Abstract
Previously, defined naturally occurring isoforms of allergenic proteins were classified as hypoallergens and therefore suggested as an agent for immunotherapy in the future. In this paper, we report for the first time the molecular background of hypoallergenicity by comparing the immunological behavior of hyperallergenic Betula verrucosa major Ag 1a (Bet v 1a) and hypoallergenic Bet v 1d, two isoforms of the major birch pollen allergen Betula verrucosa 1. Despite their cross-reactivity, Bet v 1a and Bet v 1d differ in their capacity to induce protective Ab responses in BALB/c mice. Both isoforms induced similar specific IgE levels, but only Bet v 1d expressed relevant titers of serum IgGs and IgAs. Interestingly, hypoallergenic Bet v 1d activated dendritic cells more efficiently, followed by the production of increased amounts of Th1- as well as Th2-type cytokines. Surprisingly, compared with Bet v 1a, Bet v 1d-immunized mice showed a decreased proliferation of regulatory T cells. Crystallographic studies and dynamic light scattering revealed that Bet v 1d demonstrated a high tendency to form disulfide-linked aggregates due to a serine to cysteine exchange at residue 113. We conclude that aggregation of Bet v 1d triggers the establishment of a protective Ab titer and supports a rationale for Bet v 1d being a promising candidate for specific immunotherapy of birch pollen allergy.
Type I allergic disorders are an increasingly common disease in western countries, affecting ~25% of the population. Allergic rhinitis, also termed hay fever, is characterized by an inflammation of mucus membranes that is triggered by an IgE-mediated response against innocuous extrinsic allergens, such as pollen or outdoor molds (1). The major allergen for birch pollen allergy is the 17.4-kDa protein Betula verrucosa major Ag 1 (Bet v 1), which shows reactivity with the serum IgE from >62% of all pollinosis patients (2). The genome of birch (Betula verrucosa) encodes multiple isoforms of Bet v 1, forming a diverse set of Bet v 1 proteins in the pollen grain (3, 4). Up to now, 36 different isoforms of Bet v 1 have been characterized, originally alphabetically termed Bet v 1a to Bet v 1n and more recently renamed and enumerated Bet v 1.0101 to Bet v 1.3001 by the International Union of Immunological Societies Allergen nomenclature committee (www.allergen.org). Previously, it was shown that the different isoforms are expressed at different levels and that they differ in their reactivity to bind serum IgE from birch pollen allergic patients, with their potency in T cell activation being preserved (5). On the basis of this reactivity to IgE, Bet v 1 isoforms were divided into hyperallergenic (high IgE reactivity) and hypoallergenic (low IgE reactivity) isoforms, with hypoallergenic variants being proposed for specific immunotherapy for allergic individuals, as this should minimize the risk of side effects (6). This implies that although all isoforms are highly homologous, they must differ in some specific features that induce polarization of the immune system toward IgE synthesis and the development of type I allergies. Evidently, it is of considerable interest to comprehend the biology that accounts for the allergenicity of allergens as an important step for the development of effective therapeutic strategies. Previous attempts to elucidate common allergen-related properties led to the identification of 1) enzymatic activity, 2) specific surface features, and 3) glycosylation patterns, all of which might allow the allergen to target the innate defense system in a way that leads to the induction of a Th2 response with activation of eosinophils, mast cells, and epithelial cells together with the production of IgE (7-9). However, the exact nature of immune recognition of allergens by the innate and adaptive immune system and the specific set of receptors involved in shaping an allergic response is still elusive.
In this work, we aimed to elucidate the allergenic properties of allergenicity of Ags by comparing hyperallergenic Bet v 1a (Bet v 1.0101) and hypoallergenic Bet v 1d (Bet v 1.0401), which differ in only seven amino acid residues. First, we assessed the immunogenic properties of Bet v 1 isoforms. In immunization experiments, both, Bet v 1a and Bet v 1d induced comparable levels of serum IgE, but the hypoallergenic Bet v 1d significantly expressed higher levels of protective serum IgG and IgA Abs. Furthermore, both isoforms exhibited cross-reactivity and comparable IgE-binding properties for the sera from immunized mice. However, the uptake of Bet v 1d by bone marrow-derived dendritic cells (BMDCs) was much more efficient than that of Bet v 1a. This finding is associated with a higher expression of activation markers on BMDCs and accompanied by differences in the capacity to stimulate T cells for proliferation and differentiation in syngeneic coculture experiments. Finally, we determined the three-dimensional structure of Bet v 1d by protein crystallography and found that Bet v 1a and d are very similar and show minor differences in surface potential. However, Bet v 1d is highly prone to form aggregates, stabilized by a disulfide bond because of the serine to cysteine exchange at position 113. From our experiments, we conclude that the allergenicity of an Ag is partially determined by its structural properties and intrinsic features, which dictates its ability to stimulate APCs.
Materials and Methods
Mice
BALB/c mice were obtained from Charles River Laboratories (Wilmington, MA) and C57BL/10ScNcr mice (deletion of the Tlr4 gene) from MaxPlanck-Institut (Freiburg, Germany). Mice were bred and maintained in the animal facility at the University of Salzburg according to the institutional and national guidelines for animal care and use.
Expression and purification of recombinant proteins
Bet v 1a/d and Bet v 1d C113S protein expression (pET28b [Novagen, Madison, WI] in Escherichia coli BL21 (DE3), grown in Luria-Bertani medium (25 mg/L kanamycin) at 37°C to an OD600 of 0.8. After the addition of 0.5 mM isopropyl β-d-thiogalactoside, expression of rBet v 1a was performed for 4 h at 37°C, and expression of rBet v 1d and rBet v 1d C113S was performed at 16°C for 18 h. rBet v 1a was purified under soluble conditions as described previously (10). Soluble rBet v 1d and rBet v 1d C113S were purified from nonclassical inclusion bodies by hydrophobic interaction chromatography using a Phenyl-Sepharose column (GE Healthcare, Vienna, Austria) followed by anion exchange chromatography using a DEAE-Sepharose column (GE Healthcare). Recombinant proteins were dialyzed against 10 mM sodium phosphate buffer (pH 7.4), freeze-dried, and stored at −20°C. Endotoxin content of recombinant proteins was < 3EU / mg protein as determined by Limulus amoebocyte lysate assay (Associates of Cape Cod, East Falmouth, MA). Bet v 1a/d, Bet v 1d C113S, and birch pollen extract (BPE, batch 012596101; Allergon, Ängelholm, Sweden AB) were separated on a 4–12% Bis/Tris SDS-PAGE under reducing/nonreducing conditions, followed by Coomassie staining. Physicochemical parameters of Bet v 1 isoforms were determined as described elsewhere (11). Immunization experiments, Ag uptake, and BMDC stimulation assays as well as dimerization analysis were also conducted with rBet v 1a and d purchased from Biomay (Vienna, Austria), which gave similar results (Bet v 1a: lot no. 22; Bet v 1d: lot no. 08a).
Immunization and serum Ab measurement
BALB/c mice were immunized with 5 μg Bet v 1a/d, Bet v 1d C113S, or BPE formulated with aluminum hydroxide (Serva, Heidelberg, Germany), according to the manufacturer’s instructions. Vaccinations were given as two 100-μl s.c. injections administered bilaterally in the lumbar region and boosted on days 14, 21, and 42. Serum was taken on day 0, 14, 21, 42, and 49, and Ab titers were determined by ELISA and β-hexosaminidase release assays.
β-Hexosaminidase release assay
RBL-2H3 were plated (4 × 104 cells/well) in 96-well and sensitized for 2 h with mouse sera. After washing the cells in Tyrode buffer (0.1% BSA; Life Technologies, Grand Island, NY), cross-linking was induced by 0.3 μg/ml Bet v 1a/d in Tyrode buffer for 30 min at 37°C. β-Hexosaminidase release was measured upon enzymatic cleavage of 4-methylumbelliferyl N-acetyl-β-d-glucosaminide (Sigma-Aldrich, St. Louis, MO) in citrate buffer (0.1 M, pH 4.5) at 360 nm/465 nm. Values are expressed as a percentage of total cellular β-hexosaminidase release after the addition of 1% Triton X-100 (Sigma-Aldrich).
Bet v 1-specific ELISA
Nunc-96-well plates were coated with 250 ng/well rBet v 1a/d, Bet v 1d C113S, and BPE. Sera were diluted in PBS containing 0.1% BSA. alkaline phosphatase (AP)-labeled rat anti-mouse IgE (BD Biosciences, San Jose, CA), mouse anti-human IgE-AP (BD Biosciences), goat anti-mouse IgG1-AP, goat anti-human IgG-AP (Zymed Laboratories, San Francisco, CA), goat anti-mouse IgG2a-AP, and goat anti-mouse IgA-AP (Southern Biotechnology Associates, Birmingham, AL) were added at a concentration of 1 μg/ml in PBS/0.1% BSA. Absorption was measured at 405 nm (492 nm as reference wavelength) after addition of p-nitrophenyl phosphate (Sigma-Aldrich). Results are expressed as arbitrary units in comparison with a pooled standard serum.
Generation and culture of BMDCs
BMDC precursors were harvested from femurs/tibias and were cultured in RPMI 1640 complete medium (10% FBS [Life Technologies], 2 mM l-glutamine, 100 U/ml penicillin, and 0.1 mg/ml streptomycin; PAA, Vienna, Austria) supplemented with 10 ng/ml GM-CSF (PeproTech, Rocky Hill, NJ). Cells were seeded in a concentration of 1 × 106 cells/ml. After 3 d, 50% of the medium was replaced, and the cells were cultured for a total of 7 d. As confirmed by flow cytometry, the population of cells was predominantly CD11c positive.
Proliferation assay
Proliferation assays were initiated 1 wk following the booster vaccination. BMDCs were stimulated overnight with 3 μg/ml of the respective Ag. Erythrocytes were lysed with Pharmlyse (BD Biosciences). CD4+ splenocytes were isolated (CD4+ T Cell Isolation Kit; Miltenyi Biotec, Auburn, CA) subsequently CFSE stained (10 μM; Invitrogen) and coincubated with 1 × 105 Bet v 1a/d-pulsed BMDCs in a 96-well plate in RPMI 1640 complete medium. At day 3, cells were stained according to the manufacturer’s recommendations. For intracellular cytokine staining, cells were preincubated with 1 μg/ml brefeldin A (Sigma-Aldrich) for 4 h prior to staining. The following Abs were used: anti-mouse FoxP3 (clone FJK-16s; eBioscience, San Diego, CA), anti-mouse CD25 allophycocyanin (clone: PC61.5; eBioscience), anti-mouse CD4 FITC (clone RM4-5; eBioscience), anti-mouse CD4 allophycocyanin (clone RM4-5; Biolegend, San Diego, CA), anti-mouse IL-13 PE (clone eBio13A; eBioscience), anti-mouse IFN-γ PE-Cy7 (clone XMG1.2; eBioscience), or matching isotype controls (BD Biosciences/eBioscience) and analyzed by flow cytometry. Supernatants were collected at day 3 and stored at −80°C. Results from three individual mice in triplicate wells were combined to yield a mean ± SD for each immunization group.
In vitro stimulation of BMDC and Ag-uptake assay
BMDCs (2 × 105) were loaded with 1.5 μg/ml Bet v 1a/d-FITC, Bet v 1d C113S-FITC, and OVA-FITC (Sigma-Aldrich) in RPMI 1640 complete medium at 37°C prior to labeling cells on ice with Abs against CD80 PE (clone 16-10A1), CD86 PE (clone GL1), MHC class II (MHC II)(MHC II)-PE (clone M5/114.15.2), CD11c-allophycocyanin or -FITC (clone HL3), or matching isotype controls (all from BD Biosciences) and CD70 PE (clone FR70), OX40 ligand (clone RM134L), IL-6 PE (clone MP5-20F3), and IL-12/IL23p40 PE (clone C15.6) (all from Biolegend). Bet v 1-FITC labeling was done by DRFZ Charité (Berlin, Germany) (FITC/protein labeling ratio: Bet v 1a:3.6/Bet v 1d:3.2). For endocytosis measurements, Bet v 1a/d was conjugated with pHRodo succinimidyle ester (Invitrogen, Carlsbad, CA), according to the instructions of the manufacturer. BMDCs were loaded with 1.5 μg/ml Bet v1a/d – pHRodo, and Ag endocytosis was analyzed as described above.
Cytokine analysis
The supernatants of BMDC-T cell cocultures were collected after 3 d, and specific levels of IL-2, IL-4, IL-5, IFN-γ, and TNF-α were determined using mouse Th1/Th2 cytokine cytometric bead array (CBA) (BD Biosciences) following the manufacturer’s instructions.
Flow cytometry
Data acquisition was performed on a FACSCantoII (BD Biosciences) and analyzed using FlowJo software. Statistical analyses were performed using paired/unpaired Student t test.
Generation of Bet v 1-specific hybridoma
Mice were immunized with a mixture of 2.5 μg Bet v 1a/d. Hybridomas were produced by fusing splenocytes with mouse myeloma cells (X63.Ag8.653). Screening for Ab production and isotype was done on microtiter plates against Bet v 1a/d, and positive clones were expanded by limited dilutions.
Crystallography
The Bet v 1d protein was concentrated to 8 mg/ml by centrifugal ultrafiltration (Millipore, Bedford, MA) and crystallized using the vapor diffusion method. Crystals grew in Hampton Crystal Screen 2 no. 13 (100 mM sodium acetate, 20 mM ammonium sulfate, and 30% Peg-MME 2000 [pH 4.6]). The crystals were measured at 100 K on an in-house rotating anode diffractometer (Bruker) equipped with a MAR345 image plate detector and an oxford cryosystem. Crystals diffracted to 2.80Å and indexed in the monoclinic space group P21 with a = 32.970, b = 57.010, c = 38.930, a = 90.00, b = 92.27, and g = 90.00. Crystallographic data processing was performed with MOSFLM (12) and SCALA. The structure of Bet v 1d (Fig. 8) was determined by the molecular replacement method using program Molrep and the Bet v 1l structure as the search model (Protein Data Bank: 1fm4; Fold & Function Assignment System score −94, sequence identity 97%) (13, 14). Structure refinement and model building was performed in REFMAC5 and O (15). The final model includes one Bet v 1d monomer (residues 3–158), 20 water molecules, and unidentified density in the central cavity. The Bet v 1d structure is very similar to Bet v 1a and superposes with a root mean square deviation of 0.36Å. Structural differences are within the crystallographic error because of the lower resolution of Bet v 1d. The Ramachandran plot, produced by Procheck 3.4, shows that 96.8% of the residues are in the most favored regions and 3.2% in additional allowed regions. Model quality and rotamers were checked with NQ Flipper (16). Figures were prepared with PYMOL (DeLano Scientific, Palo Alto, CA). (Coordinates and structure factors have been deposited at Protein Data Bank [www.pdb.org] with the accession number 3K78.)
FIGURE 8.
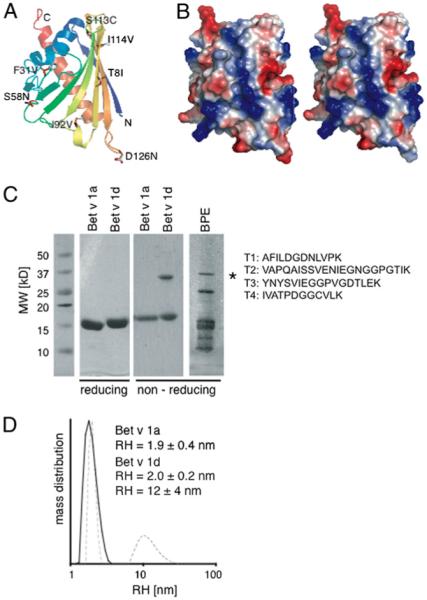
Crystal structure of Bet v1d. A, Ribbon diagram color-coded from N terminus (blue) to C terminus (red) showing residues that differ between isoforms Bet v 1a and Bet v 1d in sticks. B, Electrostatic surface potential map (blue positive, red negative) of Bet v 1a left and Bet v 1d right. C, Coomassie brilliant blue-stained reducing and nonreducing SDS-PAGE of Bet v 1a and d and aqueous BPE. Bands corresponding to dimerized Bet v 1 are marked with an asterisk. The band from BPE corresponding to dimeric Bet v 1 was analyzed by nanoliquid chromatography-tandem mass spectrometry–based sequencing. Four Bet v 1 derived peptides were identified (T1, A22–K33; T2, V34–K55; T3, Y82–K98; T4, I105–K116), with T1, T3, and T4 being specific for Bet v 1d. D, DLS of Bet v 1a (solid black line) versus Bet v 1d (dashed gray line) in RPMI 1640 at 37°C (RH, hydrodynamic radius).
Mass spectrometry-based protein identification
Twenty micrograms of aqueous BPE separated on SDS gel (Bio-Rad, Hercules, CA). A protein band migrating at height of dimeric rBet v 1d (34 kDa) was cutout and digested using the ProteoExtract Trpysin Digestion Kit (Calbiochem, San Diego, CA). Tryptic peptides were separated by reversed-phase capillary HPLC coupled with an electrospray ionization–quadrupole time-of-flight mass spectrometer (CapLC-QT of Ulitma Global, Micromass-Waters) as described previously (17). Resulting double mass spectrometry data were searched against SwissProt knowledgebase release of January 2008 using the ProteinLynx Global Server 2.2.5 software package (Waters, Milford, MA) with automatic data validation.
High-performance–size exclusion chromatography and dynamic light scattering
Homogeneity and aggregation behavior of Bet v 1a and Bet v 1d in solution were analyzed by high-performance–size exclusion chromatography online coupled with a TDA302 (Viscotek, Houston, TX) right-angle light scattering detector and by dynamic light scattering (DLS) on a DLS802 (Viscotek) system upon centrifugation for 10 min at 14,000 × g as described previously (11).
Results
Bet v 1 isoforms differ in their capacity to induce IgG1, IgG2a, and IgA responses in mice, which is independent of their relative abundance in pollen
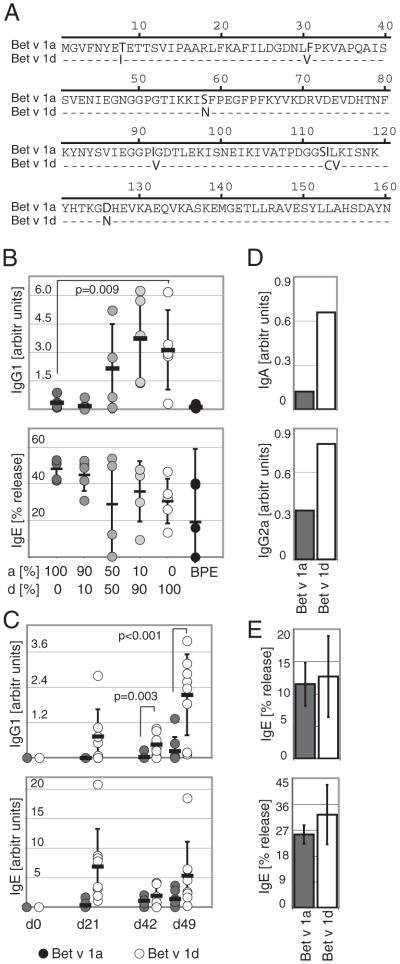
Previous experiments indicated an intrinsic difference of Bet v 1 isoforms in their capacity to induce the production of serum IgE in humans (5). To exclude the possibility of allergenicity being simply a matter of differences in the relative abundance of the two isoforms, we first investigated the humoral response toward hyperallergenic Bet v 1a and hypoallergenic Bet v 1d in mice using a standardized immunization regimen. We primary immunized mice s.c. with either recombinant Bet v 1a, Bet v 1d, defined mixtures of Bet v 1a/d, or BPE, followed by three booster immunizations and determined specific serum Ab titers using ELISA and β-hexosaminidase secretion assays. In both assays, serum IgE titers did not significantly differ between Bet v 1a and Bet v 1d-immunized animals (Fig. 1B, lower graph, for RBL assay: 100% Bet v 1a, 48.1 ± 5.6%; 100% Bet v 1d, 30.5 ± 12.2%; and Fig. 1C, lower graph, for specific ELISA: day 0, 0.0 ± 0.0; day 21, 0.3 ± 0.7 versus 6.9 ± 6.3; day 42, 1.1 ± 0.7 versus 1.9 ± 1.8; day 49, 1.4 ± 1.2 versus 5.3 ± 5.7). The IgE levels shown in Fig. 1C, lower panel, were also analyzed by hexosaminidase release assay (Fig. 1E), proving that both assays yield to comparable results (Fig. 1E, upper graph: IgE levels at day 42, 11.48 ± 3.36% [Bet v 1a] versus 12.67 ± 6.27% [Bet v 1d]; lower graph: IgE levels at day 49: 25.44 ± 3.20% [Bet v 1a] versus 32.35 ± 10.33% [Bet v 1d]).
FIGURE 1.
Specific Ab production toward Bet v 1a and d in mice. A, Sequence alignment of Bet v 1a and Bet v 1d. Different residues are indicated in bold. B, Mice were immunized with different mixtures of rBet v 1a and Bet v 1d or with BPE. The composition of each Bet v 1a/d mixture used for immunization is indicated. Specific IgG1 and IgE serum titers were determined by ELISA and β-hexosaminidase release assay at day 49, using an equimolar mixture of Bet v 1a/d as specific Ag. C, Mice were immunized with either Bet v 1a (dark gray circles) or Bet v 1d (○). Specific IgG1 and IgE serum titers were determined on days 0, 21, 42, and 49. (D) Specific IgA and IgG2a titers were determined from pooled sera of day 49 from mice described in C. E, Specific IgE titers from mice described in C were determined by β-hexosaminidase release assay on day 42 (upper panel) and day 49 (lower panel). Titers were determined from individual mice and mean values are shown as bars ± SD. B–D, Each mouse is represented by a circle (n = 5 or 8/group), and pooled sera are shown as bars. Values are shown as arbitrary units in comparison with a pooled standard serum. Mean values are given as dash ± SD; values of p are indicated in graph (unpaired Student t test).
However, specific IgG1, IgG2a, and IgA titers were much higher in Bet v 1d-immunized mice (Fig. 1C upper graph, for specific IgG1: day 0, 0.0 ± 0.0; day 21, 0.7 ± 0.9 versus 0.0 ± 0.0; day 42, 0.5 ± 0.4 versus 0.03 ± 0.1; day 49, 2.1 ± 1.4 versus 0.2 ± 0.5; Fig. 1D for specific IgA: 0.66 versus 0.12; and specific IgG2a: 0.79 versus 0.34).
Accordingly, we observed a tight correlation between the magnitude of the Bet v 1-specific IgG1 response and the ratio of Bet v 1d to Bet v 1a used for immunization (Fig. 1B, upper graph; 0% Bet v 1d, 0.351 ± 0.310; 10% Bet v 1d, 0.175 ± 0.261; 50% Bet v 1d, 2.159 ± 2.323; 90% Bet v 1d, 3.735 ± 2.091; 100% Bet v 1d, 3.127 ± 2.091; BPE, 0.123 ± 0.095). Immunization experiments with BPE consistently showed that the far more abundant occurrence of Bet v 1a in natural Bet v 1 (Bet v 1a: Bet v 1 d = 35%: 10% [5]) led to an equally low IgG1 response as observed for Bet v 1a-immunized animals (BPE, 0.123 ± 0.095) (Fig. 1B, upper graph).
Cross-reactivity of Bet v 1 isoforms is comparable for IgG1 and IgE Abs
Next we tested the cross reactivity of serum Abs toward Bet v 1a and Bet v 1d. Interestingly, we found that IgG1 and IgE Abs from Bet v 1a- or Bet v 1d-immunized mice were comparably reactive with both isoforms (Fig. 2: Bet v 1 a/d cross-reactive IgG1 of Bet v 1a-immunized mice, 0.2 ± 0.5 versus 0.4 ± 0.5 and Bet v 1dimmunized mice, 2.1 ± 1.4 versus 2.4 ± 1.1, respectively; Bet v 1a/d cross-reactive IgE of Bet v 1a-immunized mice, 22.6 ± 3.2 versus 21.8 ± 1.9 and Bet v 1d-immunized mice, 25.9 ± 2.2 versus 25.4 ± 1.8, respectively).
FIGURE 2.
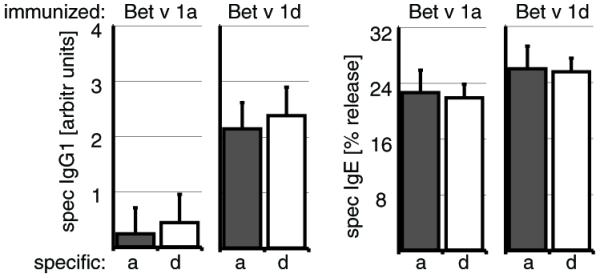
The sera from immunized mice are highly cross-reactive for Bet v 1a and Bet v 1d. Mice were immunized with either Bet v 1a (left panel) or Bet v 1d (right panel) as described in Fig. 1D, and specific serum IgG1 and IgE titers were analyzed for reactivity toward Bet v 1a (dark gray) or Bet v 1d (□) at day 49 postimmunization by ELISA and β-hexosaminidase release assay, respectively. Values are shown as arbitrary units in comparison with a standard serum. (n = 5/group).
To more precisely elucidate whether cross-reactivity is either based on serum Abs that bind both isoforms or on a mixture of Bet v 1a- or Bet v 1d-specific Abs, we screened for hybridomas from mice immunized with a mixture of Bet v 1a and Bet v 1d. Analysis of five specific clones (isotypes: IgG1 and IgM) revealed that all mAbs tested reacted with both isoforms to a similar extent (Supplemental Table I).
We next asked whether cross-reactivity is restricted to our murine model or whether it is also observed in the human serum. We therefore analyzed serum IgE and IgG from four allergic individuals for specific binding toward recombinant Bet v 1a or Bet v 1d using ELISA. Again, we could clearly show that also serum Abs from all tested allergic individuals react to a similar extent with both Bet v 1 isoforms (Supplemental Fig. 1), proving that data gained from our murine model can be applied to the human system (Supplemental Fig. 1: Bet v 1a and Bet v 1d-specific IgG levels, 0.410 ± 0.2 versus 0.348 ± 0.06; Bet v 1a- and Bet v 1d-specific IgE levels, 0.615 ± 0.35 versus 0.768 ± 0.54) (Supplemental Fig. 1).
Bet v 1 isoforms display distinct patterns of uptake by BMDCs
In order to understand the striking difference in IgG and IgA production between Bet v 1a- and Bet v 1d-immunized mice, we analyzed the response of DCs toward the two Bet v 1 isoforms. We generated mouse BMDCs and incubated them at physiological temperature with FITC-conjugated Bet v 1a and FITC-conjugated Bet v 1d, respectively. At defined time points, we stained the BMDCs on ice for the DC marker CD11c and determined the amount of FITC-conjugated Bet v 1a and d captured by BMDCs using flow cytometry. As shown in Fig. 3A, uptake of FITC-Bet v 1d occurred at much higher rate than of Bet v 1a. Upon incubation for 5 h, 17.2 ± 2.6% of the CD11c+ BMDCs were Bet v 1a positive as opposed to 32.4 ± 2.5% for Bet v 1d (p < 0.001). At 16 h of incubation, the difference in Ag uptake was even higher (13.2 ± 0.9% for Bet v 1a versus 35.8 ± 2.9% for Bet v 1d, p < 0.001; n = 9). LPS contamination of the recombinant Bet v 1 isoforms was excluded by limulus-assays (<3 EU/mg protein; data not shown). Nevertheless, also we performed the Bet v 1-uptake experiment with BMDCs from C57BL/10ScNCr mice, which lack the expression of TLR4, the innate receptor for LPS. Again, the uptake of Bet v 1a was significantly slower than the uptake of Bet v 1d, implying that LPS contamination and subsequent TLR4 activation is not responsible for the observed results (Fig. 3B: t = 5 h, 32.9 ± 3.5% Bet v 1a versus 52.3 ± 5.8% Bet v 1d, p = 0.004; t = 26 h, 12.0 ± 1.8% Bet v 1a versus 22.6 ± 2.1% Bet v 1d, p = 0.001; n = 3).
FIGURE 3.
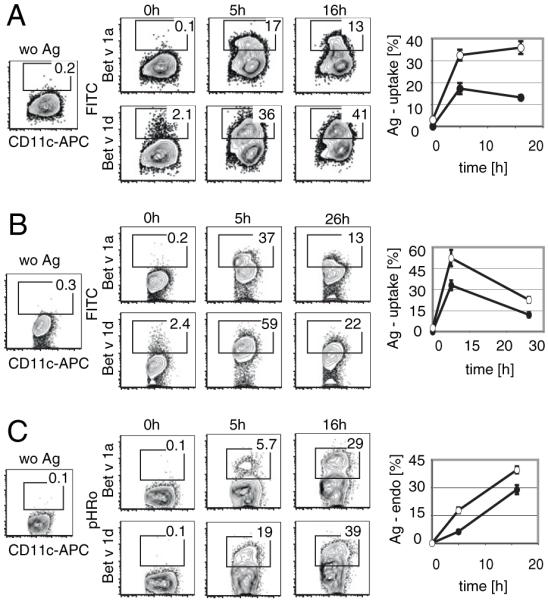
Ag uptake of BMDCs. A, BMDCs (BALB/c) were incubated with either FITC-labeled Bet v 1a or Bet v 1d and uptake of Ag was determined. A representative FACS profile of BMDCs is shown. Cells were pregated for live cells, and the percentage of Bet v 1a-FITC– or Bet v 1d-FITC–positive cells among the CD11c-positive population is indicated within each plot. B, BMDCs from C57BL/10ScNcr mice were incubated with FITC-labeled Bet v 1a or Bet v 1d and assayed as described in A. C, BMDCs from BALB/c mice were incubated with pHRodo-conjugated Bet v 1a or Bet v 1d, and internalization of specific Ag was monitored. Shown is the percentage of Bet v 1a (●)- and Bet v 1d (○)-positive cells at 0, 5, and 16 or 26 h postincubation with specific Ag (n = 5). Mean ± SD; values of p (unpaired Student t test) are indicated in graph.
Additionally, we measured BMDC-mediated endocytosis of Bet v 1a and Bet v 1d after conjugation with pHRodo, a rhodamine-based dye that is fluorescent at acidic environment, thus, being suitable for measuring endocytosis. As shown in Fig. 3C, the endocytosis of Bet v 1d-pHRodo is much more efficient than of Bet v 1a-pHRodo reflected in a higher percentage of pHRodo-positive BMDCs 5 and 16 h postaddition of Ag (t = 5 h, 6.3 ± 1.0% Bet v 1a versus 17.8 ± 1.6% Bet v 1d, p < 0.001; t = 16 h, 28.9 ± 2.5% Bet v 1a versus 39.6 ± 1.9% Bet v 1d, p < 0.001; n = 5).
We also compared uptake of Bet v 1a/d with that of OVA as a model Ag (Supplemental Fig. 2). Therefore, we used OVA-FITC to stimulate BMDCs and determined the fraction of FITC-positive BMDCs after 26 h. We found that OVA was captured at similar efficacy as Bet v 1a, with 31.2 ± 2.2% of the BMDCs being positive for OVA-FITC at t = 26 h, 32.74 ± 2.4% in case of Bet v 1a-FITC (p = 0.2) and 48.12 ± 3.2% for Bet v 1d-FITC (p < 0.001) (Supplemental Fig. 2A). Also, expression of CD80 and MHC II on OVA-FITC–positive BMDCs was comparable with Bet v 1a (Supplemental Fig. 2B: CD80, 48.2 ± 8.3% [OVA] versus 42.9 ± 6.3% [Bet v 1a] p = 0.2 versus 85.1 ± 2.9% [Bet v 1d] and MHC II, 3.6 ± 0.9% [OVA] versus 5.6 ± 2.2% [Bet v 1a] p = 0.1 versus 13.3 ± 1.2% [Bet v 1d]).
The expression of activation markers and costimulatory cytokines in BMDCs is different depending on the Bet v 1 isoform used for stimulation
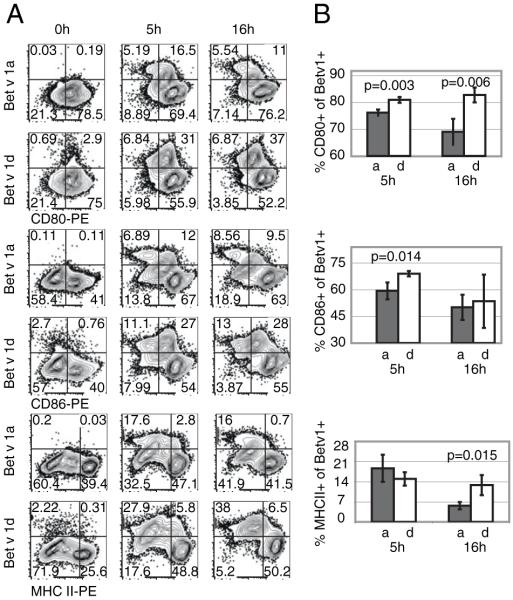
To further evaluate the pattern of BMDC activation in dependence on the Bet v 1 isoform, we examined the expression of activation markers CD80, CD86, and MHC II using flow cytometry. We incubated BMDCs for 0, 5, and 16 h with FITC-Bet v 1a or FITC-Bet v 1d at 37°C and subsequently determined the expression of CD80, CD86, and MHC II using PE-conjugated specific Abs. We observed significant differences in the expression profile of activation markers (Fig. 4). After 5 h of incubation with specific Ag, significantly fewer BMDCs were positive for CD80 (76.2 ± 1.1%) and CD86 (59.3 ± 4.7%,) when stimulated with Bet v 1a as opposed to Bet v 1d-stimulated BMDCs (80.9 ± 1.1% and 69.0 ± 1.5%, respectively; p = 0.003 and p = 0.014). After 16 h, significantly higher MHC II and CD80 expression levels were measured for Bet v 1d-pulsed BMDCs (12.8 ± 3.5% and 5.7 ± 1.3%; p = 0.015; 82.8 ± 2.8% and 69.0 ± 4.8%, respectively; p = 0.006). Thus, Bet v 1d induces higher expression rates for all three measured activation markers.
FIGURE 4.
Expression of activation markers by BMDCs upon incubation with Bet v 1a and Bet v 1d. BMDCs were incubated with FITC-conjugated Bet v 1a or d. At different time points (t = 0, 5, and 16 h), cells were stained for CD80, CD86, or MHC II. Cells were gated for CD11c expression, and the amount of CD80, CD86, and MHC II expression was determined. A, A representative FACS profile of CD11c-positive cells. The percentage of cells within each quadrant is indicated. B, Statistical analysis of three independent experiments. Shown are the percentage of CD80 (upper graph)-, CD86 (middle graph)-, and MHC II (lower graph)-expressing cells within the Bet v 1a/d-FITC–positive BMDCs at 5 and 16 h postincubation with specific FITC-conjugated Ag. Mean ± SD; values of p are indicated in graph (unpaired Student t test).
As CD80/86-independent costimulation of T cells can be achieved by the TNF-family members OX40L and CD70 on APCs (18), we also evaluated expression levels of these costimulatory molecules on BMDCs upon stimulation with either Bet v 1a or Bet v 1d. As shown in Supplemental Fig. 3, we could not detect any differences in OX40L or CD70 surface levels on BMDCs.
To more precisely determine BMDC activation, we also analyzed cytokine production upon Bet v 1 isoform-dependent BMDC stimulation. We therefore incubated BMDCs for 26h with FITC-Bet v 1a or d at 37°C and subsequently determined intracellular levels of IL-6 and IL-12/23 by flow cytometry. Although levels of intracellular IL-12/23 in BMDCs were comparable between the differentially stimulated BMDCs, we interestingly observed an increase in IL-6 production upon incubation of BMDCs with Bet v 1a. Within the Bet v 1a-FITC–positive fraction, 8.93 ± 0.31% of BMDCs were positive for IL-6, whereas only 3.87 ± 0.72% (p = 0.002) of the Bet v 1d-FITC–positive BMDCs were IL-6 positive (Fig. 5).
FIGURE 5.
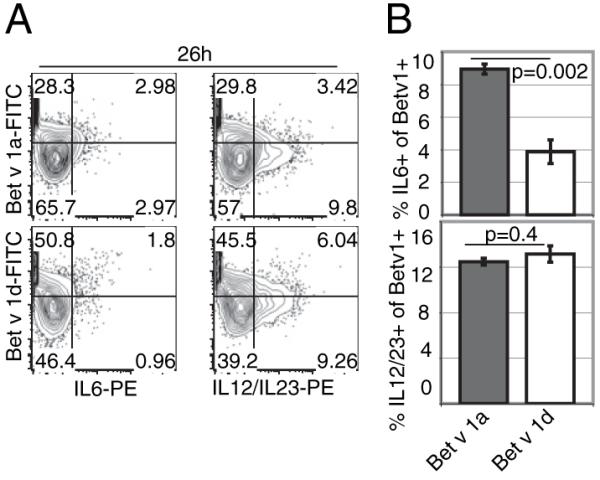
IL-6 and IL-12/23 cytokine production by BMDCs upon incubation with Bet v 1a and Bet v 1d. BMDCs were incubated with FITC-conjugated Bet v 1a or d. After 26 h, cells were stained for intracellular IL-6 and IL-12/23 and anti-mouse CD11c Ab. Cells were gated for CD11c expression, and the amount of IL-6 and IL-12/23 production was determined. A, A representative FACS profile is shown. The percentage of cells within each quadrant is indicated. B, Statistical analysis of three independent experiments. Shown is the percentage of IL-6– and IL-12/23–expressing cells within the Bet v 1a/d-FITC–positive BMDCs at 26 h postincubation with specific Ag. Mean ± SD; values of p are indicated in graph (unpaired Student t test).
Bet v 1a and Bet v 1d differ in their capacity to induce BMDC-mediated T cell proliferation and differentiation in vitro
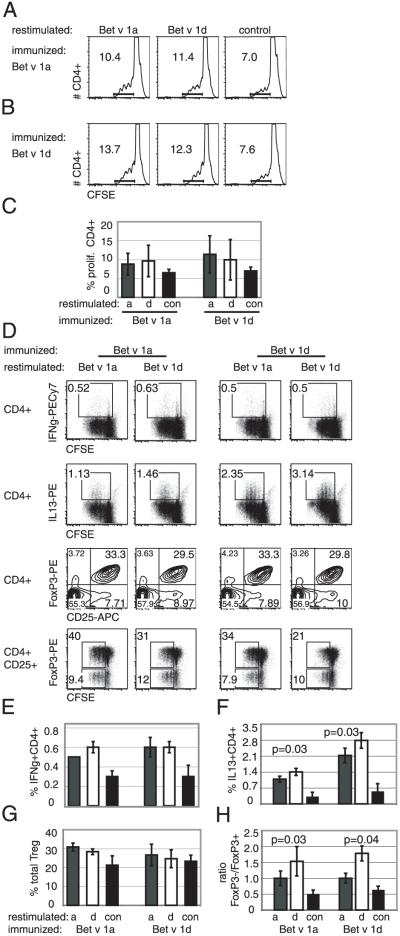
We next asked, whether the distinct pattern of BMDC activation affects the proliferation of T cells in syngeneic BMDC–T cell coculture assays. MACS-sorted splenic CD4 Th cells from either Bet v 1a- or Bet v 1d-immunized mice were CFSE loaded and coincubated with Bet v 1a- or Bet v 1d-pulsed BMDCs. Interestingly, the elevated expression of activation markers on Bet v 1d-loaded BMDCs did not accompany with an overall increase of specific CD4+ T cell proliferation. When CD4 T cells from Bet v 1a-immunized animals were used, a substantial percentage of CD4 T cells proliferated in response to Bet v 1a-pulsed BMDCs (8.8 ± 2.9%) as well as to Bet v 1d-pulsed BMDCs (9.6 ± 4.2%) (Fig. 6A, 6C). Vice versa, CD4 T cells from Bet v 1d-immunized animals proliferated comparably in response to Bet v 1a (11.4 ± 4.9%) and Bet v 1d-pulsed BMDCs (9.9 ± 5.3%) (Fig. 6B, 6C) Without specific Ag, 6.5 ± 0.9% (T cells from Bet v 1a-immunized mice) and 6.9 ± 1.0% (T cells from Bet v 1d-immunized mice) of T cells proliferated (Fig. 6C).
FIGURE 6.
Different proliferation of specific Th1, Th2, and Tregs in coculture experiments with Bet v 1a- or Bet v 1d-pulsed BMDCs. A, BMDCs were incubated with specific Ag (Bet v 1a, left panel; Bet v 1d, middle panel; or no Ag, right panel) and were cocultered with CFSE-labeled syngeneic CD4+ T cells purified from Bet v 1a (A)- or Bet v 1d (B)-immunized mice. Proliferation of Bet v 1-specific T cells was determined after 3 d as decrease of CFSE intensity within the CD4-positive cell population as indicated in each histogram. Representative FACS profiles from four independent experiments are shown (C); statistical analysis of four independent experiments. D, BMDCs were incubated with specific Ag (restim. Bet v 1a or Bet v 1d) and were cocultered with CFSE-labeled syngeneic CD4+ T cells purified from Bet v 1a (left panel)- or Bet v 1d (right panel)-immunized mice (imm. Bet v 1a or Bet v 1d). After 3 d, proliferation of T cells was determined, and differentiation into Th cell subsets was analyzed using staining with specific Abs (Treg: CD4, CD25, FoxP3; Th1: IFN-γ; Th2: IL-13). Representative FACS profiles show the distribution and proliferation of the different Th subsets. E–H, Statistical analysis of experiments from D; H, ratio of proliferating CD4+ CD25+FoxP3− to CD4+CD25+FoxP3+ T cells is shown. Proliferating cells were defined as cells within the rectangular gates of FACS plots from bottom panel in D; n =6. Mean ± SD; values of p indicated in graph (Wilcoxon test).
To analyze in vitro differentiation into distinct Th1, Th2, and regulatory T cell (Treg) subsets, we additionally stained the cells from the coculture experiment with specific Abs against IFN-γ, IL-13, and CD25/FoxP3. The amount of IFN-γ–producing Th1 cells was slightly higher for T cells from Bet v 1a-immunized mice cocultured with Bet v 1d-stimulated BMDCs (0.6 ± 0.04% versus 0.5 ± 0.0%; p = 0.1 using Wilcoxon test) (Fig. 6D, upper panel, 6E). Most interestingly, the de novo generation of IL-13–positive Th2 cells was heightened significantly by Bet v 1d-pulsed BMDCs and peaked at four to five cell divisions (Bet v 1a-immunized mice: 1.4 ± 0.2% IL13+CD4+ cells for BMDCs restimulated with Bet v 1d versus 1.1 ± 0.1% IL13+CD4+ cells for BMDCs restimulated with Bet v 1a [p = 0.03 using Wilcoxon test] versus 0.3 ± 0.2% IL13+ CD4+ cells for unstimulated BMDCs); Bet v 1d-immunized mice, 2.8 ± 0.3% IL13+CD4+ cells for BMDCs restimulated with Bet v 1d versus 2.1 ± 0.3% IL13+CD4+ cells for BMDCs restimulated with Bet v 1a [p = 0.03 using Wilcoxon t test] versus 0.6 ± 0.3% IL13+ CD4+ cells for unstimulated BMDCs; Fig. 6D, second panel, 6F).
Conversely, a slightly higher total amount of Tregs was observed when Bet v 1a was used for loading BMDCs in our in vitro assay in comparison with loading with Bet v 1d (30.9 ± 2.1% versus 28.4 ± 1.5%; [p = 0.1] with T cells from Bet v 1a-immunized mice and 26.7 ± 5.7% versus 24.7 ± 4.7%, [p = 0.1] with T cells from Bet v 1d-immunized mice, respectively; Fig. 6D, third panel, 6G).
However, a significantly higher proliferation of specific FoxP3+CD25+ Tregs was achieved when BMDCs were loaded with Bet v 1a as opposed to Bet v 1d; (Fig. 6D, bottom panel), reflected in a lower ratio of FoxP3−/FoxP3+–proliferating CD25+ cells (Bet v 1a-immunized mice: 1.0 ± 0.2 versus 1.5 ± 0.5, p = 0.03; Bet v 1d-immunized mice: 1.0 ± 0.2 versus 1.8 ± 0.2, p = 0.04; controls: 0.5 ± 0.1 and 0.6 ± 0.1;values of p determined by Wilcoxon-test) (Fig. 6H).
In addition, we determined the cytokine profile from the supernatants of the coculture experiments using Th1/Th2 CBA. In T cells from Bet v 1a-immunized mice we observed an increase of IFN-γ, IL-4, and IL-5 in the coculture supernatants when restimulated with Bet v 1d (IFN-γ, 26.0 ± 2.9 pg/ml versus 9.8 ± 1.1 pg/ml; IL-4, 7.4 ± 1.0 pg/ml versus 5.6 ± 1.0 pg/ml; IL-5, 202.3 ± 22.8 pg/ml versus 107.3 ± 14.0 pg/ml), whereas comparable amounts of TNF-α (60.4 ± 10.7 pg/ml versus 53.2 ± 11.5 pg/ml) and IL-2 (14.8 ± 4.8 pg/ml versus 15.1 ± 4.4 pg/ml) were determined. In coculture experiments using T cells from Bet v 1d-immunized mice, all cytokines examined were significantly increased using Bet v 1d-pulsed BMDCs (Fig. 7: TNF-a, 101.9 ± 3.6 pg/ml versus 77.5 ± 9.8 pg/ml; IFN-γ, 96.0 ± 35.4 pg/ml versus 32.5 ± 11.5 pg/ml; IL-5, 490.2 ± 32.4 pg/ml versus 287.2 ± 18.8 pg/ml; IL-4, 49.8 ± 24.5 pg/ml versus 18.1 ± 7.6 pg/ml and IL-2, 71.6 ± 15.3 pg/ml versus 16.0 ± 5.2 pg/ml; n = 4).
FIGURE 7.
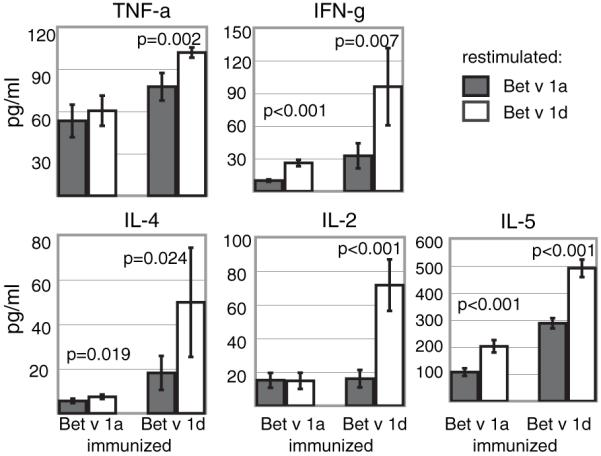
Cytokine production of T cells primed by Bet v 1a- or Bet v 1d-pulsed BMDCs. Cell culture supernatants from coculture experiments described in Fig. 5 were analyzed for the presence of specific cytokines using CBA assays. Shown are absolute amounts of the respective cytokine (mean ± SD; n = 4). (Abbreviations used as in Fig. 5.)
We conclude that the overall immune response to Bet v 1d, reflected by DC Ag uptake and presentation as well as subsequent T cell proliferation and cytokine secretion, is faster and more prominent compared with Bet v 1a.
Protein crystallography of Bet v 1d reveals an overall three-dimensional structure similar to Bet v 1a with minor differences in electrostatic surface potential and an increased ability to form aggregates
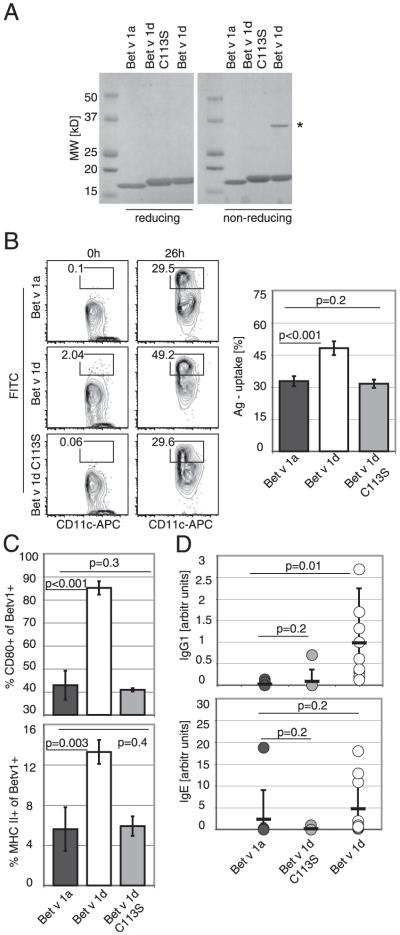
To explore whether structural changes are responsible for the observed differences in BMDC stimulation, we determined the three-dimensional structure of Bet v 1d by X-ray crystallography and compared it with the previously published structure of Bet v 1a (19). As expected, the crystal structures of Bet v 1 isoforms a and d are very similar, because all amino acid exchanges can be easily accommodated in the structure (Fig. 8A). This is not surprising given the fact that both isoforms are naturally occurring in pollen. Analysis of the electrostatic surface potential indicates that mutation D126N results in a small reduction of negative surface potential in Bet v 1d (Fig. 8B). Although, this small change may alter affinity and recognition of Bet v 1d by Abs specific for Bet v 1a, it is very unlikely to be the main cause for the reduced allergenicity of Bet v 1d. In fact, we did not detect a difference in Ab recognition between the two isoforms. Furthermore, we investigated protein stability and aggregation behavior of Bet v 1a and d at conditions resembling immunological experiments. SDS-PAGE analysis revealed that Bet v 1d shows an increased tendency for self-aggregation. Coomassie brilliant blue staining of recombinant isoforms showed clear bands at 17 and 34 kDa corresponding to monomeric and dimeric protein only for Bet v 1d but not for Bet v 1a (Fig. 8C).
Dimeric Bet v 1d could also be identified in native BPEs (Fig. 8C). Using mass spectrometry, three of four peptides were exclusively of Bet v 1d origin (T1, AFILDGDNLVPK; T3, YNYSVIEGGPVGDTLEK; T4, IVATPDGGCVLK; T1 corresponds to amino acids A22–K33, T3 corresponds to amino acids Y82–K98, and T4 corresponds to amino acids I105–K116; amino acid sequences of Bet v 1a/d are shown in Fig. 1A). The fourth peptide (T2, VAPQAISSVENIEGNGGPGTIK) could potentially derive from both isoforms. Despite the far higher abundance of Bet v 1a within BPEs, no Bet v 1a-specific peptide could be detected in the 34-kDa dimeric fraction.
Because dimeric Bet v 1d is not observed under reducing conditions, Bet v 1d forms disulfide-linked dimers because of the serine to cysteine exchange at position 113. Structural analysis showed that Cys113 is not surface exposed and thus only available for disulfide formation when Bet v 1d is at least partly unfolded. Online high-performance–size exclusion chromatography–light scattering, and DLS experiments revealed a higher polydispersity of Bet v 1d when compared with Bet v 1a indicating formation of aggregates. This elevated aggregation tendency was even more pronounced in the RPMI 1640 medium used for BMDC-uptake experiments (Fig. 8D). Consequently, increased aggregation due to stabilization of unfolded Bet v 1d contributes to enhanced BMDC activation.
Remutation of Cys113 of Bet v 1d abrogates dimerization and reverts Bet v 1d-specific immunological differences to Bet v 1a.
To show that increased aggregation, accompanied by altered immunologic properties correlates with Cys113, we remutated Cys113 to serine and generated protein Bet v 1d[Cys113Ser]. Indeed, analysis by SDS-PAGE revealed that the purified Bet v 1d[Cys113Ser] has lost its capacity to form homodimers (Fig 9A).
FIGURE 9.
Mutation of Cys113 of Bet v 1d abrogates dimerization and reverts Bet v 1d-specific immunological properties. A, Bet v 1a, Bet v 1d, and Bet v 1d[Cys113Ser] were loaded on a reducing or nonreducing SDS-PAGE. The asterisk marks dimeric Bet v 1d. B, Ag uptake by BMDCs. BMDCs were incubated with FITC-Bet v 1a, FITC-Bet v 1d, or FITC-Bet v 1d[Cys113Ser]. One representative FACS profile is shown. Statistical analysis is given as mean ± SD; values of p are indicated in graph (n = 6). C, Expression of activation markers by BMDCs. Cells were gated for CD11c expression and the amount of CD80 and MHC II surface expression was determined. The graph shows the percentage of CD80 and MHC II-expressing cells within the Bet v 1-FITC–positive BMDCs. Mean ± SD; values of p are indicated in graph (unpaired Student t test) n = 3. D, Specific Ab response in mice. Mice were immunized with either Bet v 1a (dark gray circles), Bet v 1d[Cys113Ser] (light gray circles) or Bet v 1d (○) and boosted 2 wk after primary immunization. Serum was taken at day 21, and specific IgG1 and IgE serum titers were determined (n = 7–10). Values are shown as arbitrary units in comparison with a pooled standard serum. Mean values are given as dash ± SD; values of p are indicated in graph (unpaired Student t test).
Additionally, we tested the mutant protein Bet v 1d[Cys113Ser] for uptake by BMDCs (Fig 9B) and determined an uptake efficacy of Bet v 1d[Cys113Ser] resembling that of Bet v 1a (Bet v 1a 32.7 ± 2.4% versus Bet v 1d 48.1 ± 3.2%, p < 0.001 versus Bet v 1d[Cys113Ser] 31.3 ± 1.9%, p = 0.2). Also analysis of the activation markers on stimulated BMDCs indicated that Bet v 1d[Cys113Ser] is comparable to Bet v 1a (CD80, 42.9 ± 6.3% for Bet v 1a, 85.1 ± 2.9% for Bet v 1d [p < 0.001] and 40.9 ± 0.7% for Bet v 1d[Cys113Ser] [p = 0.3]; MHC II, 5.6 ± 2.2% for Bet v 1a, 13.3 ± 1.2% for Bet v 1d [p = 0.003] and 5.9 ± 1% for Bet v 1d[Cys113Ser] [p = 0.4]) (Fig. 9C).
Finally, we immunized mice with Bet v 1d[Cys113Ser] and compared specific serum IgG1 and IgE levels to Bet v 1a- and Bet v 1d-immunized mice on day 21. Intriguingly, immunization with Bet v 1d[Cys113Ser] completely impeded the generation of high titers of specific serum IgG1, which we found characteristic for hypoallergenic Bet v 1d. Conversely, specific IgE levels remained comparable between Bet v 1a-, Bet v 1d-, and Bet v 1d[Cys113Ser]-immunized mice (Fig. 9D; Bet v 1-specific IgG1, 0.03 ± 0.05 for Bet v 1a versus 0.76 ± 0.81 for Bet v 1d [p = 0.01] versus 0.12 ± 0.34 for Bet v 1d[Cys113Ser] [p = 0.2]; Bet v 1-specific IgE, 2.4 ± 6.6 for Bet v 1a versus 5.8 ± 7.1 for Bet v 1d [p = 0.2] versus 0.2 ± 0.4 for Bet v 1d[Cys113Ser] [p = 0.2]).
We conclude that disulfide-based aggregation of Bet v 1d strongly increases immunogenicity and its potency to elicit a protective IgG1 response (Fig. 10).
FIGURE 10.
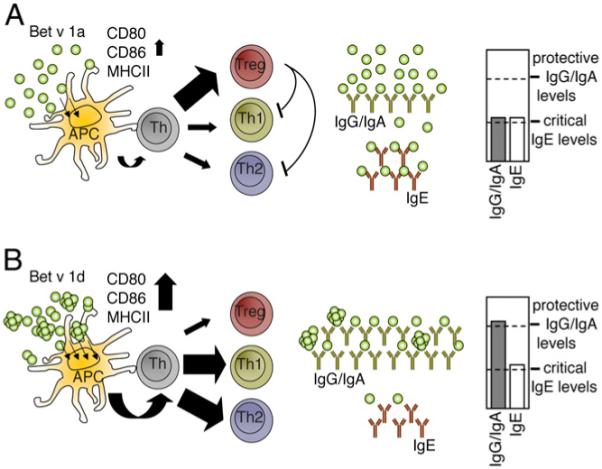
Illustration of the Ab response toward Bet v 1a (A) and Bet v1d (B). A, Monomeric Bet v 1a provides only low stimulus to APCs, leading to incomplete APC activation and therefore to a higher induction of Tregs with only moderate Ab levels. Because of the absence of a protective IgG/IgA Ab response, serum IgE levels are sufficient to trigger allergic symptoms. B, As a result of self-aggregation, Bet v 1d is more immunogenic reflected by the induction of higher costimulatory molecules, thereby inducing a strong Th1/Th2-dependent Ab response with high levels of protective IgG/IgA Abs for Ag clearance.
Discussion
Naturally occurring and engineered recombinant allergens are becoming promising tools for immunotherapy of type I allergies, although the underlying mechanism is still matter of debate. Explanations range from a shift in the cytokine profile, impeding eosinophile recruitment and T cell-dependent late-phase responses, up to the induction of a protective IgG response in allergic individuals, thereby masking the specific allergen and thus avoiding its binding to specific effector cell-bound IgE (6, 20-22). To minimize the risk of side effects, allergen variants with reduced IgE binding capacity but preserved T cell reactivity—termed hypoallergenic derivatives—are recommended for safer immunotherapy (23, 24). Hypoallergens can either be genetically engineered “in vitro” or can emerge as natural isoforms from a distinct allergen. Regarding Bet v 1, the major allergen in birch pollen allergy, several natural isoforms exist, which show different IgE reactivity despite similar T cell reactivity (5). Apparently, it is of major interest to investigate how isoform-specific differences in the primary, secondary, or tertiary structure of a protein can influence an IgE response and thus contribute to the establishment of allergic diseases. In this study, we investigated the specific immunological properties that define an allergen by comparing hyperallergenic Bet v 1a and hypoallergenic Bet v 1d. Because it was shown that naturally occurring Bet v 1 proteins have no posttranslational modifications (4), the use of E. coli-produced recombinant Bet isoforms is legitimate. First, we asked whether the allergenicity of Bet v 1 isoforms can simply be attributed to their relative abundance in birch pollen (3). Therefore, we immunized mice with purified Bet v 1a and Bet v 1d, and we found that Bet v 1a induced a significantly weaker IgG1, IgG2a, and IgA response, whereas the amount of serum IgE was comparable (Fig. 1). In addition, we observed high cross-reactivity of serum Abs toward Bet v 1a and Bet v 1d (Fig. 2 and Supplemental Fig. 1), which we also found for mAbs derived from Bet v 1-specific hybridomas (Supplemental Table I). Hence, our results clearly indicate that hypoallergenic Bet v 1d has not lost its property to bind IgE but rather has gained the ability to induce a stronger immune response, as reflected by the presence of high-serum IgG and IgA Ab titers. These findings are in contrast with previous reports that ascribe hypoallergenicity primarily to the inability to bind human IgE from birch pollen-sensitized individuals, because of the exchange of seven amino acids that are exposed on the surface of the protein (2, 5). To investigate the nature of the observed difference in immunogenicity of the two isoforms, we assessed their intrinsic property to stimulate BMDCs. Strikingly, we found that Bet v 1d was captured TLR4 independently and internalized by BMDCs at a higher rate (Fig. 3). Because the model Ag/allergen OVA (25) was captured at similar levels as Bet v 1a together with similar expression of CD80 and MHC II on BMDCs (Supplemental Fig. 2), we hypothesize that weak DC activation is a general feature of allergens.
Furthermore, the expression of activation markers CD80, CD86, and MHC II was significantly higher in BMDCs stimulated with Bet v 1d as compared with Bet v 1a (Fig. 4), whereas IL-6 production was more pronounced in BMDCs stimulated with Bet v 1a (Fig. 5). Interestingly, increased IL-6 levels have been associated with human atopic asthma, and recently, IL-6 was proposed to be essential for mucus hypersecretion by airway epithelial cells in response to inhaled allergens (26), which would suit to our finding of increased IL-6 production triggered by hyperallergenic Bet v 1a. In addition, IL-6 was shown to dampen Ag-mediated DC maturation/activation (27). Taken together with our data, it is conceivable that insufficient DC activation increases the propensity for allergic immune responses.
DCs play a pivotal role in the immune response by taking up Ag and providing information about its pathogenicity to other immune cells, thereby activating or tolerizing Ag-specific T cells and producing proinflammatory cytokines (28). Ag uptake is performed either by macro-pinocytosis or specifically by receptor-mediated endocytosis by virtue of FcRs, by multiple lectin domain-bearing transmembrane receptors like mannose receptor or DEC205 molecule, or by surface glycoproteins (29). Although, it is not yet fully understood how DCs distinctively instruct the T cells to differentiate, recent reports described a substantial influence of DCs on the Th1/Th2 polarization, depending on the nature of antigenic stimulus (30-32). It has also been suggested that DCs derived from different tissue are characterized by different T cell-polarizing properties (33-35) in addition to the finding that DCs from atopic individuals exhibit a significantly altered response to antigenic stimulation, associated with more effective production of Th2-type cytokines (36, 37). Upon stimulation with TLR9-dependent stimuli, DCs from atopic subjects respond with a vastly reduced production of IFN-α (38). As DCs are potent producers of the Th1-supportive cytokine IL-12, DCs from atopic individuals were reported to induce Th2 differentiation by an insufficient production of IL-12 (39), which contrasts other findings that DCs derived form allergic patients show increased levels of IL-13 production when stimulated in vitro with allergenic determinants (40), which triggers Th2 differentiation independent from IL-12 (36). In our own experiments, we could not detect any difference in the IL-12/23 production by BMDCs upon stimulation with Bet v 1a or Bet v 1d (Fig. 5). It is also discussed that in atopic patients, the presence of allergic mediators like histamine modulates the capacity of DCs to instruct T cells for differentiation (41). Our finding that Bet v 1d is more effectively captured by BMDCs indicates that aggregation facilitates nonspecific uptake or specific binding to distinct transmembrane receptors.
We also observed an altered T cell proliferation and differentiation in syngeneic BMDC-T cell coculture experiments when Bet v 1a or Bet v 1d was used as Ag. Although the overall proliferation of Bet v 1-specific T cells was not dramatically different in response to Bet v 1a- or Bet v 1d-pulsed BMDCs (Fig. 6C), Bet v 1d induced an increase in the generation of IL-13–producing Th1 and 2 cells and a decrease in the proliferation of FoxP3+CD25+ Tregs (Fig. 6E–H). This finding was surprising, because it contrasts recent reports on allergen-specific Tregs as being crucial for the healthy nonatopic immune response and that the generation of specific Tregs is a key event for successful immunotherapy (42, 43). Alternatively, a too early suppression of the immune response by Tregs, as seen for Bet v 1a, might negatively influence the induction of a protective Ig isotype repertoire. Our observations lead to the interpretation that Bet v 1a is less immunogenic than Bet v 1d, reflected in minor activation of BMDCs in vitro and thus leading to the induction of lower Th2 numbers and higher levels of Tregs. Accordingly, previous studies have already demonstrated that Treg differentiation is driven by reduced expression of CD80 and CD86 costimulatory molecules on BMDCs (44, 45).
Also, the profile of cytokine expression of T cells from coculture assays varied according to the Ag used for BMDCs pulsing. T cells from Bet v 1a-immunized mice cultured in the presence of Bet v 1d-loaded BMDCs responded with the secretion of significantly higher amounts of IFN-γ, IL-4, and IL-5 compared with BMDCs pulsed with Bet v 1a. Using T cells form Bet v 1d-immunized mice in coculture with Bet v 1d-pulsed BMDCs, all five cytokines were significantly increased compared with Bet v 1a-loaded BMDCs (Fig. 7).
We next looked for differences in the three-dimensional structure of Bet v 1 isoforms. However, the X-ray crystal structure of Bet v 1d revealed no significant difference to the structure of Bet v 1a (Fig. 8). However, analysis of the electrostatic surface potential indicated that mutation D125N resulted in a small reduction of negative surface potential in Bet v 1d. Although this minimal exchange may alter affinity and recognition of Bet v 1d by Igs, it is very unlikely to have an effect on dendritic phagocytosis. It is, however, conceivable that increased aggregation, which is indeed observed for purified Bet v1d is the reason for this phenomenon. This increased self-aggregation is either caused by altered surface potentials or decreased protein stability. SDS-PAGE analysis revealed that a significant fraction of Bet v 1d forms disulfide-linked dimers because of the serine to cysteine exchange at position 113. In fact, high molecular weight aggregates (30–100 Bet v 1d molecules) were observed by DLS under physiological conditions. Formation of such aggregates may be promoted by partial unfolding stabilized by cysteine bridging. We further supported this hypothesis by mutating the cysteine at position 113 of Bet v 1d back to serine. The resulting protein Bet v 1d[cys113ser] exhibited a similar behavior as Bet v 1a. Both are primarily monomeric, ineffective in stimulating BMDCs and fail to elicit high titers of protective IgG1 in immunized mice (Fig 9). We conclude that this difference in aggregation could well account for the observed dissimilarity in BMDC stimulation and the diverse ability to induce a protective IgG and IgA response upon immunization. Accordingly, a recent publication showed that polymerization of Ag leads to increased stimulation of APCs and thus to higher immunogenicity (46). Interestingly, Schöll et al. (47) recently showed that also Bet v 1a forms dimers when highly concentrated, with only dimerized but not monomeric Bet v 1a being able to induce the production of specific serum IgE in mice. From these data, the authors concluded that dimerization of Bet v 1a is the basis for its allergenicity. However, accompanying IgG levels, which give information about the immunogenicity of Bet v 1 dimers were not addressed (47). From our own findings, we rather interpret aggregation of Bet v 1 as a marker for its immunogenicity, inversely correlating with allergenicity. Consequently, Bet v 1a, which is primarily monomeric, leads to only incomplete BMDC activation and thus to the predominant production of specific serum IgE, whereas increased aggregation—as shown for Bet v 1d—induces strong BMDC stimulation and a profound humoral immune response upon immunization.
In summary, our findings indicate that hypoallergenic Bet v 1d is not impaired in its ability to evoke an IgE response in mice, but more potent in triggering the production of protective IgG and IgA Ab isotypes, because of a more efficient activation of APCs, most likely due to increased aggregate formation. Our data suggest that a detailed examination of allergen uptake and activation of APCs will provide insights into the molecular basis for allergy and a better understanding of the properties specific for allergenic determinants.
Supplementary Material
Acknowledgments
We thank M. A. Freudenberg (Max-Planck-Institut) for providing the C57BL/10ScNcr mice. We also thank R. Geisberger and D. Hebenstreit for helpful discussion and critical reading of the manuscript.
The study was supported by the Christian Doppler Laboratory for Allergy Diagnosis and Therapy, the Austrian Science Fund Project P-19017, and the Australian National Bank (OENB Grant 11710).
Abbreviations used in this paper
- AP
alkaline phosphatase
- Bet v 1
Betula verrucosa major Ag 1
- BMDC
bone marrow-derived dendritic cell
- BPE
birch pollen extract
- CBA
cytometric bead array
- DLS
dynamic light scattering
- MHC II
MHC class II
- Treg
regulatory T cell
Footnotes
The sequence presented in this article has been submitted to the Protein Data Bank under accession number 3K 78.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner S, Radauer C, Bublin M, Hoffmann-Sommergruber K, Kopp T, Greisenegger EK, Vogel L, Vieths S, Scheiner O, Breiteneder H. Naturally occurring hypoallergenic Bet v 1 isoforms fail to induce IgE responses in individuals with birch pollen allergy. J. Allergy Clin. Immunol. 2008;121:246–252. doi: 10.1016/j.jaci.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Schenk MF, Gilissen LJ, Esselink GD, Smulders MJ. Seven different genes encode a diverse mixture of isoforms of Bet v 1, the major birch pollen allergen. BMC Genomics. 2006;7:168. doi: 10.1186/1471-2164-7-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swoboda I, Jilek A, Ferreira F, Engel E, Hoffmann-Sommergruber K, Scheiner O, Kraft D, Breiteneder H, Pittenauer E, Schmid E, et al. Isoforms of Bet v 1, the major birch pollen allergen, analyzed by liquid chromatography, mass spectrometry, and cDNA cloning. J. Biol. Chem. 1995;270:2607–2613. doi: 10.1074/jbc.270.6.2607. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira F, Hirtenlehner K, Jilek A, Godnik-Cvar J, Breiteneder H, Grimm R, Hoffmann-Sommergruber K, Scheiner O, Kraft D, Breitenbach M, et al. Dissection of immunoglobulin E and T lymphocyte reactivity of isoforms of the major birch pollen allergen Bet v 1: potential use of hypoallergenic isoforms for immunotherapy. J. Exp. Med. 1996;183:599–609. doi: 10.1084/jem.183.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valenta R, Kraft D. From allergen structure to new forms of allergen-specific immunotherapy. Curr. Opin. Immunol. 2002;14:718–727. doi: 10.1016/s0952-7915(02)00402-8. [DOI] [PubMed] [Google Scholar]
- 7.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat. Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shakib F, Ghaemmaghami AM, Sewell HF. The molecular basis of allergenicity. Trends Immunol. 2008;29:633–642. doi: 10.1016/j.it.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Pomés A. Allergen structures and biologic functions: the cutting edge of allergy research. Curr. Allergy Asthma Rep. 2008;8:425–432. doi: 10.1007/s11882-008-0082-y. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann-Sommergruber K, Susani M, Ferreira F, Jertschin P, Ahorn H, Steiner R, Kraft D, Scheiner O, Breiteneder H. High-level expression and purification of the major birch pollen allergen, Bet v 1. Protein Expr. Purif. 1997;9:33–39. doi: 10.1006/prep.1996.0671. [DOI] [PubMed] [Google Scholar]
- 11.Himly M, Nony E, Chabre H, Van Overtvelt L, Neubauer A, van Ree R, Buchheit KH, Vieths S, Moingeon P, Ferreira F. Standardization of allergen products. 1. Detailed characterization of GMP-produced recombinant Bet v 1.0101 as biological reference preparation. Allergy. 2009;64:1038–1045. doi: 10.1111/j.1398-9995.2009.01957.x. [DOI] [PubMed] [Google Scholar]
- 12.Leslie A. Recent changes to the MOSFLM package for processing film and image plate data. Newsletter on Protein Crystallography. 1992;26 [Google Scholar]
- 13.Jaroszewski L, Li W, Godzik A. In search for more accurate alignments in the twilight zone. Protein Sci. 2002;11:1702–1713. doi: 10.1110/ps.4820102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marković-Housley Z, Degano M, Lamba D, von Roepenack-Lahaye E, Clemens S, Susani M, Ferreira F, Scheiner O, Breiteneder H. Crystal structure of a hypoallergenic isoform of the major birch pollen allergen Bet v 1 and its likely biological function as a plant steroid carrier. J. Mol. Biol. 2003;325:123–133. doi: 10.1016/s0022-2836(02)01197-x. [DOI] [PubMed] [Google Scholar]
- 15.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 16.Weichenberger CX, Sippl MJ. NQ-Flipper: recognition and correction of erroneous asparagine and glutamine side-chain rotamers in protein structures. Nucleic Acids Res. 2007;35:W403–W406. doi: 10.1093/nar/gkm263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Himly M, Nony E, Chabre H, Van Overtvelt L, Neubauer A, van Ree R, Buchheit KH, Vieths S, Moingeon P, Ferreira F. Standardization of allergen products: 1. Detailed characterization of GMP-produced recombinant Bet v 1.0101 as biological reference preparation. Allergy. 2009;64:1038–1045. doi: 10.1111/j.1398-9995.2009.01957.x. [DOI] [PubMed] [Google Scholar]
- 18.Kober J, Leitner J, Klauser C, Woitek R, Majdic O, Stöckl J, Herndler-Brandstetter D, Grubeck-Loebenstein B, Reipert BM, Pickl WF, et al. The capacity of the TNF family members 4-1BBL, OX40L, CD70, GITRL, CD30L and LIGHT to costimulate human T cells. Eur. J. Immunol. 2008;38:2678–2688. doi: 10.1002/eji.200838250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gajhede M, Osmark P, Poulsen FM, Ipsen H, Larsen JN, Joost van Neerven RJ, Schou C, Løwenstein H, Spangfort MD. X-ray and NMR structure of Bet v 1, the origin of birch pollen allergy. Nat. Struct. Biol. 1996;3:1040–1045. doi: 10.1038/nsb1296-1040. [DOI] [PubMed] [Google Scholar]
- 20.Saint-Remy JM. Novel approaches in immunotherapy. Clin. Rev. Allergy. 1994;12:23–42. doi: 10.1007/BF02815508. [DOI] [PubMed] [Google Scholar]
- 21.Meissner N, Kochs S, Coutelle J, Kussebi F, Baumgarten C, Løwenstein H, Kunkel G, Renz H. Modified T-cell activation pattern during specific immunotherapy (SIT) in cat-allergic patients. Clin. Exp. Allergy. 1999;29:618–625. doi: 10.1046/j.1365-2222.1999.00562.x. [DOI] [PubMed] [Google Scholar]
- 22.Valenta R, Ball T, Focke M, Linhart B, Mothes N, Niederberger V, Spitzauer S, Swoboda I, Vrtala S, Westritschnig K, Kraft D. Immunotherapy of allergic disease. Adv. Immunol. 2004;82:105–153. doi: 10.1016/S0065-2776(04)82003-0. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira F, Ebner C, Kramer B, Casari G, Briza P, Kungl AJ, Grimm R, Jahn-Schmid B, Breiteneder H, Kraft D, et al. Modulation of IgE reactivity of allergens by site-directed mutagenesis: potential use of hypoallergenic variants for immunotherapy. FASEB J. 1998;12:231–242. doi: 10.1096/fasebj.12.2.231. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira F, Hirthenlehner K, Briza P, Breiteneder H, Scheiner O, Kraft D, Breitenbach M, Ebner C. Isoforms of atopic allergens with reduced allergenicity but conserved T cell antigenicity: possible use for specific immunotherapy. Int. Arch. Allergy Immunol. 1997;113:125–127. doi: 10.1159/000237524. [DOI] [PubMed] [Google Scholar]
- 25.Elsayed S, Apold J, Holen E, Vik H, Florvaag E, Dybendal T. The structural requirements of epitopes with IgE binding capacity demonstrated by three major allergens from fish, egg and tree pollen. Scand. J. Clin. Lab. Invest. Suppl. 1991;204:17–31. [PubMed] [Google Scholar]
- 26.Neveu WA, Allard JB, Dienz O, Wargo MJ, Ciliberto G, Whittaker LA, Rincon M. IL-6 is required for airway mucus production induced by inhaled fungal allergens. J. Immunol. 2009;183:1732–1738. doi: 10.4049/jimmunol.0802923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, Kamimura D, Ueda N, Iwakura Y, Ishihara K, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J. Immunol. 2004;173:3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 28.Bousso P. T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nat Rev Immunol. 2008;8:675–684. doi: 10.1038/nri2379. [DOI] [PubMed] [Google Scholar]
- 29.Novak N, Haberstok J, Geiger E, Bieber T. Dendritic cells in allergy. Allergy. 1999;54:792–803. doi: 10.1034/j.1398-9995.1999.00101.x. [DOI] [PubMed] [Google Scholar]
- 30.Lombardi V, Van Overtvelt L, Horiot S, Moingeon P. Human dendritic cells stimulated via TLR7 and/or TLR8 induce the sequential production of Il-10, IFN-γ, and IL-17A by naive CD4+ T cells. J. Immunol. 2009;182:3372–3379. doi: 10.4049/jimmunol.0801969. [DOI] [PubMed] [Google Scholar]
- 31.Farkas L, Kvale EO, Johansen FE, Jahnsen FL, Lund-Johansen F. Plasmacytoid dendritic cells activate allergen-specific TH2 memory cells: modulation by CpG oligodeoxynucleotides. J. Allergy Clin. Immunol. 2004;114:436–443. doi: 10.1016/j.jaci.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 32.Larangé A, Antonios D, Pallardy M, Kerdine-Römer S. TLR7 and TLR8 agonists trigger different signaling pathways for human dendritic cell maturation. J. Leukoc. Biol. 2009;85:673–683. doi: 10.1189/jlb.0808504. [DOI] [PubMed] [Google Scholar]
- 33.Kapsenberg ML, Hilkens CM, Wierenga EA, Kalinski P. The paradigm of type 1 and type 2 antigen-presenting cells. Implications for atopic allergy. Clin. Exp. Allergy. 1999;29(Suppl 2):33–36. [PubMed] [Google Scholar]
- 34.Feili-Hariri M, Falkner DH, Morel PA. Polarization of naive T cells into Th1 or Th2 by distinct cytokine-driven murine dendritic cell populations: implications for immunotherapy. J. Leukoc. Biol. 2005;78:656–664. doi: 10.1189/jlb.1104631. [DOI] [PubMed] [Google Scholar]
- 35.Hibi M, Hachimura S, Ise W, Sato A, Yoshida T, Takayama T, Sasaki K, Senga T, Hashizume S, Totsuka M, Kaminogawa S. Dendritic cells from spleen, mesenteric lymph node and Peyer’s patch can induce the production of both IL-4 and IFN-γ from primary cultures of naive CD4+ T cells in a dose-dependent manner. Cytotechnology. 2003;43:49–55. doi: 10.1023/B:CYTO.0000039906.15156.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellinghausen I, Brand U, Knop J, Saloga J. Comparison of allergen-stimulated dendritic cells from atopic and nonatopic donors dissecting their effect on autologous naive and memory T helper cells of such donors. J. Allergy Clin. Immunol. 2000;105:988–996. doi: 10.1067/mai.2000.105526. [DOI] [PubMed] [Google Scholar]
- 37.Kapsenberg ML, Hilkens CM, van Der Pouw Kraan TC, Wierenga EA, Kalinski P. Atopic allergy: a failure of antigen-presenting cells to properly polarize helper T cells? Am. J. Respir. Crit. Care Med. 2000;162:S76–S80. doi: 10.1164/ajrccm.162.supplement_2.ras-4. [DOI] [PubMed] [Google Scholar]
- 38.Tversky JR, Le TV, Bieneman AP, Chichester KL, Hamilton RG, Schroeder JT. Human blood dendritic cells from allergic subjects have impaired capacity to produce interferon-α via Toll-like receptor 9. Clin. Exp. Allergy. 2008;38:781–788. doi: 10.1111/j.1365-2222.2008.02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reider N, Reider D, Ebner S, Holzmann S, Herold M, Fritsch P, Romani N. Dendritic cells contribute to the development of atopy by an insufficiency in IL-12 production. J. Allergy Clin. Immunol. 2002;109:89–95. doi: 10.1067/mai.2002.120556. [DOI] [PubMed] [Google Scholar]
- 40.Bellinghausen I, Brand P, Böttcher I, Klostermann B, Knop J, Saloga J. Production of interleukin-13 by human dendritic cells after stimulation with protein allergens is a key factor for induction of T helper 2 cytokines and is associated with activation of signal transducer and activator of transcription-6. Immunology. 2003;108:167–176. doi: 10.1046/j.1365-2567.2003.01576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazzoni A, Young HA, Spitzer JH, Visintin A, Segal DM. Histamine regulates cytokine production in maturing dendritic cells, resulting in altered T cell polarization. J. Clin. Invest. 2001;108:1865–1873. doi: 10.1172/JCI13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J. Allergy Clin. Immunol. 2009;123:735–746. doi: 10.1016/j.jaci.2009.02.030. quiz 747-738. [DOI] [PubMed] [Google Scholar]
- 43.Miyara M, Wing K, Sakaguchi S. Therapeutic approaches to allergy and autoimmunity based on FoxP3+ regulatory T-cell activation and expansion. J. Allergy Clin. Immunol. 2009;123:749–755. doi: 10.1016/j.jaci.2009.03.001. quiz 756-747. [DOI] [PubMed] [Google Scholar]
- 44.Li M, Zhang X, Zheng X, Lian D, Zhang ZX, Ge W, Yang J, Vladau C, Suzuki M, Chen D, et al. Immune modulation and tolerance induction by RelB-silenced dendritic cells through RNA interference. J. Immunol. 2007;178:5480–5487. doi: 10.4049/jimmunol.178.9.5480. [DOI] [PubMed] [Google Scholar]
- 45.Roncarolo MG, Levings MK, Traversari C. Differentiation of T regulatory cells by immature dendritic cells. J. Exp. Med. 2001;193:F5–F9. doi: 10.1084/jem.193.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ilyinskii PO, Thoidis G, Sherman MY, Shneider A. Adjuvant potential of aggregate-forming polyglutamine domains. Vaccine. 2008;26:3223–3226. doi: 10.1016/j.vaccine.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 47.Schöll I, Kalkura N, Shedziankova Y, Bergmann A, Verdino P, Knittelfelder R, Kopp T, Hantusch B, Betzel C, Dierks K, et al. Dimerization of the major birch pollen allergen Bet v 1 is important for its in vivo IgE-cross-linking potential in mice. J. Immunol. 2005;175:6645–6650. doi: 10.4049/jimmunol.175.10.6645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.