Abstract
In the asymmetric unit of the title compound, C19H17NO3, there are two crystallographically independent molecules, which are connected to each other by O—H⋯O hydrogen bonds, forming molecular chains as well as cyclic centrosymmetric R 2 2(16) dimers.
Related literature
For background literature, see: Barker et al. (2008 ▶); Gade (2002 ▶); Linton & Hamilton (1997 ▶); Valeur & Leray (2000 ▶); Wabnitz & Spencer (2002 ▶). For related structures, see: Gowda et al. (2000 ▶, 2006 ▶, 2007 ▶). For hydrogen-bond motifs, see: Bernstein et al. (1995 ▶). For bond-length data, see: Fun et al. (2008 ▶).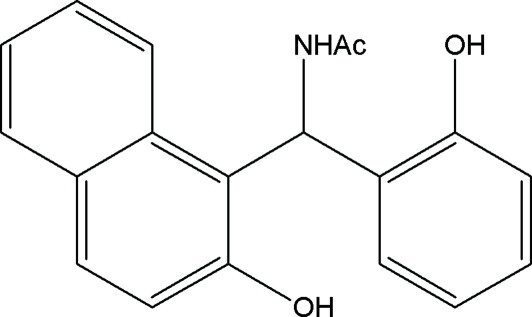
Experimental
Crystal data
C19H17NO3
M r = 307.34
Monoclinic,

a = 21.286 (5) Å
b = 17.9288 (4) Å
c = 19.524 (7) Å
β = 121.428 (1)°
V = 6358 (3) Å3
Z = 16
Mo Kα radiation
μ = 0.09 mm−1
T = 293 K
0.20 × 0.16 × 0.16 mm
Data collection
Bruker Kappa APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2004 ▶) T min = 0.980, T max = 0.986
33278 measured reflections
6464 independent reflections
4384 reflections with I > 2σ(I)
R int = 0.034
Refinement
R[F 2 > 2σ(F 2)] = 0.045
wR(F 2) = 0.146
S = 1.02
6464 reflections
417 parameters
H-atom parameters constrained
Δρmax = 0.47 e Å−3
Δρmin = −0.50 e Å−3
Data collection: APEX2 (Bruker, 2004 ▶); cell refinement: APEX2 and SAINT (Bruker, 2004 ▶); data reduction: SAINT and XPREP (Bruker, 2004 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶); software used to prepare material for publication: SHELXL97 and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809007983/pv2141sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809007983/pv2141Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1⋯O3i | 0.82 | 1.99 | 2.792 (2) | 166 |
| O2A—H2A1⋯O3ii | 0.82 | 1.89 | 2.702 (2) | 170 |
| O2—H2⋯O3Aiii | 0.82 | 1.81 | 2.588 (2) | 159 |
| O1A—H1A1⋯O3A | 0.82 | 2.16 | 2.928 (2) | 156 |
Symmetry codes: (i) 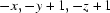 ; (ii)
; (ii) 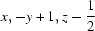 ; (iii)
; (iii)  .
.
Acknowledgments
The authors thank Dr Babu Vargheese, SAIF, IIT, Madras, India, for his help in collecting the X-ray intensity data. MNM and ASP thank Dr J. Jothi Kumar, Principal of Presidency College, Chennai, India, for providing computer and internet facilities.
supplementary crystallographic information
Comment
β-Acetamido ketones serve as potential intermediates in the synthesis of natural products and antibiotics (Wabnitz & Spencer, 2002). Due to the nucleophilic nature of benzylic hydroxyl groups these are usually protected during multi-step organic synthesis (Barker et al., 2008). Amide moiety and their metal ion complexes are widely used for their properties and potential applications (Gade, 2002; Valeur & Leray, 2000; Linton & Hamilton, 1997). The amide linkage [–NHC(O)-] is known to be strong enough to form and maintain protein architectures and has been utilized to create various molecular devices for a spectrum of purposes in organic chemistry. The effect of substituents on the solid state structures of N-aromatic amides have been described in the literature (Gowda et al., 2000, 2006, 2007). As part of our investigations on acetamide derivatives, the title compound, (I), has been prepared and its crystal structure is presented here.
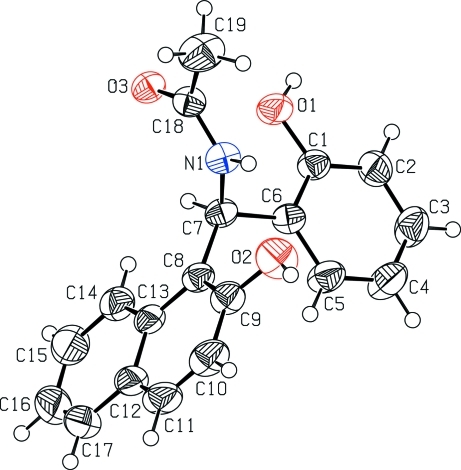
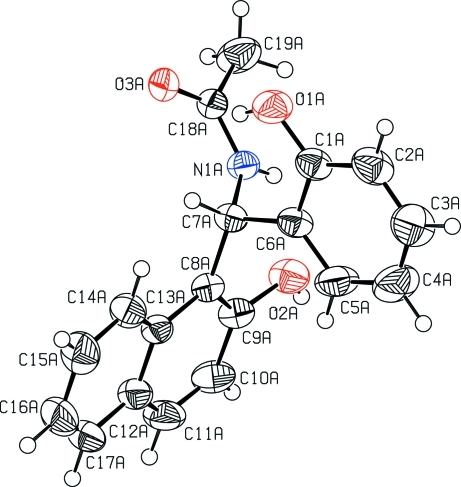
Figs. 1 and 2 show the molecular structures and conformations of the two crystallographically independent molecules, A (C1—C19, N1, O1, O2, O3) and B (C1A—C19A, N1A, O1A, O2A, O3A), in the asymmetric unit of (I), with the atomic numbering scheme. The bond lengths and angles in the two independent molecules agree with each other. The normal probability plot analyses (International Tables for X-ray Crystallography, 1974, Vol. IV, pp. 293–309) for both bond lengths and angles show that the differences between the two symmetry independent molecules are of a statistical nature. The bond distances of C18 = O3 and C18A = O3A [1.245 (2) and 1.244 (2) Å] for the molecules A and B, respectively, which are typical for double bonds (Fun et al., 2008).
In the molecules A and B, benzene and naphthalene rings are individually planar as expected. The deviations of the atoms O2 and O2A from the least-squares plane of the naphthalene rings are -0.075 (1) and 0.164 (1) Å. The deviations of the atoms O1 and O1A from the least-squares plane of the benzene rings are 0.056 (1) and 0.021 (1) Å. The dihedral angles between the naphthalene ring system and benzene rings are 75.7 (1) and 82.9 (1)° for molecules A and B respectively, and those between the fused rings are 0.3 (1) and 2.8 (1) °.
The crystal packing is stabilized by strong O—H···O inter and intramolecular hydrogen bonds and each molecule has a week intramolecular C—H···O interaction (Table 1). Considering only A-type molecules, atom O1 acts as a donar in a strong intermolecular O—H···O interaction via H1 with acetamido atom O3 of a symmetry related molecule, generating centrosymmetric hydrogen bonded dimers with a cyclic R22(16) ring system (Bernstein et al., 1995) (Fig. 3). The interlinking of A and B molecules via strong O—H···O hydrogen bond generates infinite chains running along c axis. The atoms O3 and O3a act as a acceptors for all inter and intramolecular interactions.
Experimental
A mixture of 2-hydroxybenzaldehyde (10 mmol), β-naphthol (10 mmol) and iodine (0.4 mmol, 4 mol%) were mixed in acetonitrile (5 ml). To the suspension acetyl chloride (2.8 mmol, 0.2 ml) was added and the reaction mixture was stirred at room temperature for 6 h. After the completion of the reaction (as monitored by TLC), saturated sodium thiosulfate solution (5 ml) was added. The precipitated solid was filtered and dried. The dried sample was washed with diethyl ether (2 × 10 ml) and again dried. Single crystals of the title compound suitable for X-ray diffraction were obtained by slow evaporation of a solution in ethanol.
Refinement
All H atoms were positioned geometrically, with N—H = 0.86, O—H = 0.82 and C—H = 0.93, 0.98 and 0.96 Å, for aromatic, methylene and methyl H-atoms, respectively, and constrained to ride on their parent atoms, with Uiso(H) = xUeq(C, N), where x = 1.5 for methyl H, and x = 1.2 for all other H atoms.
Figures
Fig. 1.
One of the two independent molecules in the asymmetric unit of (I), showing the atom-numbering scheme. Displacement ellipsoids are drawn at the 30% probability level.
Fig. 2.
The other independent molecules in the asymmetric unit of (I), showing the atom-numbering scheme. Displacement ellipsoids are drawn at the 30% probability level.
Fig. 3.
Part of the crystal structure of (I), showing the R22(16) rings. For the sake of clarity, H atoms not involved in the hydrogen bonding have been omitted for clarity. Hydrogen bonding is shown as dashed lines. [Symmetry codes: (*) -x, -y + 1, -z + 1]
Crystal data
| C19H17NO3 | F(000) = 2592 |
| Mr = 307.34 | Dx = 1.284 Mg m−3 |
| Monoclinic, C2/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -C 2yc | Cell parameters from 6464 reflections |
| a = 21.286 (5) Å | θ = 2.5–25° |
| b = 17.9288 (4) Å | µ = 0.09 mm−1 |
| c = 19.524 (7) Å | T = 293 K |
| β = 121.428 (1)° | Prism, colourless |
| V = 6358 (3) Å3 | 0.20 × 0.16 × 0.16 mm |
| Z = 16 |
Data collection
| Bruker Kappa APEXII CCD diffractometer | 6464 independent reflections |
| Radiation source: fine-focus sealed tube | 4384 reflections with I > 2σ(I) |
| graphite | Rint = 0.034 |
| ω and φ scans | θmax = 26.4°, θmin = 2.2° |
| Absorption correction: multi-scan (SADABS; Bruker, 2004) | h = −26→26 |
| Tmin = 0.980, Tmax = 0.986 | k = −22→22 |
| 33278 measured reflections | l = −24→23 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.045 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.146 | H-atom parameters constrained |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0747P)2 + 2.6626P] where P = (Fo2 + 2Fc2)/3 |
| 6464 reflections | (Δ/σ)max = 0.005 |
| 417 parameters | Δρmax = 0.47 e Å−3 |
| 0 restraints | Δρmin = −0.49 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.05068 (7) | 0.50195 (8) | 0.59220 (8) | 0.0556 (4) | |
| H1 | 0.0080 | 0.5100 | 0.5793 | 0.083* | |
| O2 | 0.30227 (7) | 0.59048 (8) | 0.70861 (10) | 0.0655 (4) | |
| H2 | 0.3361 | 0.6207 | 0.7274 | 0.098* | |
| O3 | 0.09894 (7) | 0.46622 (7) | 0.47788 (7) | 0.0427 (3) | |
| N1 | 0.17949 (8) | 0.53425 (8) | 0.58664 (9) | 0.0413 (4) | |
| H1A | 0.2011 | 0.5768 | 0.6017 | 0.050* | |
| C1 | 0.09638 (10) | 0.51566 (10) | 0.67223 (11) | 0.0415 (4) | |
| C2 | 0.07009 (11) | 0.53887 (11) | 0.72002 (13) | 0.0531 (5) | |
| H2A | 0.0200 | 0.5475 | 0.6975 | 0.064* | |
| C3 | 0.11779 (12) | 0.54928 (13) | 0.80092 (14) | 0.0619 (6) | |
| H3 | 0.1000 | 0.5648 | 0.8332 | 0.074* | |
| C4 | 0.19150 (13) | 0.53670 (14) | 0.83396 (13) | 0.0659 (6) | |
| H4 | 0.2238 | 0.5436 | 0.8887 | 0.079* | |
| C5 | 0.21785 (11) | 0.51384 (12) | 0.78609 (12) | 0.0533 (5) | |
| H5 | 0.2680 | 0.5051 | 0.8092 | 0.064* | |
| C6 | 0.17118 (9) | 0.50360 (10) | 0.70431 (11) | 0.0391 (4) | |
| C7 | 0.19871 (9) | 0.47856 (9) | 0.65004 (10) | 0.0376 (4) | |
| H7 | 0.1727 | 0.4323 | 0.6237 | 0.045* | |
| C8 | 0.28056 (9) | 0.46165 (10) | 0.69505 (11) | 0.0399 (4) | |
| C9 | 0.33020 (10) | 0.52005 (11) | 0.72284 (12) | 0.0473 (5) | |
| C10 | 0.40660 (11) | 0.50776 (14) | 0.76622 (13) | 0.0600 (6) | |
| H10 | 0.4390 | 0.5479 | 0.7834 | 0.072* | |
| C11 | 0.43294 (11) | 0.43680 (15) | 0.78296 (13) | 0.0641 (6) | |
| H11 | 0.4836 | 0.4290 | 0.8120 | 0.077* | |
| C12 | 0.38526 (12) | 0.37479 (13) | 0.75730 (12) | 0.0552 (5) | |
| C13 | 0.30753 (10) | 0.38715 (11) | 0.71243 (10) | 0.0431 (4) | |
| C14 | 0.26164 (12) | 0.32330 (11) | 0.68782 (12) | 0.0531 (5) | |
| H14 | 0.2108 | 0.3296 | 0.6590 | 0.064* | |
| C15 | 0.28989 (16) | 0.25316 (13) | 0.70521 (14) | 0.0739 (7) | |
| H15 | 0.2584 | 0.2123 | 0.6876 | 0.089* | |
| C16 | 0.36600 (18) | 0.24206 (16) | 0.74933 (16) | 0.0850 (9) | |
| H16 | 0.3850 | 0.1939 | 0.7615 | 0.102* | |
| C17 | 0.41189 (15) | 0.30083 (17) | 0.77423 (14) | 0.0747 (8) | |
| H17 | 0.4624 | 0.2926 | 0.8033 | 0.090* | |
| C18 | 0.13247 (10) | 0.52537 (10) | 0.50919 (11) | 0.0398 (4) | |
| C19 | 0.12036 (14) | 0.59206 (12) | 0.45734 (14) | 0.0709 (7) | |
| H19A | 0.1426 | 0.5835 | 0.4261 | 0.106* | |
| H19B | 0.1422 | 0.6352 | 0.4906 | 0.106* | |
| H19C | 0.0685 | 0.6002 | 0.4221 | 0.106* | |
| O1A | −0.04220 (8) | 0.82226 (9) | 0.16047 (8) | 0.0572 (4) | |
| H1A1 | 0.0018 | 0.8190 | 0.1765 | 0.086* | |
| O2A | 0.05897 (8) | 0.65781 (7) | 0.02450 (8) | 0.0532 (4) | |
| H2A1 | 0.0688 | 0.6224 | 0.0051 | 0.080* | |
| O3A | 0.11589 (7) | 0.79276 (7) | 0.26185 (8) | 0.0472 (3) | |
| N1A | 0.06323 (8) | 0.72763 (8) | 0.14574 (8) | 0.0364 (3) | |
| H1A2 | 0.0553 | 0.6835 | 0.1257 | 0.044* | |
| C1A | −0.08229 (10) | 0.80834 (10) | 0.08044 (12) | 0.0444 (4) | |
| C2A | −0.15812 (11) | 0.80967 (13) | 0.03873 (14) | 0.0591 (6) | |
| H2A2 | −0.1826 | 0.8196 | 0.0656 | 0.071* | |
| C3A | −0.19743 (13) | 0.79645 (16) | −0.04221 (16) | 0.0768 (8) | |
| H3A | −0.2486 | 0.7973 | −0.0700 | 0.092* | |
| C4A | −0.16199 (13) | 0.78190 (18) | −0.08270 (15) | 0.0820 (8) | |
| H4A | −0.1888 | 0.7730 | −0.1377 | 0.098* | |
| C5A | −0.08569 (12) | 0.78056 (14) | −0.04061 (12) | 0.0613 (6) | |
| H5A | −0.0617 | 0.7708 | −0.0680 | 0.074* | |
| C6A | −0.04459 (10) | 0.79339 (10) | 0.04089 (11) | 0.0412 (4) | |
| C7A | 0.03910 (9) | 0.79041 (9) | 0.08923 (10) | 0.0361 (4) | |
| H7A | 0.0562 | 0.8361 | 0.1214 | 0.043* | |
| C8A | 0.07419 (9) | 0.78832 (9) | 0.03854 (10) | 0.0361 (4) | |
| C9A | 0.08438 (10) | 0.72246 (10) | 0.00967 (11) | 0.0421 (4) | |
| C10A | 0.11902 (12) | 0.72005 (12) | −0.03502 (12) | 0.0547 (5) | |
| H10A | 0.1268 | 0.6744 | −0.0520 | 0.066* | |
| C11A | 0.14087 (12) | 0.78344 (13) | −0.05326 (12) | 0.0573 (6) | |
| H11A | 0.1644 | 0.7810 | −0.0821 | 0.069* | |
| C12A | 0.12871 (10) | 0.85341 (11) | −0.02947 (11) | 0.0471 (5) | |
| C13A | 0.09502 (9) | 0.85622 (10) | 0.01715 (10) | 0.0385 (4) | |
| C14A | 0.08239 (10) | 0.92775 (10) | 0.03835 (12) | 0.0478 (5) | |
| H14A | 0.0605 | 0.9315 | 0.0689 | 0.057* | |
| C15A | 0.10157 (12) | 0.99152 (12) | 0.01502 (14) | 0.0618 (6) | |
| H15A | 0.0927 | 1.0378 | 0.0299 | 0.074* | |
| C16A | 0.13439 (13) | 0.98752 (14) | −0.03111 (15) | 0.0678 (7) | |
| H16A | 0.1471 | 1.0310 | −0.0471 | 0.081* | |
| C17A | 0.14749 (12) | 0.92046 (14) | −0.05222 (13) | 0.0609 (6) | |
| H17A | 0.1695 | 0.9183 | −0.0826 | 0.073* | |
| C18A | 0.09592 (9) | 0.73285 (9) | 0.22438 (10) | 0.0376 (4) | |
| C19A | 0.10774 (14) | 0.66185 (12) | 0.26910 (13) | 0.0664 (6) | |
| H19D | 0.1576 | 0.6455 | 0.2912 | 0.100* | |
| H19E | 0.0746 | 0.6245 | 0.2332 | 0.100* | |
| H19F | 0.0986 | 0.6698 | 0.3118 | 0.100* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0317 (7) | 0.0809 (10) | 0.0451 (8) | 0.0032 (6) | 0.0138 (6) | −0.0079 (7) |
| O2 | 0.0455 (8) | 0.0528 (9) | 0.0865 (11) | −0.0171 (6) | 0.0263 (8) | −0.0101 (8) |
| O3 | 0.0459 (7) | 0.0437 (7) | 0.0373 (7) | −0.0037 (5) | 0.0210 (6) | 0.0021 (5) |
| N1 | 0.0413 (8) | 0.0384 (8) | 0.0412 (9) | −0.0073 (6) | 0.0195 (7) | 0.0029 (6) |
| C1 | 0.0359 (10) | 0.0454 (10) | 0.0392 (10) | −0.0024 (7) | 0.0170 (8) | −0.0011 (8) |
| C2 | 0.0417 (11) | 0.0628 (13) | 0.0575 (13) | 0.0017 (9) | 0.0278 (10) | −0.0055 (10) |
| C3 | 0.0575 (14) | 0.0827 (16) | 0.0557 (14) | −0.0054 (11) | 0.0367 (12) | −0.0149 (11) |
| C4 | 0.0558 (14) | 0.0982 (18) | 0.0403 (12) | −0.0084 (12) | 0.0226 (11) | −0.0121 (11) |
| C5 | 0.0383 (10) | 0.0776 (14) | 0.0405 (12) | −0.0030 (9) | 0.0181 (9) | −0.0024 (10) |
| C6 | 0.0347 (9) | 0.0431 (9) | 0.0384 (10) | −0.0037 (7) | 0.0183 (8) | 0.0017 (8) |
| C7 | 0.0344 (9) | 0.0394 (9) | 0.0353 (10) | −0.0045 (7) | 0.0156 (8) | 0.0017 (7) |
| C8 | 0.0336 (9) | 0.0510 (10) | 0.0345 (10) | −0.0024 (7) | 0.0173 (8) | −0.0013 (8) |
| C9 | 0.0376 (10) | 0.0579 (12) | 0.0466 (12) | −0.0060 (8) | 0.0221 (9) | −0.0049 (9) |
| C10 | 0.0359 (11) | 0.0898 (17) | 0.0515 (13) | −0.0125 (11) | 0.0208 (10) | −0.0118 (11) |
| C11 | 0.0339 (11) | 0.108 (2) | 0.0452 (13) | 0.0087 (11) | 0.0170 (9) | −0.0039 (12) |
| C12 | 0.0496 (12) | 0.0800 (15) | 0.0343 (11) | 0.0183 (11) | 0.0207 (9) | 0.0040 (10) |
| C13 | 0.0440 (10) | 0.0565 (11) | 0.0288 (9) | 0.0069 (8) | 0.0190 (8) | 0.0016 (8) |
| C14 | 0.0631 (13) | 0.0502 (11) | 0.0416 (11) | 0.0043 (9) | 0.0243 (10) | 0.0037 (9) |
| C15 | 0.103 (2) | 0.0525 (13) | 0.0569 (15) | 0.0120 (12) | 0.0349 (14) | 0.0057 (11) |
| C16 | 0.116 (2) | 0.0699 (17) | 0.0615 (16) | 0.0430 (17) | 0.0412 (17) | 0.0142 (14) |
| C17 | 0.0735 (16) | 0.096 (2) | 0.0478 (14) | 0.0407 (15) | 0.0266 (12) | 0.0112 (13) |
| C18 | 0.0392 (10) | 0.0421 (10) | 0.0400 (11) | 0.0004 (7) | 0.0220 (8) | 0.0062 (8) |
| C19 | 0.0904 (17) | 0.0539 (13) | 0.0561 (14) | −0.0071 (11) | 0.0296 (13) | 0.0165 (11) |
| O1A | 0.0482 (8) | 0.0822 (10) | 0.0491 (9) | 0.0035 (7) | 0.0308 (7) | −0.0040 (7) |
| O2A | 0.0785 (10) | 0.0377 (7) | 0.0558 (9) | −0.0008 (6) | 0.0437 (8) | −0.0059 (6) |
| O3A | 0.0497 (8) | 0.0510 (8) | 0.0430 (8) | −0.0150 (6) | 0.0257 (6) | −0.0112 (6) |
| N1A | 0.0441 (8) | 0.0343 (7) | 0.0327 (8) | 0.0001 (6) | 0.0212 (7) | −0.0004 (6) |
| C1A | 0.0443 (11) | 0.0470 (10) | 0.0448 (11) | 0.0062 (8) | 0.0253 (9) | 0.0098 (8) |
| C2A | 0.0468 (12) | 0.0752 (15) | 0.0633 (15) | 0.0115 (10) | 0.0343 (11) | 0.0171 (11) |
| C3A | 0.0406 (12) | 0.117 (2) | 0.0626 (16) | 0.0098 (12) | 0.0201 (12) | 0.0232 (14) |
| C4A | 0.0492 (14) | 0.140 (3) | 0.0415 (13) | 0.0071 (14) | 0.0133 (11) | 0.0125 (14) |
| C5A | 0.0530 (13) | 0.0939 (17) | 0.0376 (12) | 0.0075 (11) | 0.0242 (10) | 0.0092 (11) |
| C6A | 0.0427 (10) | 0.0449 (10) | 0.0398 (10) | 0.0053 (7) | 0.0241 (8) | 0.0085 (8) |
| C7A | 0.0433 (10) | 0.0335 (8) | 0.0362 (10) | 0.0019 (7) | 0.0241 (8) | 0.0024 (7) |
| C8A | 0.0378 (9) | 0.0396 (9) | 0.0314 (9) | 0.0033 (7) | 0.0184 (8) | 0.0018 (7) |
| C9A | 0.0482 (11) | 0.0436 (10) | 0.0361 (10) | 0.0038 (8) | 0.0231 (9) | 0.0012 (8) |
| C10A | 0.0682 (14) | 0.0587 (13) | 0.0486 (12) | 0.0087 (10) | 0.0384 (11) | −0.0051 (10) |
| C11A | 0.0640 (14) | 0.0759 (15) | 0.0486 (13) | 0.0025 (11) | 0.0410 (11) | −0.0010 (10) |
| C12A | 0.0430 (11) | 0.0617 (12) | 0.0361 (10) | −0.0028 (8) | 0.0203 (9) | 0.0056 (9) |
| C13A | 0.0350 (9) | 0.0464 (10) | 0.0310 (9) | −0.0001 (7) | 0.0151 (8) | 0.0038 (7) |
| C14A | 0.0511 (11) | 0.0443 (10) | 0.0497 (12) | 0.0021 (8) | 0.0275 (10) | 0.0048 (9) |
| C15A | 0.0647 (14) | 0.0450 (11) | 0.0678 (15) | −0.0017 (9) | 0.0291 (12) | 0.0083 (10) |
| C16A | 0.0716 (15) | 0.0637 (15) | 0.0650 (15) | −0.0153 (11) | 0.0335 (13) | 0.0165 (12) |
| C17A | 0.0608 (13) | 0.0790 (16) | 0.0479 (13) | −0.0136 (11) | 0.0319 (11) | 0.0091 (11) |
| C18A | 0.0360 (9) | 0.0429 (10) | 0.0353 (10) | −0.0027 (7) | 0.0194 (8) | −0.0010 (8) |
| C19A | 0.0892 (17) | 0.0554 (13) | 0.0411 (12) | −0.0012 (11) | 0.0245 (12) | 0.0092 (10) |
Geometric parameters (Å, °)
| O1—C1 | 1.365 (2) | O1A—H1A1 | 0.8200 |
| O1—H1 | 0.8200 | O2A—C9A | 1.372 (2) |
| O2—C9 | 1.361 (2) | O2A—H2A1 | 0.8200 |
| O2—H2 | 0.8200 | O3A—C18A | 1.243 (2) |
| O3—C18 | 1.246 (2) | N1A—C18A | 1.318 (2) |
| N1—C18 | 1.317 (2) | N1A—C7A | 1.469 (2) |
| N1—C7 | 1.473 (2) | N1A—H1A2 | 0.8600 |
| N1—H1A | 0.8600 | C1A—C2A | 1.378 (3) |
| C1—C2 | 1.379 (3) | C1A—C6A | 1.400 (3) |
| C1—C6 | 1.391 (2) | C2A—C3A | 1.369 (3) |
| C2—C3 | 1.374 (3) | C2A—H2A2 | 0.9300 |
| C2—H2A | 0.9300 | C3A—C4A | 1.373 (4) |
| C3—C4 | 1.369 (3) | C3A—H3A | 0.9300 |
| C3—H3 | 0.9300 | C4A—C5A | 1.386 (3) |
| C4—C5 | 1.380 (3) | C4A—H4A | 0.9300 |
| C4—H4 | 0.9300 | C5A—C6A | 1.378 (3) |
| C5—C6 | 1.384 (3) | C5A—H5A | 0.9300 |
| C5—H5 | 0.9300 | C6A—C7A | 1.521 (2) |
| C6—C7 | 1.521 (3) | C7A—C8A | 1.520 (2) |
| C7—C8 | 1.518 (2) | C7A—H7A | 0.9800 |
| C7—H7 | 0.9800 | C8A—C9A | 1.374 (2) |
| C8—C9 | 1.382 (3) | C8A—C13A | 1.430 (2) |
| C8—C13 | 1.423 (3) | C9A—C10A | 1.406 (3) |
| C9—C10 | 1.405 (3) | C10A—C11A | 1.344 (3) |
| C10—C11 | 1.360 (3) | C10A—H10A | 0.9300 |
| C10—H10 | 0.9300 | C11A—C12A | 1.408 (3) |
| C11—C12 | 1.410 (3) | C11A—H11A | 0.9300 |
| C11—H11 | 0.9300 | C12A—C17A | 1.410 (3) |
| C12—C17 | 1.412 (3) | C12A—C13A | 1.423 (3) |
| C12—C13 | 1.429 (3) | C13A—C14A | 1.416 (3) |
| C13—C14 | 1.416 (3) | C14A—C15A | 1.370 (3) |
| C14—C15 | 1.358 (3) | C14A—H14A | 0.9300 |
| C14—H14 | 0.9300 | C15A—C16A | 1.400 (4) |
| C15—C16 | 1.397 (4) | C15A—H15A | 0.9300 |
| C15—H15 | 0.9300 | C16A—C17A | 1.347 (3) |
| C16—C17 | 1.344 (4) | C16A—H16A | 0.9300 |
| C16—H16 | 0.9300 | C17A—H17A | 0.9300 |
| C17—H17 | 0.9300 | C18A—O3A | 1.243 (2) |
| C18—O3 | 1.246 (2) | C18A—O3A | 1.243 (2) |
| C18—C19 | 1.499 (3) | C18A—C19A | 1.489 (3) |
| C19—H19A | 0.9600 | C19A—H19D | 0.9600 |
| C19—H19B | 0.9600 | C19A—H19E | 0.9600 |
| C19—H19C | 0.9600 | C19A—H19F | 0.9600 |
| O1A—C1A | 1.357 (2) | ||
| C1—O1—H1 | 109.5 | C9A—O2A—H2A1 | 109.5 |
| C9—O2—H2 | 109.5 | C18A—N1A—C7A | 125.89 (14) |
| C18—N1—C7 | 126.49 (14) | C18A—N1A—H1A2 | 117.1 |
| C18—N1—H1A | 116.8 | C7A—N1A—H1A2 | 117.1 |
| C7—N1—H1A | 116.8 | O1A—C1A—C2A | 121.13 (18) |
| O1—C1—C2 | 122.04 (16) | O1A—C1A—C6A | 118.25 (16) |
| O1—C1—C6 | 116.91 (17) | C2A—C1A—C6A | 120.61 (19) |
| C2—C1—C6 | 121.03 (17) | C3A—C2A—C1A | 120.1 (2) |
| C3—C2—C1 | 120.10 (18) | C3A—C2A—H2A2 | 119.9 |
| C3—C2—H2A | 120.0 | C1A—C2A—H2A2 | 119.9 |
| C1—C2—H2A | 120.0 | C2A—C3A—C4A | 120.6 (2) |
| C4—C3—C2 | 119.9 (2) | C2A—C3A—H3A | 119.7 |
| C4—C3—H3 | 120.1 | C4A—C3A—H3A | 119.7 |
| C2—C3—H3 | 120.1 | C3A—C4A—C5A | 119.2 (2) |
| C3—C4—C5 | 120.1 (2) | C3A—C4A—H4A | 120.4 |
| C3—C4—H4 | 120.0 | C5A—C4A—H4A | 120.4 |
| C5—C4—H4 | 120.0 | C6A—C5A—C4A | 121.6 (2) |
| C6—C5—C4 | 121.34 (19) | C6A—C5A—H5A | 119.2 |
| C6—C5—H5 | 119.3 | C4A—C5A—H5A | 119.2 |
| C4—C5—H5 | 119.3 | C5A—C6A—C1A | 117.88 (17) |
| C5—C6—C1 | 117.60 (18) | C5A—C6A—C7A | 122.95 (17) |
| C5—C6—C7 | 122.49 (16) | C1A—C6A—C7A | 119.16 (16) |
| C1—C6—C7 | 119.90 (16) | N1A—C7A—C8A | 111.85 (13) |
| N1—C7—C8 | 110.73 (14) | N1A—C7A—C6A | 109.32 (13) |
| N1—C7—C6 | 110.26 (14) | C8A—C7A—C6A | 114.26 (14) |
| C8—C7—C6 | 113.48 (14) | N1A—C7A—H7A | 107.0 |
| N1—C7—H7 | 107.4 | C8A—C7A—H7A | 107.0 |
| C8—C7—H7 | 107.4 | C6A—C7A—H7A | 107.0 |
| C6—C7—H7 | 107.4 | C9A—C8A—C13A | 118.19 (16) |
| C9—C8—C13 | 119.13 (17) | C9A—C8A—C7A | 121.65 (15) |
| C9—C8—C7 | 119.21 (16) | C13A—C8A—C7A | 120.11 (15) |
| C13—C8—C7 | 121.61 (15) | O2A—C9A—C8A | 118.40 (16) |
| O2—C9—C8 | 117.37 (16) | O2A—C9A—C10A | 119.78 (16) |
| O2—C9—C10 | 120.90 (18) | C8A—C9A—C10A | 121.81 (17) |
| C8—C9—C10 | 121.71 (19) | C11A—C10A—C9A | 120.26 (18) |
| C11—C10—C9 | 119.6 (2) | C11A—C10A—H10A | 119.9 |
| C11—C10—H10 | 120.2 | C9A—C10A—H10A | 119.9 |
| C9—C10—H10 | 120.2 | C10A—C11A—C12A | 121.17 (19) |
| C10—C11—C12 | 121.49 (19) | C10A—C11A—H11A | 119.4 |
| C10—C11—H11 | 119.3 | C12A—C11A—H11A | 119.4 |
| C12—C11—H11 | 119.3 | C11A—C12A—C17A | 121.7 (2) |
| C11—C12—C17 | 122.1 (2) | C11A—C12A—C13A | 118.89 (17) |
| C11—C12—C13 | 118.98 (19) | C17A—C12A—C13A | 119.44 (19) |
| C17—C12—C13 | 119.0 (2) | C14A—C13A—C12A | 117.06 (16) |
| C14—C13—C8 | 123.81 (17) | C14A—C13A—C8A | 123.40 (17) |
| C14—C13—C12 | 117.12 (18) | C12A—C13A—C8A | 119.53 (16) |
| C8—C13—C12 | 119.06 (18) | C15A—C14A—C13A | 121.6 (2) |
| C15—C14—C13 | 121.8 (2) | C15A—C14A—H14A | 119.2 |
| C15—C14—H14 | 119.1 | C13A—C14A—H14A | 119.2 |
| C13—C14—H14 | 119.1 | C14A—C15A—C16A | 120.4 (2) |
| C14—C15—C16 | 120.4 (3) | C14A—C15A—H15A | 119.8 |
| C14—C15—H15 | 119.8 | C16A—C15A—H15A | 119.8 |
| C16—C15—H15 | 119.8 | C17A—C16A—C15A | 119.7 (2) |
| C17—C16—C15 | 120.1 (2) | C17A—C16A—H16A | 120.2 |
| C17—C16—H16 | 119.9 | C15A—C16A—H16A | 120.2 |
| C15—C16—H16 | 119.9 | C16A—C17A—C12A | 121.8 (2) |
| C16—C17—C12 | 121.6 (2) | C16A—C17A—H17A | 119.1 |
| C16—C17—H17 | 119.2 | C12A—C17A—H17A | 119.1 |
| C12—C17—H17 | 119.2 | O3A—C18A—N1A | 123.91 (16) |
| O3—C18—N1 | 124.33 (16) | O3A—C18A—N1A | 123.91 (16) |
| O3—C18—N1 | 124.33 (16) | O3A—C18A—N1A | 123.91 (16) |
| O3—C18—C19 | 119.64 (17) | O3A—C18A—C19A | 119.49 (17) |
| O3—C18—C19 | 119.64 (17) | O3A—C18A—C19A | 119.49 (17) |
| N1—C18—C19 | 116.02 (17) | O3A—C18A—C19A | 119.49 (17) |
| C18—C19—H19A | 109.5 | N1A—C18A—C19A | 116.59 (16) |
| C18—C19—H19B | 109.5 | C18A—C19A—H19D | 109.5 |
| H19A—C19—H19B | 109.5 | C18A—C19A—H19E | 109.5 |
| C18—C19—H19C | 109.5 | H19D—C19A—H19E | 109.5 |
| H19A—C19—H19C | 109.5 | C18A—C19A—H19F | 109.5 |
| H19B—C19—H19C | 109.5 | H19D—C19A—H19F | 109.5 |
| C1A—O1A—H1A1 | 109.5 | H19E—C19A—H19F | 109.5 |
| O1—C1—C2—C3 | 177.50 (19) | C6A—C1A—C2A—C3A | −0.1 (3) |
| C6—C1—C2—C3 | −1.1 (3) | C1A—C2A—C3A—C4A | −0.1 (4) |
| C1—C2—C3—C4 | 0.1 (3) | C2A—C3A—C4A—C5A | 0.1 (4) |
| C2—C3—C4—C5 | 0.2 (4) | C3A—C4A—C5A—C6A | 0.1 (4) |
| C3—C4—C5—C6 | 0.4 (4) | C4A—C5A—C6A—C1A | −0.3 (3) |
| C4—C5—C6—C1 | −1.3 (3) | C4A—C5A—C6A—C7A | 178.4 (2) |
| C4—C5—C6—C7 | 179.9 (2) | O1A—C1A—C6A—C5A | −178.92 (18) |
| O1—C1—C6—C5 | −177.01 (17) | C2A—C1A—C6A—C5A | 0.3 (3) |
| C2—C1—C6—C5 | 1.7 (3) | O1A—C1A—C6A—C7A | 2.3 (2) |
| O1—C1—C6—C7 | 1.8 (2) | C2A—C1A—C6A—C7A | −178.44 (17) |
| C2—C1—C6—C7 | −179.54 (17) | C18A—N1A—C7A—C8A | 122.33 (17) |
| C18—N1—C7—C8 | 120.86 (18) | C18A—N1A—C7A—C6A | −110.10 (18) |
| C18—N1—C7—C6 | −112.71 (19) | C5A—C6A—C7A—N1A | −114.0 (2) |
| C5—C6—C7—N1 | −121.32 (19) | C1A—C6A—C7A—N1A | 64.7 (2) |
| C1—C6—C7—N1 | 59.9 (2) | C5A—C6A—C7A—C8A | 12.2 (2) |
| C5—C6—C7—C8 | 3.5 (2) | C1A—C6A—C7A—C8A | −169.11 (15) |
| C1—C6—C7—C8 | −175.20 (15) | N1A—C7A—C8A—C9A | 39.0 (2) |
| N1—C7—C8—C9 | 49.0 (2) | C6A—C7A—C8A—C9A | −85.9 (2) |
| C6—C7—C8—C9 | −75.7 (2) | N1A—C7A—C8A—C13A | −143.40 (15) |
| N1—C7—C8—C13 | −133.86 (17) | C6A—C7A—C8A—C13A | 91.73 (19) |
| C6—C7—C8—C13 | 101.53 (19) | C13A—C8A—C9A—O2A | −174.72 (15) |
| C13—C8—C9—O2 | −176.66 (17) | C7A—C8A—C9A—O2A | 2.9 (3) |
| C7—C8—C9—O2 | 0.6 (3) | C13A—C8A—C9A—C10A | 4.5 (3) |
| C13—C8—C9—C10 | 1.5 (3) | C7A—C8A—C9A—C10A | −177.87 (17) |
| C7—C8—C9—C10 | 178.75 (18) | O2A—C9A—C10A—C11A | 176.72 (19) |
| O2—C9—C10—C11 | 176.8 (2) | C8A—C9A—C10A—C11A | −2.5 (3) |
| C8—C9—C10—C11 | −1.3 (3) | C9A—C10A—C11A—C12A | −1.0 (3) |
| C9—C10—C11—C12 | 0.4 (3) | C10A—C11A—C12A—C17A | −176.2 (2) |
| C10—C11—C12—C17 | 179.9 (2) | C10A—C11A—C12A—C13A | 2.3 (3) |
| C10—C11—C12—C13 | 0.2 (3) | C11A—C12A—C13A—C14A | −178.70 (18) |
| C9—C8—C13—C14 | 179.16 (18) | C17A—C12A—C13A—C14A | −0.2 (3) |
| C7—C8—C13—C14 | 2.0 (3) | C11A—C12A—C13A—C8A | −0.2 (3) |
| C9—C8—C13—C12 | −0.8 (3) | C17A—C12A—C13A—C8A | 178.39 (17) |
| C7—C8—C13—C12 | −177.97 (17) | C9A—C8A—C13A—C14A | 175.33 (17) |
| C11—C12—C13—C14 | 179.99 (19) | C7A—C8A—C13A—C14A | −2.4 (3) |
| C17—C12—C13—C14 | 0.3 (3) | C9A—C8A—C13A—C12A | −3.1 (2) |
| C11—C12—C13—C8 | −0.1 (3) | C7A—C8A—C13A—C12A | 179.18 (15) |
| C17—C12—C13—C8 | −179.79 (19) | C12A—C13A—C14A—C15A | 0.1 (3) |
| C8—C13—C14—C15 | 179.4 (2) | C8A—C13A—C14A—C15A | −178.39 (18) |
| C12—C13—C14—C15 | −0.7 (3) | C13A—C14A—C15A—C16A | 0.2 (3) |
| C13—C14—C15—C16 | 0.9 (4) | C14A—C15A—C16A—C17A | −0.4 (3) |
| C14—C15—C16—C17 | −0.7 (4) | C15A—C16A—C17A—C12A | 0.3 (4) |
| C15—C16—C17—C12 | 0.3 (4) | C11A—C12A—C17A—C16A | 178.5 (2) |
| C11—C12—C17—C16 | −179.8 (2) | C13A—C12A—C17A—C16A | −0.1 (3) |
| C13—C12—C17—C16 | −0.1 (4) | C7A—N1A—C18A—O3A | −5.8 (3) |
| C7—N1—C18—O3 | −2.7 (3) | C7A—N1A—C18A—O3A | −5.8 (3) |
| C7—N1—C18—O3 | −2.7 (3) | C7A—N1A—C18A—O3A | −5.8 (3) |
| C7—N1—C18—C19 | 177.57 (19) | C7A—N1A—C18A—C19A | 173.15 (18) |
| O1A—C1A—C2A—C3A | 179.1 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1···O3i | 0.82 | 1.99 | 2.792 (2) | 166 |
| O2A—H2A1···O3ii | 0.82 | 1.89 | 2.702 (2) | 170 |
| O2—H2···O3Aiii | 0.82 | 1.81 | 2.588 (2) | 159 |
| O1A—H1A1···O3A | 0.82 | 2.16 | 2.928 (2) | 156 |
| C7—H7···O3 | 0.98 | 2.51 | 2.901 (2) | 104 |
| C7A—H7A···O3A | 0.98 | 2.47 | 2.879 (2) | 105 |
Symmetry codes: (i) −x, −y+1, −z+1; (ii) x, −y+1, z−1/2; (iii) −x+1/2, −y+3/2, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: PV2141).
References
- Barker, D., Lehmann, A. L., Mai, A., Khan, G. S. & Ng, E. (2008). Tetrahedron Lett.49, 1660–1664.
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N. L. (1995). Angew. Chem. Int. Ed. Engl.34, 1555–1573.
- Bruker (2004). APEX2, SAINT, XPREP and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Fun, H.-K., Jebas, S. R., Jana, S., Chakrabarty, R. & Goswami, S. (2008). Acta Cryst. E64, o699. [DOI] [PMC free article] [PubMed]
- Gade, L. H. (2002). Acc. Chem. Res.35, 575–582. [DOI] [PubMed]
- Gowda, B. T., Foro, S. & Fuess, H. (2007). Acta Cryst. E63, o3364.
- Gowda, B. T., Paulus, H. & Fuess, H. (2000). Z. Naturforsch. Teil A, 55, 711–720.
- Gowda, B. T., Paulus, H., Kozisek, J., Tokarcik, M. & Fuess, H. (2006). Z. Naturforsch. Teil A, 61, 675–682.
- Linton, B. & Hamilton, A. D. (1997). Chem. Rev.97, 1669–1680. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Valeur, B. & Leray, I. (2000). Coord. Chem. Rev.205, 3–40.
- Wabnitz, T. C. & Spencer, J. B. (2002). Tetrahedron Lett.43, 3891–3894.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809007983/pv2141sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809007983/pv2141Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report