities. Cyanobacteria are approximately 50% of the phytoplankton community of the open ocean covering over half of the earth (Li et al., 1983; Itturiaga and Mitchell, 1986). It is plausible that humans have been exposed to some level of BMAA throughout their evolutionary history. The increase in cyanobacteria blooms as a result of human activities is probably increasing this exposure, but by how much is not yet known. Our research is now expanding from this initial exploratory analysis to determine the distribution of BMAA in aquatic food webs and seafood in both natural ecosystems and eutrophic ecosystems.
Neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and Amyotrophic Lateral Sclerosis (ALS) are also increasing (Kokmen, et al., 1993; Gauthier, 1997; Chio, 2005). Increased longevity alone may not account for all of this increase. Heritability of these diseases is low, accounting for less that 10% of cases. While certain genetic factors are known to influence susceptibility to these diseases, some unknown environmental factors probably play a major role. BMAA may be a significant environmental factor. Cyanobacteria and the presence of BMAA in portions of aquatic food webs used as human food could be a significant human health hazard.
5. CONCLUSIONS
These data on BMAA concentrations in animals in South Florida waters indicate that the situation in Guam is not unique. These data further suggest that BMAA could be found in high concentrations in aquatic animals in many areas of the world where cyanobacteria blooms occur. This possibility includes not only natural water bodies, but also nutrient rich habitats such as aquaculture ponds and flooded rice fields that have high concentrations of cyanobacteria (Paerl and Tucker, 1995; Massout, 1999; Kankaanpaa et al., 2005; Zimba, 2008). Agricultural soils can also have high concentrations of cyanobacteria (Ramsey and Ball, 1983; Shimmel and Darley, 1985; Whitton, 2000).
As an increasing human population on the earth increases the flux of sewage, agricultural fertilizer, animal wastes, and eroded soil into various aquatic habitats, it is predicted that blooms of cyanobacteria will increase. It is predicted that human exposure to cyanobacteria and BMAA will increase, leading to a possible increased incidence of neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and Amyotrophic Lateral Sclerosis (ALS).
ACKNOWLEDGEMENTS
We would like to acknowledge John Cassani, Maria Criales, John Lamkin, and Aswani Volety for some of the animal samples used in this study, and Margaret Basile for help in the laboratory. Nutrient and water flow data were obtained from the South Florida Water Management District and Lee County Environmental Laboratory. We thank Lora Fleming for reviewing an earlier draft of this paper. We would like to acknowledge the Cove Point Foundation, National Science Foundation (#OCE0742285 and #OCE0432368) and National Institute of Environmental Health Sciences (#1 P50 ES12736) for their financial support of this research. Partial support for this study was provided by gifts in kind from the Institute for Ethnomedicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allen CN, Omelchenko I, Ross SM, Spencer P. The neurotoxin beta-N-methylamino-L-alanine (BMAA) interacts with the strychnine-insensitive glycine modulatory site of the N-methyl-D-aspartate receptor. Neuropharm. 1995;34:651–658. doi: 10.1016/0028-3908(95)00043-6. [DOI] [PubMed] [Google Scholar]
- Anderson DM, Glibert PM, Burkholder JM. Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries. 2002;25:704–726. [Google Scholar]
- Anderson DM, et al. Harmful algal blooms and eutrophication: Examining linkages from selected coastal regions of the United States. Harmful Algae. 2008;8:39–53. doi: 10.1016/j.hal.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnot JA, Gobas FAPC. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Env. Rev. 2006;14:257–297. [Google Scholar]
- Banack SA, Cox PA. Biomagnification of cycad neurotoxins in flying foxes. Neurol. 2003;61:387–389. doi: 10.1212/01.wnl.0000078320.18564.9f. [DOI] [PubMed] [Google Scholar]
- Banack SA, Murch SJ, Cox PA. Neurotoxic flying foxes as dietary items for the Chamorro people, Marianas Islands. J Ethnopharm. 2006;106:97–104. doi: 10.1016/j.jep.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Bodeanu N. Algal blooms and the development of the main phytoplanktonic species at the Romanian Black Sea littoral in conditions of intensification of the eutrophication process. In: Vollenweider RA, Marchetti R, Viviani R, editors. Marine Coastal Eutrophication. Elsevier; Amsterdam: 1992. pp. 891–906. [Google Scholar]
- Brand LE. The transport of terrestrial nutrients to South Florida coastal waters. In: Porter JW, Porter KG, editors. The Everglades, Florida Bay, and Coral Reefs of the Florida Keys. CRC Press; Boca Raton, Florida: 2002. pp. 353–406. [Google Scholar]
- Brand LE, Suzuki M. Distribution of benthic chlorophyll in Florida Bay sediments. 1999 Florida Bay and Adjacent Marine Systems Science Conference; Key Largo, FL. Nov. 1999.1999. p. 129. [Google Scholar]
- Carmichael WW. Health effects of toxin producing cyanobacteria: The cyanoHABS. Human Ecol. Risk Assess. 2001;7:1393–1407. [Google Scholar]
- Carmichael WW, Falconer IR. Diseases related to freshwater blue-green algal toxins, and control measures. In: Falconer IR, editor. Algal Toxins in Seafood and Drinking Water. Academic Press; 1993. pp. 187–209. [Google Scholar]
- Carmichael WW, Azevedo MFO, An JS, Molica RJR, Jochimsen EM, Lau S, Rinehart KL, Shaw GR, Eagelsham GK. Human fatalities from cyanobacteria: Chemical and biological evidence for cyanotoxins. Env. Health Persp. 2001;109:663–668. doi: 10.1289/ehp.01109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chio A. Mortality trends in ALS: an increasingly intricate puzzle. The Lancet Neurol. 2005;4:453–454. doi: 10.1016/S1474-4422(05)70125-3. [DOI] [PubMed] [Google Scholar]
- Chorus I, Bartram J. Toxic Cyanobacteria in Water: A Guide to Their Public Heath Consequences, Monitoring and Management. E&FN Spoon; London: 1999. [Google Scholar]
- Cociasu A, Dorogan L, Humborg C, Popa L. Long-term ecological changes in Romanian coastal waters of the Black Sea. Mar. Poll. Bull. 1996;32:32–38. [Google Scholar]
- Connolly JP, Pedersen CJ. A thermodynamic-based evaluation of organic chemical accumulation in aquatic organisms. Env. Sci. Tech. 1988;22:99–103. doi: 10.1021/es00166a011. [DOI] [PubMed] [Google Scholar]
- Cooper SR, Brush GS. Long-term history of Chesapeake Bay anoxia. Science. 1991;254:992–995. doi: 10.1126/science.254.5034.992. [DOI] [PubMed] [Google Scholar]
- Copani A, Canonico PL, Nicoletti F. Beta-N-methylamino-L-alanine (L-BMAA) is a potent agonist of “metabolotropic” glutamate receptors. Eur. J. Pharmocol. 1990;181:327–328. doi: 10.1016/0014-2999(90)90100-k. [DOI] [PubMed] [Google Scholar]
- Cox PA, Sacks OW. Cycad neurotoxins, consumption of flying foxes, and ALS-PDC disease in Guam. Neurology. 2002;58:956–9. doi: 10.1212/wnl.58.6.956. [DOI] [PubMed] [Google Scholar]
- Cox PA, Banack SA, Murch SJ. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc. Natl. Acad. Sci. USA. 2003;100:13380–13383. doi: 10.1073/pnas.2235808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox PA, Banack SA, Murch SJ, Rasmussen U, Tien G, Bidigare RR, Metcalf JS, Morrison LF, Codd GA, Bergman B. Diverse taxa of cyanobacteria produce beta-N-methylamino-L-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA. 2005;102:5074–5078. doi: 10.1073/pnas.0501526102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKanel J, Morse JW. The chemistry of orthophosphate uptake from seawater onto calcite and aragonite. Geochim. Cosmochim. Acta. 1978;42:1335–1340. [Google Scholar]
- DeMaria K. Changes in the Florida Keys Marine Ecosystem based upon Interviews with Experienced Residents. The Nature Conservancy and Center for Marine Conservation; 1996. p. 105. [Google Scholar]
- Dunn MS, Camien MN, Eiduson S, Malin RB. The nutritive value of canned foods. I. Amino acid content of fish and meat products. J. Nutr. 1949;39:177–185. doi: 10.1093/jn/39.2.177. [DOI] [PubMed] [Google Scholar]
- Esterhuizen M, Downing TG. ß-N-methylamino-L alanine (BMAA) in novel South African cyanobacterial isolates. Ecotox. Env. Safety. 2008;71:309–313. doi: 10.1016/j.ecoenv.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Falconer IR. Health effects associated with controlled exposures to cyanobacterial toxins. In: Hudnell HK, editor. Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs. Springer; 2008. pp. 607–612.pp. 950 [DOI] [PubMed] [Google Scholar]
- Fountoulakis M, Lahm H-W. Hydrolysis and amino acid composition analysis of proteins. J Chromatogr. 1998;826:109–134. doi: 10.1016/s0021-9673(98)00721-3. [DOI] [PubMed] [Google Scholar]
- Funari E, Testai E. Human health risk assessment related to cyanotoxins exposure. Crit. Rev. Tox. 2008;38:97–125. doi: 10.1080/10408440701749454. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Panisset M, Nalbantoglu J, Poirier J. Alzheimer’s disease: current knowledge, management and research. Can. Med. Assoc. J. 1997;157:1047–1052. [PMC free article] [PubMed] [Google Scholar]
- Glibert PM, Seitzinger S, Heil CA, Burkholder JM, Parrow MW, Codispoti LA, Kelly V. The role of eutrophication in the global proliferation of harmful algal blooms. Oceanogr. 2005;18:198–209. [Google Scholar]
- Hallegraeff GM. A review of harmful algal blooms and their apparent global increase. Phycologia. 1993;32:79–99. [Google Scholar]
- Harding LW, Perry ES. Long-term increase of phytoplankton biomass in Chesapeake Bay, 1950-1994. Mar. Ecol. Prog. Ser. 1997;157:39–52. [Google Scholar]
- Heisler J, et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae. 2008;8:3–13. doi: 10.1016/j.hal.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibelings BW, Chorus I. Accumulation of cyanobacterial toxins in freshwater “seafood” and its consequences for public health: A review. Env. Poll. 2007;150:177–192. doi: 10.1016/j.envpol.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Ibelings BW, Havens KE. Cyanobacterial toxins: A qualitative meta-analyis of concentrations, dosage and effects in freshwater, estuarine and marine biota. In: Hudnell HK, editor. Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs. Springer; 2008. pp. 675–732.pp. 950 [DOI] [PubMed] [Google Scholar]
- Ibelings BW, Havens K, Codd GA, Dyble J, Landsberg J, Coveney M, Fournie JW, Hilborn ED. Ecosystem effects workgroup report. In: Hudnell HK, editor. Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs. Springer; 2008. pp. 655–674.pp. 950 [DOI] [PubMed] [Google Scholar]
- Itturiaga R, Mitchell BG. Chroococcoid cyanobacteria: A significant component in the food web dynamics of the open ocean. Mar. Ecol. Progr. Ser. 1986;28:291–297. [Google Scholar]
- Justic D, Rabalais NN, Turner RE, Dortch Q. Changes in nutrient structure of river-dominated coastal waters: Stoichiometric nutrient balance and its consequences. Est. Coast. Shelf Sci. 1995;40:339–356. [Google Scholar]
- Kankaanpaa HT, Holliday J, Schroder H, Goddard TJ, von Fister R, Carmichael WW. Cyanobacteria and prawn farming in northern New South Wales, Australia – a case study on cyanobacteria diversity and hepatotoxin bioaccumulation. Tox. Appl. Pharm. 2005;203:243–256. doi: 10.1016/j.taap.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Karamyan VT, Speth RC. Animal models of BMAA neurotoxicity: A critical review. Life Sci. 2008;82:233–246. doi: 10.1016/j.lfs.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Kelly BC, Ikonomou MG, Blair JD, Morin AE, Gobas FAPC. Food web-specific biomagnifications of persistent organic pollutants. Science. 2007;317:236–238. doi: 10.1126/science.1138275. [DOI] [PubMed] [Google Scholar]
- Kitano Y, Okumura M, Idogaki M. Uptake of phosphate ions by calcium carbonate. Geochem. J. 1978;12:29–37. [Google Scholar]
- Kokmen E, Beard CM, O’Bien PC, Offord KP, Kurland LT. Is the incidence of dementing illness changing? Neurol. 1993;43:1887–1892. doi: 10.1212/wnl.43.10.1887. [DOI] [PubMed] [Google Scholar]
- Larsson U, Elmgren R, Wulff F. Eutrophication and the Baltic Sea: Causes and Consequences. Ambio. 1985;14:9–14. [Google Scholar]
- Li WK, Rao DV, Harrison WG, Smith JC, Cullen JJ, Irwin B, Platt T. Autotrophic picoplankton in the tropical ocean. Science. 1983;219:292–295. doi: 10.1126/science.219.4582.292. [DOI] [PubMed] [Google Scholar]
- Lobner D, Piana PM, Salous AK, Peoples RW. β-NMethylamino-l-alanine enhances neurotoxicity through multiple mechanisms. Neurobiol. Dis. 2007;25:360–366. doi: 10.1016/j.nbd.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay D. Correlation of bioconcentration factors. Env. Sci. Tech. 1982;16:274–278. doi: 10.1021/es00099a008. [DOI] [PubMed] [Google Scholar]
- Mash DC. Cyanobacterial toxins in neurodegeneraton. Cont. Life Learn. Neurol. 2008;14:138–149. [Google Scholar]
- Massout L. Cyanobacteria management in aquaculture ponds: A review. In: Charpy L, Larkum AWD, editors. Marine Cyanobacteria. Bulletin de L’Institut Oceanographique 19, Monaco Musee Oceanographique; 1999. pp. 579–584. [Google Scholar]
- Mee LD. The Black Sea in Crisis: A need for concerted international action. Ambio. 1992;21:278–285. [Google Scholar]
- Metcalf JS, Banack SA, Lindsay J, Morrison LF, Cox PA, Codd GA. Co-occurrence of ß-N-methylamino-L-alanine, a neurotoxic amino acid with other cyanobacterial toxins in British waterbodies, 1990-2004. Env. Microbiol. 2008;10:702–708. doi: 10.1111/j.1462-2920.2007.01492.x. [DOI] [PubMed] [Google Scholar]
- Moffat AS. Global nitrogen overload problem grows critical. Science. 1998;279:988–989. [Google Scholar]
- Moore RE. Cyclic peptides and depsipeptides from cyanobacteria: A review. J. Ind. Microbiol. 1996;16:134–143. doi: 10.1007/BF01570074. [DOI] [PubMed] [Google Scholar]
- Murch SJ, Cox PA, Banack SA. A mechanism for slow release of biomagnified cyanobacterial neurotoxins and neurodegenerative disease in Guam. Proc. Nat. Acad. Sci. 2004;101:12228–11131. doi: 10.1073/pnas.0404926101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murch SJ, Cox PA, et al. Occurrence of beta-methylamino-l-alanine (BMAA) in ALS/PDC patients from Guam. Acta Neurol. Scand. 2004;110:267–269. doi: 10.1111/j.1600-0404.2004.00320.x. [DOI] [PubMed] [Google Scholar]
- Nehring D. Eutrophication in the Baltic Sea. In: Vollenweider RA, Marchetti R, Viviani R, editors. Marine Coastal Eutrophication. Elsevier; Amsterdam: 1992. pp. 673–682. [Google Scholar]
- Nixon SW. Coastal eutrophication: A definition, social causes, and future concerns. Ophelia. 1995;41:199–220. [Google Scholar]
- Pablo J, Banack SA, Cox PA, Johnson TE, Papapetropoulos S, Bradley WG, Buck A, Mash DC. Cyanobacterial neurotoxin BMAA in ALS and Alzheimer disease. Acta Neurol. Scand. 2009 doi: 10.1111/j.1600-0404.2008.01150.x. DOI 10.1111/J.1600-0404.2008.01150.x. [DOI] [PubMed] [Google Scholar]
- Paerl H. Nutrient and other environmental controls of harmful cyanobacterial blooms along the freshwater – marine continuum. In: Hudnell HK, editor. Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs. Springer; 2008. pp. 217–237.pp. 950 [DOI] [PubMed] [Google Scholar]
- Paerl HW, Tucker CS. Ecology of blue-green algae in aquaculture ponds. J. World Aq. Soc. 1995;26:109–131. [Google Scholar]
- Pilotto LS. Epidemiology of cyanobacteria and their toxins. In: Hudnell HK, editor. Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs. Springer; 2008. pp. 639–649.pp. 950 [Google Scholar]
- Ramsey AJ, Ball KT. Estimation of algae in New Zealand pasture soil and litter by culturing and by chlorophyll a extraction. N. Z. J. Sci. 1983;26:493–503. [Google Scholar]
- Rao SD, Banack SA, Cox PA, Weiss JH. BMAA selectively injures motor neurons via AMPA/kainate receptor activation. Exp. Neurol. 2006;201:244–252. doi: 10.1016/j.expneurol.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Richardson K, Jorgensen BB. Eutrophication in Coastal Marine Ecosystems. Coastal and Estuarine Studies. Vol. 52. American Geophysical Union; 1996. Eutrophication: Definition, history and effects; pp. 1–19. [Google Scholar]
- Richter KE, Mena EE. L-beta-methylaminoalanine inhibits [3H]glutamate binding in the presence of bicarbonate ions. Brain Res. 1989;492:385–588. doi: 10.1016/0006-8993(89)90925-6. [DOI] [PubMed] [Google Scholar]
- Smith SE, Meldrum BS. Receptor site specificity for the acute effects of beta-N-methylamino-alanine in mice. Eur. J. Pharmacol. 1990;187:131–134. doi: 10.1016/0014-2999(90)90350-f. [DOI] [PubMed] [Google Scholar]
- Shimmel KM, Darley WM. Productivity and density of soil algae in an agricultural system. Ecology. 1985;66:1439–1447. [Google Scholar]
- Sivonen K, Borner T. Bioactive compounds produced by cyanobacteria. In: Herrero A, Flores E, editors. The Cyanobacteria: Molecular Biology, Genomics and Evolution. Caister Academic Press; 2008. pp. 159–197.pp. 484 [Google Scholar]
- Smayda TJ. Novel and nuisance phytoplankton blooms in the sea: Evidence for a global epidemic. In: Graneli E, Sundstrom B, Edler L, Anderson DM, editors. Toxic Marine Phytoplankton. Elsevier Science; 1990. pp. 29–40. [Google Scholar]
- Spencer PS, Nunn PB, Hugon J, Ludolph AC, Ross SM, Roy DN, Robertson RC. Guam amyotrophic lateral sclerosis-Parkinsonism-dementia linked to a plant excitant neurotoxin. Science. 1987;2237:517–522. doi: 10.1126/science.3603037. [DOI] [PubMed] [Google Scholar]
- Stewart I, Seawright AA, Shaw GR. Cyanobacterial poisoning in livestock, wild mammals and birds – an overview. In: Hudnell HK, editor. Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs. Springer; 2008. pp. 613–637.pp. 950 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Billups B, Rossi D, Sarantis M, Hamann M, Attwell D. The role of glutamate transporters in glutamate homeostasis in the brain. J. Exp. Biol. 1997;200:401–409. doi: 10.1242/jeb.200.2.401. [DOI] [PubMed] [Google Scholar]
- Top Z, Brand LE, Corbett RD, Burnett W, Chanton J. Helium as a tracer of groundwater input into Florida Bay. J. Coastal Res. 2001;17:859–868. [Google Scholar]
- Turner RE, Rabalais NN. Changes in Mississippi River water quality this century: Implications for coastal food webs. Bioscience. 1991;41:140–147. [Google Scholar]
- Turner RE, Rabalais NN. Coastal eutrophication near the Mississippi river delta. Nature. 1994;368:619–621. [Google Scholar]
- Vollenweider RA. Coastal marine eutrophication: Principles and control. In: Vollenweider RA, Marchetti R, Viviani R, editors. Marine Coastal Eutrophication. Elsevier; Amsterdam: 1992a. pp. 1–20. [Google Scholar]
- Vollenweider RA. Eutrophication, structure and dynamics of a marine coastal system: results of a ten-year monitoring along the Emilia-Romagna coast (Northwest Adriatic Sea) In: Vollenweider RA, Marchetti R, Viviani R, editors. Marine Coastal Eutrophication. Elsevier; Amsterdam: 1992b. pp. 63–106. [Google Scholar]
- Weiss JH, Choi DW. β-N-methylamino-L-alanine neurotoxicity: requirement for bicarbonate as a cofactor. Science. 1988;241:973–975. doi: 10.1126/science.3136549. [DOI] [PubMed] [Google Scholar]
- Weiss JH, Kaoh JY, Choi DW. Neurotoxicity of beta-N-methylamino-L-alanine (BMAA) and beta-N-oxalylamino-L-alanine (BOAA) on cultured cortical neuron. Brain Res. 1989;497:64–71. doi: 10.1016/0006-8993(89)90970-0. [DOI] [PubMed] [Google Scholar]
- Welker M, von Dohren H. Cyanobacterial peptides – Nature’s own combinatorial biosynthesis. FEMS Microbiol. Rev. 2006;30:530–563. doi: 10.1111/j.1574-6976.2006.00022.x. [DOI] [PubMed] [Google Scholar]
- Whitton BA. Soils and rice fields. In: Whitton BA, Potts M, editors. The Ecology of Cyanobacteria. Kluwer Academic Publishers; 2000. pp. 233–255.pp. 669 [Google Scholar]
- Xie L, Xie P, Guo L, Li L, Miyabara Y, Park H-D. Organ distribution and bioaccumulation of microcystins in freshwater fish at different trophic levels from the eutrophic Lake Chaohu, China. Env. Tox. 2005;20:293–300. doi: 10.1002/tox.20120. [DOI] [PubMed] [Google Scholar]
- Yunger LM, Cramer RD. Measurement and correlation of partition coefficients of polar amino acids. Mole. Pharm. 1981;20:602–608. [PubMed] [Google Scholar]
- Zimba PV. ARS research on harmful algal blooms in SE USA aquaculture impoundments. In: Hudnell HK, editor. Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs. Springer; 2008. pp. 577–578.pp. 950 [Google Scholar]