Summary
We present a survey on the distribution and habitat range of P. necessarius subspecies asymbioticus (PnecC), an important taxon in the water column of freshwater systems. We systematically sampled stagnant freshwater habitats in a heterogeneous 2000 km2 area, together with ecologically different habitats outside this area. In total, 137 lakes, ponds and puddles were investigated, which represent an enormous diversity of habitats differing, i.e., in depth (<10 cm – 171 m) and pH (3.9 – 8.5). PnecC was detected by cultivation-independent methods in all investigated habitats, and its presence was confirmed by cultivation of strains from selected habitats including the most extreme ones. The determined relative abundance of the subspecies ranged from slightly above 0% to 67% (average 14.5% ± 14.3%), and the highest observed absolute abundance was 5.3×106 cells mL−1. Statistical analyses revealed that the abundance of PnecC is partially controlled by factors linked to concentrations of humic substances, which might support the hypothesis that these bacteria utilize photodegradation products of humic substances. .
Statistical analyses revealed that the abundance of PnecC is partially controlled by low conductivity and pH and factors linked to concentrations of humic substances.
Based on the revealed statistical relationships, an average relative abundance of this subspecies of 20% in global freshwater habitats was extrapolated. Our study provides important implications for the current debate on ubiquity and biogeography in microorganisms.
Keywords: ubiquity, Polynucleobacter, FISH, pH, humic habitat
Introduction
Systematic investigations on the distribution of species or species-like taxa of free-living bacteria across freshwater habitats are basically missing as opposed to investigations on marine systems (Morris et al., 2002; Selje et al., 2004; Johnson et al., 2006), even though the physico-chemical conditions vary much more strongly across freshwater habitats than in marine systems. For instance, the biologically highly important pH values of most natural freshwater habitats (lakes including bog lakes, ponds, and running water systems) are ranging at least from 3 to 10, while the pH of marine systems varies only slightly around pH 8.1. The lack of systematic surveys across freshwater systems hampers the identification of globally important species or genera of freshwater bacteria, as well as the identification of taxon-specific habitat requirements.
In the study presented here, we investigated systematically the distribution and abundance of a phylogenetically narrow group of bacteria, Polynucleobacter necessarius subspecies asymbioticus (Hahn et al., in press), comparable to >99% 16S rRNA sequence similarity operational taxonomic units (OTUs), in lentic freshwater habitats (pH range 3.9 – 8.5) of a heterogeneous 2000 km2 area located in Central Europe. This survey covered almost completely all stagnant freshwater habitats > 1 hectare surface area (lakes) and also included numerous habitats in the size range of 0.0001 to 1 ha (puddles and ponds) within this area. Several habitats located outside of the primary study area were included in the survey in order to enlarge the ecological diversity of the investigated habitats. Altogether, 137 habitats were sampled. The sampled habitats differed in age, size, depth, altitude, landscape position, organic and inorganic water chemistry, retention time, stability, trophic status, etc., and therefore represent an enormous diversity of lentic freshwater systems.
The studied taxon, Polynucleobacter necessarius subspecies asymbioticus is affiliated with the Betaproteobacteria and represents free-living strains within the ecologically heterogeneous species P. necessarius (Heckmann and Schmidt, 1987). The second subspecies, P. necessarius subsp. necessarius within this species contains exclusively obligate endosymbionts of the benthic ciliate Euplotes aediculatus (Heckmann and Schmidt, 1987; Hahn et al., in press). Vannini et al. (2007) demonstrated that the endosymbionts and the free-living strains, i.e. members of the two subspecies, differ fundamentally and strictly in their lifestyle. The obligate endosymbionts have to be assumed to be absent from the water column of stagnant freshwater systems since their ciliate host possesses a benthic lifestyle, while strains affiliated with P. nec. subsp. asymbioticus inhabit as free-living non-motile planktonic cells the water column of freshwater systems. Due to the strict differences in lifestyle and (micro)habitat preferences, it is likely that all P. necessarius bacteria previously detected in the water columns of freshwater habitats (e.g., Bahr et al., 1996; Hiorns et al., 1997; Zwart et al., 2002) represent members of the subspecies “asymbioticus”. Throughout this paper, we will use the term PnecC bacteria for bacteria belonging to the subspecies P. necessarius subsp. asymbioticus. This term was previously introduced for bacteria affiliated with subcluster C of the Polynucleobacter cluster (Hahn, 2003), which is synonymous to the species P. necessarius according to its recent emended description (Hahn et al., in press). Since we apply the term PnecC bacteria in this paper exclusively to planktonic strains, it is synonymous to the subspecies “asymbioticus”. Note that bacteria affiliated with the other three species-like subclusters of the Polynucleobacter cluster are not targeted by this study.
PnecC bacteria have been frequently detected with cultivation-independent methods in the water column of freshwater habitats (e.g., Bahr et al., 1996; Hiorns et al., 1997; Zwart et al., 2002; Burkert et al., 2003), but were never reported from offshore marine or soil systems. The taxon is known to appear with variable cell numbers across habitats (Salcher et al., 2008; Alonso et al., in press; Buck et al., 2009), ranging from <1% (Wu and Hahn, 2006a) to 60% of total bacterial numbers (Hahn et al., 2005). Detection of this subspecies on all continents (Crump et al., 2003; Hahn, 2003; Pearce et al., 2003; Alonso et al., in press; Hahn et al., unpublished data) demonstrates a cosmopolitan distribution of the taxon (Hahn, 2003). Furthermore, PnecC bacteria were detected in an ecologically broad variety of freshwater habitats including acidic lakes (Burkert et al., 2003, Hahn et al., 2005), lakes acidified by acid rain (Percent, et al., 2008), alkaline lakes (Wu and Hahn, 2006a), Arctic and Antarctic lakes (Crump et al., 2003; Pearce et al., 2003), high-altitude lakes located above 5000 m.a.s.l. (Wu et al., 2006), and tropical habitats (Hahn et al., unpublished data).
The goals of the presented study were (i) to reveal the habitat range of PnecC bacteria at a regional scale, (ii) to characterize the widths of physico-chemical gradients (pH, conductivity, and DOC) covered by the subspecies, and (iii) to identify environmental parameters controlling the abundance of the taxon in its natural habitats.
Results
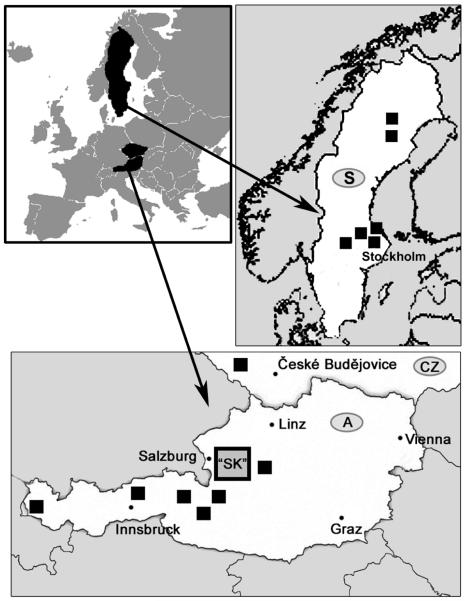
Freshwater habitats distributed over an area of more than 2000 square kilometers located in Central Europe (Fig. 1) were systematically sampled to obtain a representative coverage of the whole geographical region (Salzkammergut area, SK). This major set of habitats was supplemented by sampling of other habitats, namely humic ponds located in Austria mostly at altitudes >1000 m.a.s.l. (designation “humic”), clearwater lakes located at high altitudes (2100-2300 m.a.s.l.) on granitic bedrock (designation “granitic”), intermediate and raised bog systems (designation “bog”), artificially acidified lakes with high aluminum content located in the Šumava Mountains, Czech Republic (designated “acidified”), and boreal lakes representing a humic gradient located in Sweden (designated “boreal”).
Fig. 1.
Geographic location of the ~2000 km2 primary study area (SK) and the additional sampling sites in Central (Austria and the Czech Republic) and Northern Europe (Sweden).
Ecological diversity of sampled freshwater systems
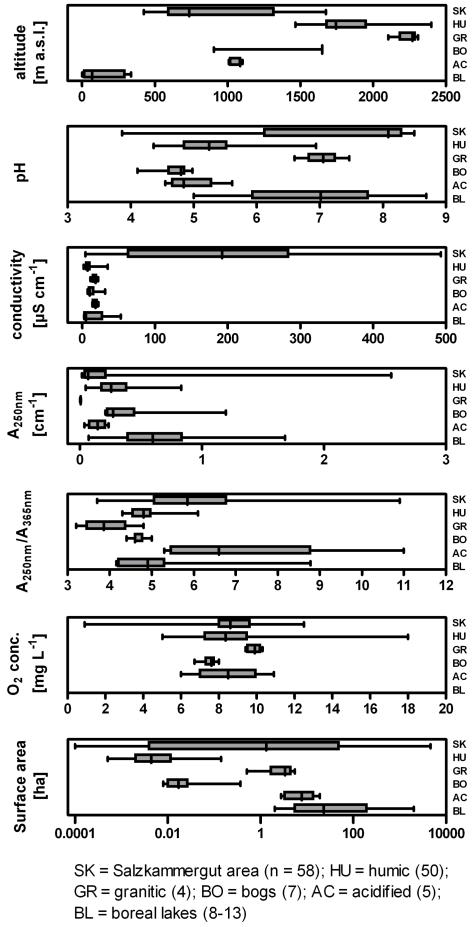
The smallest investigated habitats, i.e. puddles (#55-58, 53 in Table S1, Supplementary Material), were not larger than 0.0001 hectare (Fig. 2), the largest, i.e. ultra-oligotrophic Lake Attersee (#21), possess an area of around 4620 hectares. The set of investigated habitats is numerically dominated by systems < 1 ha (Table 1), which well reflects the numerical importance of smaller habitats in the global size distribution of stagnant freshwater habitats (Downing et al., 2006). The investigated habitats also differed strongly in maximum depths, which ranged from a few centimeters to 171 m (#21). Polymixis or only short-lived stratifications have to be assumed for all shallow habitats, while all deeper lakes possessed stable stratifications during the warmer seasons. These stratified lakes mainly represent holomictic lakes, but also include two meromictic habitat (Lake Krottensee, #13; Lake Toplitzsee, #31). The sampled habitats are located over a wide range of altitudes. Habitats in Central Europe are located at altitudes spanning from 420 m.a.s.l. (lowland habitats) to alpine sites at 2300 m.a.s.l. The boreal habitats in Sweden are located at altitudes of 1 to 335 m.a.s.l. (Fig. 3, Table S1). The sampled systems span from soft to hard water habitats (low and high concentrations of Ca- and Mg-carbonates), which mainly reflects the geology of the bedrock (granitic versus limestone). Conductivity and pH values of the habitats are strongly influenced by this inorganic water chemistry (Fig. 3 and 4A). The pH of sampled habitats ranged from highly acidic, (e.g., pH 3.9, #52) to the maximum of about 8.5 observed for several lakes located at lower altitudes on limestone bedrock (e.g., oligotrophic lake #6). Conductivity ranged from very few μS.cm−1, typical for acidic ponds rich in humic substances, to few hundreds of μS.cm−1, typical for alkaline hardwater systems like Lake Mondsee (#10, Fig. 3 and 4A). Furthermore, the employed proxies indicated that the investigated habitats varied strongly in concentrations of DOC and humic substances (Fig. 3 and 4B).
Fig. 2.

Examples of investigated habitats selected in order to illustrate the ecological diversity of the sampled systems. A, Größter Teich (# 65), an acidic humic pond located in a coniferous forest; B, Ross4 (# 57), a puddle located on a forestry road; C, Gerlos3 (# 115), a pond located on a raised bog system (Sieben Möser); D, Lake Hallstättersee (# 33), an oligotrophic alkaline hardwater lake; E, Trög2 (# 73), a small acidic humic pond surrounded by a quacking bog located close to the timber line.
Table 1.
Contribution of different size classes of lentic freshwater habitats to the 61 and 76 water bodies sampled in the Salzkammergut lake district (SK) and outside of SK, respectively
| Surface area (ha) | # of habitats in SK | # of habitats outside SK |
|---|---|---|
| > 1 (lakes) | 31 | 21 |
| 0.1 – 1 (ponds) | 5 | 4 |
| < 0.1 (ponds and puddles) | 25 | 51 |
Fig. 3.
Parameters characterizing main features of the habitats investigated for Polynucleobacter necessarius subs. asymbioticus (PnecC bacteria) and differences among groups of sampled habitats (SK – Salzkammergut, HU – humic, GR – granitic, BO – bogs, AC – acidified and BL – boreal). Box-and-Whiskers plots (A, B) are showing median, 1st and 3rd quartile and the smallest and largest values.
Fig. 4.
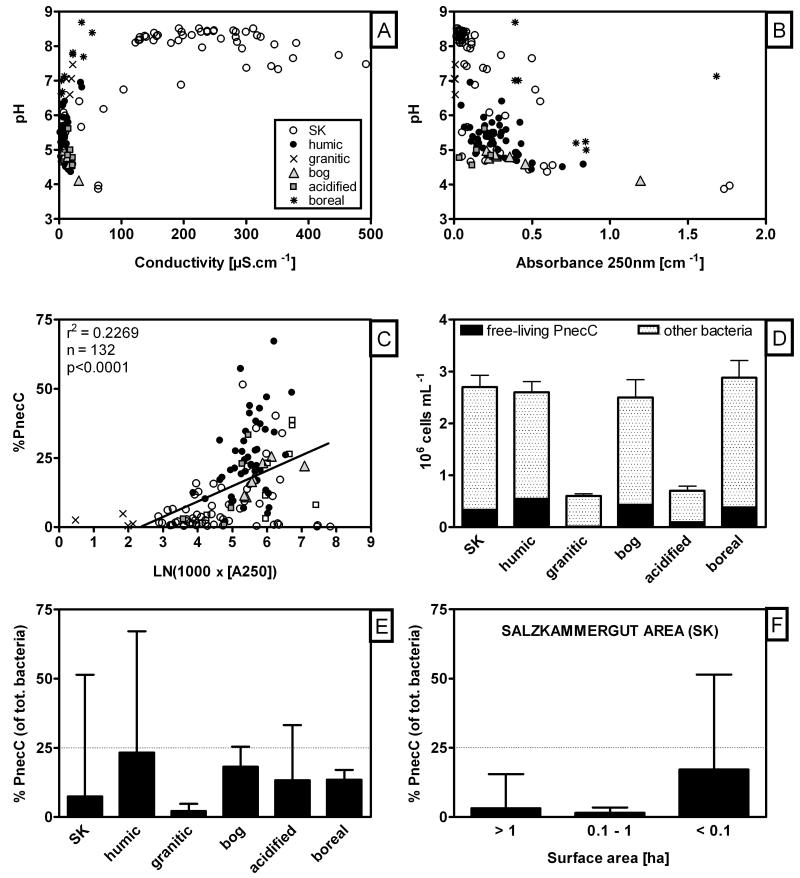
(A) Range of pH and conductivity of the sampled habitats and (B) range of pH and proxy for DOC and humic substances (A250nm). (C) Linear regression of absorption A250nm and relative PnecC abundance (% of total bacterial numbers). Note the low %PnecC values in the three samples with highest A250nm values. (D) Absolute and relative (E, F) abundances of PnecC bacteria and total bacteria in defined environmental groups.
Abundance and distribution of PnecC bacteria
Total bacterial numbers ranged from 0.22 × 106 cells mL−1 (#19) to 13 × 106 cells mL−1 (#40), although these numbers were rather exceptional values, and the average values ranged around 2.4 × 106 (± 2.02 × 106) cells mL−1 (Fig. 4D). PnecC bacteria were detected in each of the sampled habitats. In two habitats (#52 and #53) their occurrence was under the detection limits of the FISH method, however here, as well as in all other investigated habitats, PnecC bacteria were detected by a P. necessarius specific PCR assay. Enormous differences between habitats were recorded regarding both absolute and relative abundance of PnecC bacteria (Table S1 and Fig. 4). The relative abundance of PnecC bacteria (% PnecC of total bacterial numbers) spanned a very wide range from values slightly above 0 to more than 67% (#85) and the mean value was 14.5% ± 14.3% of total bacterial numbers. Similarly, absolute abundance of PnecC bacteria ranged from slightly above 0 to the maximum of 5.3 × 106 mL−1 (#40). PnecC numbers were lowest in large alkaline lakes (pH around 8) and in ponds of raised bog systems with pH around 4 (#51-53 in Table S1; Fig. 4F). The presence of PnecC bacteria in both of these habitat types was confirmed by isolation of PnecC strains (Table S1).
Factors controlling the abundance of PnecC bacteria
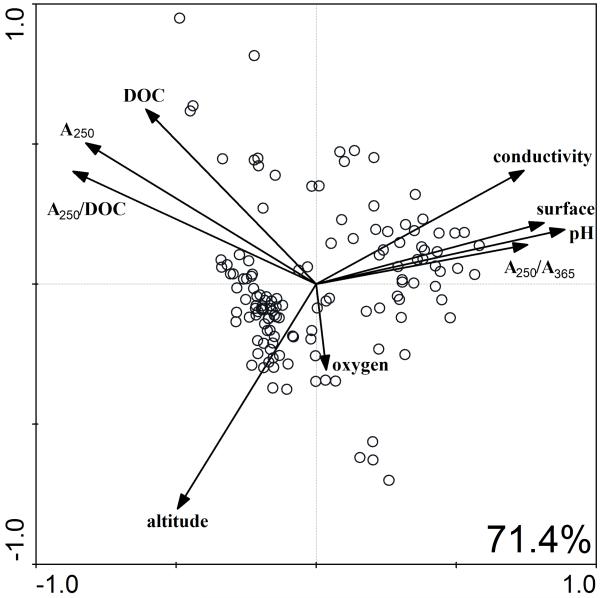
Simple statistical analyses (correlation matrices) on the whole data set of 137 habitats indicated that total heterotrophic bacterial numbers were only correlated with oxygen concentration (negative correlation, p<0.05), whereas PnecC bacteria (both absolute and relative numbers) were correlated with pH, conductivity, absorption A250nm (proxy for concentrations of humic substances), altitude and also habitat size (surface area) (Table 2). Absolute numbers of PnecC were on top of that negatively correlated with oxygen concentration (p<0.0001). Regarding PnecC bacteria, the RDA and subsequent adjusted variation partitioning analysis by CANOCO demonstrated that PnecC bacteria (both the relative and the absolute numbers) were influenced by two large groups of environmental variables, which explained together approximately half of the data variability (52.5%, p=0.002). Definition of the groups was based on calculations in Forward selection. First group comprises physical-chemical parameters (pH, conductivity, size of the water body and altitude) and the second proxies for content of humic substances (absorption A250nm, DOC, low molecular DOC (A250nm/A365nm) and aromatic humic substances (A250nm/DOC)). Due to strong mutual correlation inside of each group (for illustration see PCA graph – Fig. 5) that was observed during performance of the Forward selection, only one (the strongest) environmental factor from each group was considered for the RDA analyses, (i) pH (F = 53.1, p=0.002) and (ii) A250nm (F = 48.5, p=0.002). Interestingly, two-thirds of the explained data variability was comprised by both above-mentioned groups of factors (mutual effect) represented in the analysis by the two selected factors, while each group separately was responsible for 9 – 30% of the explained variability (i.e. 3.6 – 12% of the complete data variability) presenting a great deal of co-variation between the two different groups (Table 3). Furthermore, after filtering these strong effects of pH and humic substances out, PnecC abundance was observed to be partially influenced by oxygen concentration (F=5.0, p=0.038) with very little shared effect of the above-described groups of environmental variables (Table 3).
Table 2.
Results of the correlation analyses of single environmental factors with total bacteria, absolute and relative PnecC numbers of 137 sampled habitats calculated by GraphPad PRISM package. r = Pearson r, P value summary: p<0.05 (*), p<0.01 (**), p<0.0001(***)
| pH | conductivity | altitude | surface | A250nm | DOC | A250/DOC | A250/A365 | O2 | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
|
Total bacteria (mL−1) |
n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | r = 0,20 R2 = 0,04 * |
n.s. | r = −0,25 R2 = 0,07 ** |
|
| |||||||||
| PnecC (mL−1) | r = −0,41 R2 = 0,16 *** |
r = −0,51 R2 = 0,26 *** |
r = 0,30 R2 = 0,09 *** |
r = −0,40 R2 = 0,16 *** |
r = 0,39 R2 = 0,15 *** |
r = 0,28 R2 = 0,08 ** |
r = 0,45 R2 = 0,20 *** |
r = −0,36 R2 = 0,12 *** |
r = −0,34 R2 = 0,1188 *** |
|
| |||||||||
| PnecC (% of tot. bact.) | r = −0,59 R2 = 0,35 *** |
r = −0,53 R2 = 0,28 *** |
r = 0,38 R2 = 0,15 *** |
r = −0,44 R2 = 0,20 *** |
r = 0,48 R2 = 0,23 *** |
r = 0,42 R2 = 0,18 *** |
r = 0,53 R2 = 0,28 *** |
r = −0,42 R2 = 0,18 *** |
n.s. |
Fig.5.
Principal component analysis of the environmental characteristics of our sampled habitats (centered and normalized for species) indicating correlations among single factors (arrows) and distributions of the sampled habitats (circles). Note the broad distribution of the sampled habitats along all measured factors.
Table 3.
Summary of RDA adjusted variation partitioning analysis. This table indicates the influence of the strongest environmental variables (pH and A250nm) together with oxygen on relative and absolute PnecC numbers, how much variability (%) in our data was explained by our model and how many % of the explained variabitity it presented (*)
| total (A and B) | group A only | group B only | intersection A and B | |
|---|---|---|---|---|
| (A) pH and (B) A250nm | ||||
| PnecC (% and mL−1) | 35.1 | 7.6 (22*) | 5.7 (16*) | 21.8 (62*) |
| PnecC (mL−1) | 35.1 | 3.8 (11*) | 9.5 (27*) | 21.8 (62*) |
| PnecC (%) | 40.6 | 12 (30*) | 3.6 (9*) | 25 (62*) |
| (A) pH+A250nm (B) oxygen | ||||
| PnecC (% and mL−1) | 36.8 | 33.4 (91*) | 1.8 (5*) | 1.65 (4*) |
| PnecC (mL−1) | 37.3 | 32.6 (87*) | 2.2 (6*) | 2.5 (7*) |
| PnecC (%) | 41.7 | 39.5 (95*) | 1.1 (3*) | 1.1 (3*) |
Comparison across the investigated habitat groups revealed that PnecC bacteria were most abundant in the habitats such as: higher altitude humic ponds, bog lakes, boreal lakes and in small waterbodies of the SK area, strongly resembling the humic mountain ponds. The lowest PnecC numbers were found in the high altitude lakes on granitic bedrock and in SK habitats with surface area 0.1 – 1 ha (Fig. 4D-F).
PnecC bacteria and oxygen concentrations
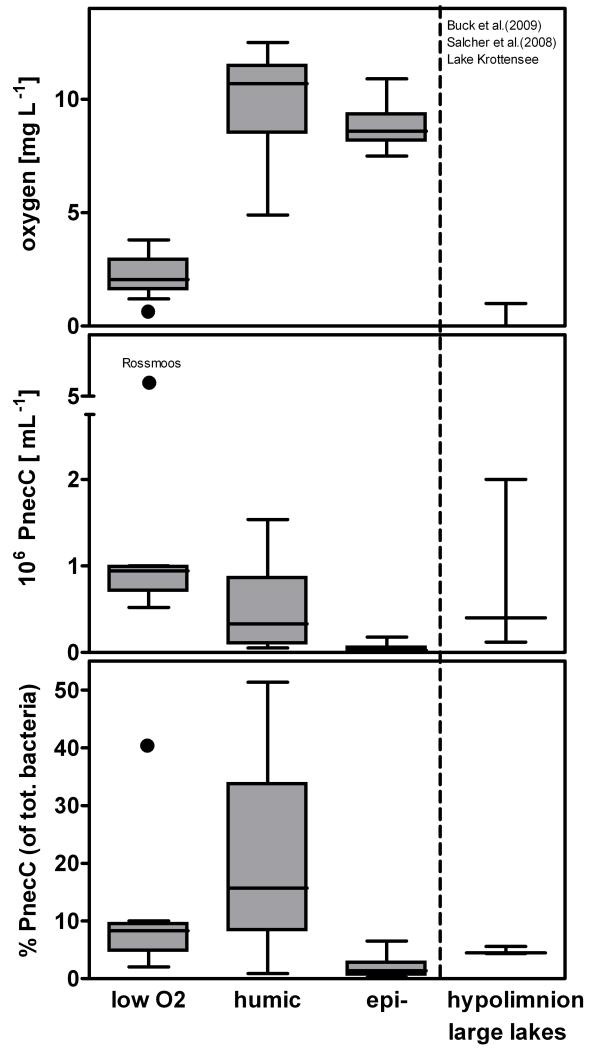
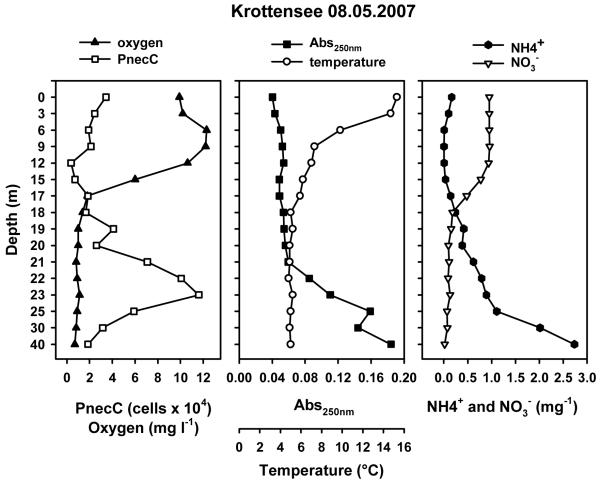
A further analysis of the observed negative correlation of PnecC bacteria with oxygen concentration revealed two strategies regarding oxygen (Fig. 6). Firstly, PnecC bacteria occurred in humic oxygen-rich environments, where they reached the highest relative abundances. Secondly, they reached high numbers in waters with oxygen-low to anoxic conditions, where the absolute numbers are comparable to humic environments, but the relative numbers are lower. For instance, high numbers of PnecC bacteria were observed in both the shallow oxygen-depleted pond Rossmoos (#40; Fig. 6) and in the permanently anoxic monimolimnion of the meromictic Lake Krottensee (#13; depth profile in Fig. 7).
Fig. 6.
Relative and absolute abundances of PnecC bacteria in environments differing in oxygen concentration. Data of anoxic hypolimnia and monimolimnia represent values from the literature (Buck et al. 2009, Salcher et al. 2008) and data from meromictic Lake Krottensee. Box-and-Whiskers plots are showing median, 1st and 3rd quartile and the minimum and maximum values. The extreme values of Rossmoos pond, a very shallow macrophyte rich pond, are depicted by closed circles.
Fig. 7.
Vertical profile of meromictic Lake Krottensee in spring 2007. Note that the used device overestimated the oxygen concentration in the monimolimnion. The oxygen concentration below depths of 18-20 m was zero.
Discussion
Ubiquity of P. necessarius subsp. asymbioticus
Polynucleobacter necessarius subsp. asymbioticus is known to have a cosmopolitan distribution (Hahn, 2003). The presence of this subspecies in typical alkaline (Hahn, 2003; Wu and Hahn, 2006b, Salcher et al., 2008) and acidic lakes and ponds (Burkert et al. 2003, Hahn et al., 2005; Percent et al., 2008) is well documented. The present study considers several additional habitat types, which were not investigated for the presence of PnecC bacteria so far. These include ponds and puddles of intermediate and raised bog systems, as well as temporary puddles on forestry roads. Surprisingly, the subspecies was detected in all 137 investigated habitats with cultivation-independent methods, and the presence of the taxon in the unusual habitat types could also be confirmed by cultivation of strains. Based on our systematic study design, we conclude that the investigated subspecies has a ubiquitous distribution in all permanent stagnant freshwater habitats in our primary study area SK. More detailed investigations are needed to reveal if the subspecies also possesses a ubiquitous distribution in temporary freshwater habitats like puddles on forestry and agricultural roads, phytotelma, barrels collecting rainwater etc. Our study exclusively considered lentic systems, however, the presence of PnecC bacteria in larger lotic systems was demonstrated previously (Sekiguchi et al., 2002; Crump et al. 1998, Crump and Hobbie, 2005). All running water systems investigated so far represent higher order systems (mainly streams), which most likely were partially fed by lentic systems. Therefore, it is unknown if first order running waters, which are exclusively fed by ground water and runoff also contain PnecC bacteria.
Based on the broad ecological range of habitats investigated in our study (Fig. 3 and Fig. 5), combined with previous detection of the subspecies on all continents and in all climatic zones (Hahn, 2003; Alonso et al., in press; Crump et al., 2003; Pearce et al., 2003; Wu et al., 2006; Watanabe et al., 2009), we conclude that it is highly likely that PnecC bacteria possess a ubiquitous distribution in permanent stagnant freshwater habitats world wide. Recent sampling of a broad variety of freshwater habitats in Central Africa supports this conclusion (Hahn et al., unpublished data). On the other hand, the presence of PnecC bacteria in saline inland waters is unlikely. Wu et al. (2006) could not detect them in lakes with salinities > 1 g.L−1, which is largely in accordance with results of ecophysiological investigations with cultivated strains (Wu et al., 2006; Hahn et al., in press). The distribution of PnecC bacteria along a salinity gradient in an estuarine lagoon is in agreement with the other observations on a low salinity tolerance of PnecC bacteria and indicates that it is unlikely that PnecC bacteria extent their habitat range to marine systems (Alonso et al., in press).
Very broad habitat range of P. necessarius subsp. asymbioticus
To our best knowledge, the report of the presence of a particular bacterial species in such a broad spectrum of freshwater systems is unique, however, this does not necessarily mean that the observed distribution is unusual for species of freshwater bacteria, because systematic investigations are very rare. For instance, a systematic investigation on the distribution of Candidatus Aquirestis calciphila (previously designated LD2 bacteria) and Candidatus Haliscomenobacter calcifugiens (previously designated GKS2-217 bacteria) across an ecologically more narrow set of 115 lentic freshwater systems revealed that both candidate species were restricted to a chemically defined range of systems (Schauer et al., 2005). The first taxon was absent in softwater systems but extended its habitat range to polysaline lakes of up to 20 g.L−1 salinity, while the second candidate species was exclusively found in softwater systems (Schauer et al., 2005; Wu et al., 2006), and both taxa were absent in systems with low pH values.
Several investigations employing the reverse line blot hybridization method with probes targeting species- or genus like taxa of freshwater bacteria revealed only for a minority of taxa an ubiquitous presence across the investigated freshwater habitats (Zwart et al., 2003; Lindström et al., 2005; Wu et al., 2006). Interestingly, all taxa with frequent detections represent rather broad phylogenetic groups (e.g., “Rhodoferax” sp. BAL47, and ACK-M1). On the other hand, a lack of detection could be caused by cell numbers below the detection limit of the method.
Environmental factors controlling abundance of PnecC bacteria
Our statistical analysis revealed that the relative and absolute abundance of PnecC bacteria is collectively influenced by environmental factors, including (i) altitude, surface area of habitats, pH, conductivity, (ii) A250nm, DOC, ratio A250nm/A365nm and A250nm /DOC and (iii) oxygen concentration. Apart from the influence of the oxygen concentration, all other influential parameters support the hypothesis that PnecC bacteria utilize substrates produced by photooxidation from humic substances (Watanabe et al., 2009). We observed significant correlations of the proxies for humic substance concentrations (A250nm, DOC) and quality of DOC (A250nm/A365nm, A250nm /DOC) with absolute and relative PnecC numbers (Table 2 and Fig. 5). Other parameters like pH and conductivity are negatively correlated with the concentration of humic substances (Fig. 5) and an interconnection of both habitat size and altitude with the proxies for humic substances is caused by the fact that the majority of the investigated humic-rich habitats are of smaller size and are located at higher altitudes.
Photooxidation of humic substances results in production of low molecular weight substances mainly represented by CO2, CO, short chain carboxyl (e.g., acetate) and carbonyl molecules (Bertilsson and Tranvik, 1998). The spectrum of organic substances utilized by PnecC bacteria (Watanabe et al., 2009; Hahn et al. in press) fits well to the spectrum of substances produced by photooxidation of humic material. Additionally, the uptake of acetate, a typical product of the photooxidation of humic substances, by PnecC bacteria was demonstrated under in situ conditions (Buck et al., 2009).
Remarkably, relative and absolute abundance of PnecC bacteria was very low (>0% – 0.5% of total bacterial numbers in the three samples with the highest estimated DOC (37 – 54 mg.L−1, Fig. 4C), which could indicate an inhibition of PnecC bacteria at higher DOC concentrations. On the other hand, the observed relative abundance of 8 % PnecC bacteria in Lake Tvigölingen (#126) with 37 mg.L−1 estimated DOC concentration (41 mg.L−1 determined by direct measurement) does not support the assumption of a general inhibition of PnecC bacteria at higher DOC concentrations.
The values of estimated DOC concentrations in our investigated habitats (mean DOC concentration 7.9 mg.L−1, 3.8% of habitats > 20 mg.L−1, maximum value 54 mg.L−1) is in a similar range as the distribution of DOC values determined for a global set of 7,514 lakes (mean DOC concentration 7.6 mg.L−1, 4.6% of habitats > 20 mg.L−1, maximum value 332 mg.L−1; Sobek et al., 2007). Thus, the relationship between DOC concentrations (A250nm) and relative abundance of PnecC bacteria revealed in our study could also be representative on a global scale. Combining the significant (P < 0.001) linear relationship observed in our study between estimated DOC concentration (A250nm) and relative PnecC abundance (Fig. 6, Fig. 4C) with the above mentioned determined mean DOC concentration of globally distributed lakes (Sobek et al., 2007) may indicates that PnecC bacteria contribute on average about 20% of the total bacterial numbers in this most likely representative global set of lakes. However, this extrapolation has to be treated cautiously, because it is unknown if the relationship between DOC concentration and %PnecC observed for European habitats holds true across other geographical regions and other climatic zones.
Eva: If the linear relationship between DOC concentrations (A250nm) and the relative abundance of PnecC bacteria revealed in our study is valid also globally, extrapolation to the above mentioned determined mean DOC concentration of globally distributed lakes (Sobek et al., 2007) indicates that PnecC bacteria could contribute on average to about 20% of the total bacterial numbers globally.
By contrast, the negative relationship of PnecC numbers to oxygen concentration cannot be explained entirely by oxygen consumption during photooxidation of DOC. PnecC bacteria were observed to occur with high numbers under environmental conditions, which exclude photo-oxidation of DOC, for instance, in the completely dark monimolimnion of Lake Krottensee (#13; Fig. 7). Previously reported PnecC populations in anoxic hypolimnia (Salcher et al., 2008; Taipale et al., 2009; Buck et al., 2009) could represent facultatively anaerobic PnecC strains (Hahn et al., in press), which grew under aerobic conditions before oxygen depletion occurred. However, the monimolimnic PnecC population in Lake Krottensee must represent a permanently anaerobically growing population since the last holomixis of this lake dates back at least decades if not centuries. Interestingly, a PnecC strain isolated from this meromictic lake from a depth of 35 meters could be cultivated aerobically, thus did not represent an obligate anaerobe.
We suggest that not all PnecC populations may exclusively rely on utilization of photodegradation products of humic substances. In shallow ponds like Rossmoos pond (#40; average water depths 10 to 20 cm) with low oxygen concentration (0.9 mg.L−1), but seemingly high anaerobic activity in the sediment (indicated by frequent release of gas bubbles), PnecC bacteria may oxidize fermentation products (e.g. organic acids like acetate) released from the sediment. Obviously, the spectrum of organic compounds produced by photooxidation of humic substances and by fermentative processes show pronounced overlaps.
Generalistic versus specialized adaptation of P. necessarius subsp. asymbioticus strains
The observed ubiquity of a so narrowly defined bacterial taxon seems to be surprising and raises questions whether PnecC bacteria are perfect generalists or if they represent an assemblage of differently adapted subgroups evolved by an adaptive radiation. Such subgroups may, for example, be adapted to low or high pH values and consequently inhabit only a subset of the range of investigated systems. Such subgroups could represent ecotypes within the subspecies P. necessarius subsp. asymbioticus, or they could represent cryptic species.
Experimental Procedures
Investigated habitats
In total 137 stagnant freshwater habitats including lakes, natural and artificial ponds, and puddles on forestry roads located in Central (Austria and Czech Republic) and Northern Europe (Sweden) were investigated (Fig. 1). Forty-five percent of the habitats are located in a four-cornered 2353 km2 area (defined by the geographic coordinates 47°58′6.00″N/13°5′44.00″E, 47°56′47.00″N/13°47′4.00″E, 47°39′48.00″N/13°59′40.00″E, 47°25′57.00″N/13°26′27.00″E). This primary study area represents the Salzkammergut lake district (SK), which is a mountainous area with numerous lakes and ponds located on the northern slope of the Alps. All but two lakes (> 1 ha) located in SK were sampled, as well as almost all smaller water bodies detectable on maps with scales of 1:50,000 (Table S1, Supplementary Material). Additionally, several smaller ponds and a few temporary puddles on forestry roads were sampled (Table 1). In total, the sampled habitats cover 5.5% of the planimetric area of SK. The entire SK area is located on limestone bedrock and consists of three separate catchment areas. Additional 76 selected habitats located outside of SK were sampled in order to enlarge the diversity of the investigated habitats (Fig. 1). This included alpine habitats located in the main range of the Austrian Alps, boreal habitats located in Sweden and artificially (acidic rain) acidified habitats in the Czech Republic characterized by high concentration of aluminum. The majority of these habitats were located on granitic bedrock. Some of the sampled habitats (including some SK habitats) are hydrologically decoupled from the underlying bedrock of their catchment area (e.g., raised bog systems) and therefore their inorganic water chemistry does not reflect the bedrock characteristics. All sampled habitats are listed in Table S1 (Supplementary Material). Habitat heterogenity is illustrated in Figs. 2 and 3.
Sampling of habitats
Almost all SK habitats were sampled in June and July 2006. Other habitats were sampled in the period 2006-2008 (mainly during the months June to September), and the Swedish habitats were sampled in 2002 (in June or October). Larger SK habitats were sampled at two to three sites and some of the habitats were sampled twice at different dates. However, distributions of PnecC across the same habitat showed a consistent, not markedly changing pattern, and we consequently decided to use data from only one sample per habitat in order to not over-represent particular habitats. Sampling of surface waters (0.5 m depths) along a transect (littoral, pelagial, littoral) across Lake Mondsee indicated a lack of differences in PnecC numbers between surface waters of the littoral and the pelagic zone, therefore, almost all habitats were sampled at a depths of about 0.5 m in the littoral zone (Swedish habitats were sampled at 0 to 1 m in the pelagic zone). Sampling of depth profiles was performed for a few habitats. Water temperature, pH, concentration of oxygen, and conductivity were measured on location. For the assessment of microbiological data, water samples were cooled and transported to the laboratory, where they were processed immediately (usually within a few hours after sampling). Subsamples were fixed with formaldehyde (2% final concentration) for determination of bacterial abundance, and with paraformaldehyde (2% final conc., for 4 hours at RT or over night at 4°C) for fluorescence in situ hybridization (FISH). Untreated samples for extraction of DNA were filtered (volumes of 200 to 500 mL) onto 0.2 μm Poretics filters and stored at −20°C until further processed.
Proxies for DOC and humic substances concentrations
The absorbance of 0.2 μm filtrated water samples were measured at 250, 365, 430 and 436 nm for estimation of DOC and humic substance concentration. Analysis of a set of 61 samples for which both the DOC concentration and the absorbance A250nm was measured demonstrated a significant linear correlation of these parameters (R2=0.9087, P<0.0001), therefore, we used A250nm values as proxies for DOC concentrations. Since 70-80% of DOC in freshwater systems are comprised by humic substances (Wetzel, 2001), A250nm values represent also a rough proxy for humic substance concentrations. Furthermore, the quality of DOC is roughly indicated by the ratio of A250nm/A365nm, because the proportion of small, presumably more labile, molecules in the DOC pool is increasing with this ratio (De Haan, 1972) and hence this parameter is inversely related to the molecular weight of dissolved organic matter (DOM) (Pages and Gadel 1990, Schwarz et al. 2002). Another DOC proxy used in this study was SUVA (A250/DOC), which is derived from SUVA254 (Weishaar et al. 2003) and is a good predictor of general chemical characteristics of DOC, namely dissolved aromatic carbon content.
Determination of total bacterial cell numbers
Formaldehyde fixed samples were stained with 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI, Sigma), filtered onto black 0.2 μm-pore-size Nuclepore filters (Millipore) and enumerated by epifluorescence microscopy (Zeiss Axioplan) under UV excitation at a magnification of 1250×. At least 500 cells were enumerated, covering the area of at least 10 microscopic fields.
Analysis of samples by fluorescence in situ hybridization (FISH)
Paraformaldehyde preserved samples were filtered onto 0.2 μm-pore-size, 47 mm diameter, Nuclepore filters (Millipore), rinsed with 1 mL of 1× PBS followed by 1 ml of sterile MilliQ water, air-dried and stored at −20°C until further processed. In situ hybridization of cut filter sections was performed following the protocol of Alfreider et al. (1996). For enumeration of P. necessarius the Cy-3-labeled probe PnecC-16S-445 (Hahn et al., 2005), specifically targeting the 16S rRNA of this taxon, was used.
DNA extraction and purification
Genomic DNA was extracted from biomass collected on 0.2 μm-pore-size filters (47 mm diameter) employing the standard phenol-chloroform extraction and subsequent ethanol precipitation, using the filters cut in halves and extracted in duplicates. Genomic DNA was purified using the Wizard DNA clean-up kit (Promega) and the concentration of DNA was measured using the NanoDrop ND-1000 UV-Vis Spectrophotometer.
Specific PCR for the detection of P. necessarius
A P. necessarius-specific PCR was performed for confirmation of the detection of P. necessarius by FISH by using the primers PnecCf-4 (sequence 5′-CAC ACT TAT CGG TTG ACA ATA A-3′) and PnecCr-5 (sequence 5′-AAC GAG CAC CAT TGC TAG Y-3′), which specifically bind at the beginning and the end of the 16S-23S ITS of P. necessarius bacteria. The conditions of the PCR reaction were as follows: Initial phase at 94°C for 3 minutes, followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 61°C for 1 min and extension at 72°C for 2 min. The final elongation at 72°C was run for 10 min.
Isolation of PnecC bacteria
For further confirmation of the presence of P. necessarius subsp. asymbioticus bacteria, strains were isolated from selected habitats by employing either the filtration-acclimatization method (Hahn et al., 2004) or the dilution-acclimatization method (Hahn et al., 2005). The 16S-23S ITS of isolated strains was sequenced for identification of the strains.
Statistical analysis
All data summarized in the Table S1 were used for the statistical analyses. The conductivity, surface, A250nm and real PnecC (#) data were logarithmically transformed prior to the analysis. Correlation analysis and graph visualisations were performed in the program package PRISM (GraphPad Software, Inc.). For multivariable analysis, the CANOCO program (Ter Braak and Smilauer, 1998) was used. Ordinations such as principal component analysis (PCA), redundancy analysis (RDA) and partial redundancy analysis (adjusted variation partitioning, Peres-Neto et al. 2006) were carried out with centering and standardization by species norm. Forward selection was used to choose significant explanatory (environmental) variables. Variables were included when p<0.05 was estimated by a Monte Carlo permutation test with 999 unrestricted permutations. The results of the analyses done in CANOCO were visualised by CanoDraw for Windows (Ter Braak and Šmilauer, 1998).
Supplementary Material
Table S1: List and details about all sampled habitats. Only data from one representative sample is shown for each habitat. Definition of groups: 1 – Salzkammergut area (SK), 2 – humic habitats, 3 – granitic bedrock, 4 – bog systems, 5 – acidified lakes.
Acknowledgements
This work was supported by the Austrian Science Fund (FWF) awarded to MWH and partially supported by the Grant Agency of the Czech Republic under research grant 208/05/0015 awarded to K. Šimek.
References
- Alonso C, Zeder M, Piccini C, Conde D, Pernthaler J. Ecophysiological differences of betaproteobacterial populations in two hydrochemically distinct compartments of a subtropical lagoon. Environ. Microb. 2009 doi: 10.1111/j.1462-2920.2008.01807.x. in press. [DOI] [PubMed] [Google Scholar]
- Bahr M, Hobbie JE, Sogin ML. Bacterial diversity in an arctic lake: a freshwaterSAR11 cluster. Aquat Microb Ecol. 1996;11:271–277. [Google Scholar]
- Bertilsson S, Tranvik LJ. Photochemically produced carboxylic acids as substrates for freshwater bacterioplankton. Limnol Oceanog. 1998;43:885–895. [Google Scholar]
- Buck U, Grossart HP, Amann R, Pernthaler J. Substrate incorporation patterns of bacterioplankton populations in stratified and mixed waters of a humic lake. Environ Microbiol. 2009 doi: 10.1111/j.1462-2920.2009.01910.x. In press, but already electronical (no paging yet) [DOI] [PubMed] [Google Scholar]
- Burkert U, Warnecke F, Babenzien D, Zwirnmann E, Pernthaler J. Members of a Readily Enriched β-Proteobacterial Clade Are Common in Surface Waters of a Humic Lake. Appl Environ Microbiol. 2003;69:6550–6559. doi: 10.1128/AEM.69.11.6550-6559.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump BC, Baross JA, Simenstad CA. Dominance of particle-attached bacteria in the Columbia River estuary, USA. Aqua Microb Ecol. 1998;14:7–18. [Google Scholar]
- Crump BC, Kling GW, Bahr M, Hobbie JE. Bacterioplankton community shifts in an arctic lake correlate with seasonal changes in organic matter source. Appl Environ Microb. 2003;69:2253–2268. doi: 10.1128/AEM.69.4.2253-2268.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump BC, Hobbie JE. Synchrony and seasonality in bacterioplankton communities of two temperate rivers. Limnol Oceanogr. 2005;50:1718–1729. [Google Scholar]
- De Haan H. Molecule-size distribution of soluble humic compounds from different natural waters. Freshwater Biol. 1972;2:235–241. [Google Scholar]
- Hahn MW. Isolation of strains belonging to the cosmopolitan Polynucleobacter necessarius cluster from freshwater habitats located in three climatic zones. Appl Environ Microbiol. 2003;69:5248–5254. doi: 10.1128/AEM.69.9.5248-5254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MW, Lang E, Brandt U, Wu QL, Scheuerl T. Emended description of the genus Polynucleobacter and the species P. necessarius and proposal of two subspecies, P. necessarius subspecies necessarius subsp. nov. and P. necessarius subsp. asymbioticus subsp. nov. 2009 doi: 10.1099/ijs.0.005801-0. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MW, Pöckl M, Wu QL. Low intraspecific diversity in a Polynucleobacter subcluster population numerically dominating bacterioplankton of a freshwater pond. Appl Environ Microbiol. 2005;71:4539–4547. doi: 10.1128/AEM.71.8.4539-4547.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MW, Stadler P, Wu QL, Pöckl M. The filtration-acclimatization method for isolation of an important fraction of the not readily cultivable bacteria. J Microb Meth. 2004;57:379–390. doi: 10.1016/j.mimet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Heckmann K, Schmidt HJ. Polynucleobacter necessarius gen. nov., sp. nov., an obligately endosymbiotic bacterium living in the cytoplasm of Euplotes. Int J Syst Bacteriol. 1987;37:456–457. [Google Scholar]
- Hiorns WD, Methé EA, Nierzwickibauer SA, Zehr JP. Bacterial diversity in Adirondack mountain lakes as revealed by 16S rRNA gene sequences. Appl Environ Microbiol. 1997;63:2957–2960. doi: 10.1128/aem.63.7.2957-2960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZI, Zinser ER, Coe A, McNulty NP, Woodward EMS, Chisholm SW. Niche partitioning among Prochlorococcus ecotypes along ocean-scale environmental gradients. Science. 2006;311:1737–1740. doi: 10.1126/science.1118052. [DOI] [PubMed] [Google Scholar]
- Lindström ES, Kamst-Van Agterveld MP, Zwart G. Distribution of typical freshwater bacterial groups is associated with pH, temperature, and lake water retention time. Appl Environ Microbiol. 2005;71:8201–8206. doi: 10.1128/AEM.71.12.8201-8206.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RM, Rappe MS, Connon SA, Vergin KL, Siebold WA, Carlson CA, Giovannoni SJ. SAR11 clade dominates ocean surface bacterioplankton communities. Nature. 2002;420:806–810. doi: 10.1038/nature01240. [DOI] [PubMed] [Google Scholar]
- Pages J, Gadel F. Dissolved organic matter and UV absorption in a tropical hyperhaline estuary. Sci. Total Environm. 1990;99:173–204. [Google Scholar]
- Pearce DA, van der Gast CJ, Lawley B, Ellis-Evans JC. Bacterioplankton community diversity in a maritime antarctic lake, determined by culture-dependent and culture-independent techniques. FEMS Microbiol. Ecol. 2003;45:59–70. doi: 10.1016/S0168-6496(03)00110-7. [DOI] [PubMed] [Google Scholar]
- Peres-Neto PR, Legendre P, Dray S, Borcard D. Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology. 2006;87(10):2614–2625. doi: 10.1890/0012-9658(2006)87[2614:vposdm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Percent SF, Frischer ME, Vescio PA, Duffy EB, Milano V, McLellan M, et al. Bacterial Community Structure of Acid-Impacted Lakes: What Controls Diversity? Appl. Environ. Microbiol. 2008;74:1856–1868. doi: 10.1128/AEM.01719-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcher MM, Pernthaler J, Zeder M, Psenner R, Posch T. Spatio-temporal niche separation of planktonic Betaproteobacteria in an oligo-mesotrophic lake. Environ Microbiol. 2008;10:2074–2086. doi: 10.1111/j.1462-2920.2008.01628.x. [DOI] [PubMed] [Google Scholar]
- Schauer M, Kamenik C, Hahn MW. Ecological differentiation within a cosmopolitan group of planktonic freshwater bacteria (SOL cluster, Saprospiraceae, Bacteroidetes) Appl Environ Microbiol. 2005;71:5900–5907. doi: 10.1128/AEM.71.10.5900-5907.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JN, Kowalczuk P, Kaczmarek S, Cota GF, Mitchel BG, Kahru M, Chavez FP. Two models for absorption by coloured dissolved organic matter (CDOM) Oceanologia. 2002;44:209–241. [Google Scholar]
- Selje N, Simon M, Brinkhoff T. A newly discovered Roseobacter cluster in temperate and polar oceans. Nature. 2004;427:445–448. doi: 10.1038/nature02272. [DOI] [PubMed] [Google Scholar]
- Sekiguchi H, Watanabe M, Nakahara T, Xu BH, Uchiyama H. Succession of bacterial community structure along the Changjiang River determined by denaturing gradient gel electrophoresis and clone library analysis. Appl Environ Microbiol. 2002;68:5142–5150. doi: 10.1128/AEM.68.10.5142-5150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobek S, Tranvik LJ, Prairie YT, Kortelainen P, Cole JJ. Patterns and regulation of dissolved organic carbon: An analysis of 7500 widely distributed lakes. Limnol. Oceanogr. 2007;52(3):1208–1219. [Google Scholar]
- Taipale S, Jones RI, Tiirola M. Vertical diversity of bacteria in an oxygen-stratified humic lake, evaluated using DNA and phospholipid analyses. Aquat Microb Ecol. 2009;55:1–16. [Google Scholar]
- Ter Braak CJF, Šmilauer P. CANOCO for Windows Version 4.02 Centre for Biometry Wageningen. CPRO-DLO. Wageningen; The Netherlands: 1998. [Google Scholar]
- Vannini C, Pöckl M, Petroni G, Wu QL, Lang E, Stackebrandt E, et al. Endosymbiosis in statu nascendi: close phylogenetic relationship between obligately endosymbiotic and obligately free-living Polynucleobacter strains (Betaproteobacteria) Environ Microbiol. 2007;9:347–359. doi: 10.1111/j.1462-2920.2006.01144.x. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Komatsu N, Ishii Y, Negishi M. Effective isolation of bacterioplankton genus Polynucleobacter from freshwater environments grown on photochemically degraded dissolved organic matter. FEMS Microb Ecol. 2009;67:57–68. doi: 10.1111/j.1574-6941.2008.00606.x. [DOI] [PubMed] [Google Scholar]
- Weishaar JL, Aiken GR, Bergamaschi BA, Fram MS, Fujii R, Mopper K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol. 2003;37:4702–4708. doi: 10.1021/es030360x. [DOI] [PubMed] [Google Scholar]
- Wetzel RG. Limnology. Lake and River Ecosystems. 3rd edn. Academic Press; San Diego, CA: 2001. [Google Scholar]
- Wu QL, Hahn MW. Differences in structure and dynamics of Polynucleobacter communities in a temperate and a subtropical lake revealed at three phylogenetic levels. FEMS Microb Ecol. 2006a;57:67–79. doi: 10.1111/j.1574-6941.2006.00105.x. [DOI] [PubMed] [Google Scholar]
- Wu QL, Hahn MW. High predictability of the seasonal dynamics of a species-like Polynucleobacter population in a freshwater lake. Environ Microbiol. 2006b;8:1660–1668. doi: 10.1111/j.1462-2920.2006.01049.x. [DOI] [PubMed] [Google Scholar]
- Wu QL, Schauer M, Kamst-Van Agterveld MP, Zwart G, Hahn MW. Bacterioplankton community composition along a salinity gradient of sixteen high mountain lakes located on the Tibetan Plateau, China. Appl Environ Microbiol. 2006;72:5478–5485. doi: 10.1128/AEM.00767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart G, Crump BC, Kamst-van Agterveld MP, Hagen F, Han S-K. Typical freshwater bacteria: an analysis of available 16S rRNA gene sequences from plankton of lakes and rivers. Aquat Microb Ecol. 2002;28:141–155. [Google Scholar]
- Zwart G, van Hannen EJ, Kamst-van Agterveld MP, van der Gucht K, Lindström ES, van Wichelen J, et al. Rapid Screening for Freshwater Bacterial Groups by Using Reverse Line Blot Hybridization. Appl Environ Microbiol. 2003;69:5875–5883. doi: 10.1128/AEM.69.10.5875-5883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: List and details about all sampled habitats. Only data from one representative sample is shown for each habitat. Definition of groups: 1 – Salzkammergut area (SK), 2 – humic habitats, 3 – granitic bedrock, 4 – bog systems, 5 – acidified lakes.