Abstract
Ion-pair reversed-phase high performance liquid chromatography (IP RP HPLC) is presented as a new, superior method for the analysis of RNA. IP RP HPLC provides a fast and reliable alternative to classical methods of RNA analysis, including separation of different RNA species, quantification and purification. RNA is stable under the analysis conditions used; degradation of RNA during the analyses was not observed. The versatility of IP RP HPLC for RNA analysis is demonstrated. Components of an RNA ladder, ranging in size from 155 to 1770 nt, were resolved. RNA transcripts of up to 5219 nt were analyzed, their integrity determined and they were quantified and purified. Purification of mRNA from total RNA is described, separating mouse rRNA from poly(A)+ mRNA. IP RP HPLC is also suitable for the separation and purification of DIG-labeled from unlabeled RNA. RNA purified by IP RP HPLC exhibits improved stability.
INTRODUCTION
A number of labor intensive and time consuming techniques are currently available for RNA isolation, purification and quantification. Quantification of RNA samples is performed by measuring their absorption at 260 nm, while the quality and integrity of RNA samples are generally determined by gel electrophoresis followed by ethidium bromide visualization (1–3). Absorption measurements, however, do not provide information about the integrity (impurities and degradation) of the RNA sample analyzed. Analysis of RNA by electrophoretic techniques creates the potential for RNA degradation by exogenous RNases due to prolonged handling. Neither one of the above methods is suitable for RNA purification. Furthermore, aliquots of RNA analyzed by either procedure are not recoverable for use in downstream applications.
Currently the only mRNA purification technology available is that of oligo(dT) selection (1,2). A number of hybridization- and affinity-based methods are available for the isolation and purification of labeled or unlabeled specific mRNA species (4–6). However, recoveries for purified mRNA species obtained with these procedures are generally low and the mRNA recovered often exhibits varying degrees of purity and integrity (due to the presence of degraded RNA, proteins or genomic DNA).
The analysis of DNA by ion-pair reversed-phase high performance liquid chromatography (IP RP HPLC) under non-denaturing, partially denaturing or fully denaturing conditions has gained widespread acceptance over the past years. IP RP HPLC under non-denaturing conditions provides a means for sequence-independent sizing of DNA fragments of up to 2000 bp (7,8). The main impact of HPLC performed under partially denaturing conditions has been in mutation detection by means of heteroduplex analysis (9,10). IP RP HPLC under fully denaturing conditions is described in the literature for analysis of oligonucleotides (11,12) and single-stranded (ss)DNA fragments of up to 100 nt (13). Under fully denaturing conditions separation of ssDNA on an alkylated poly(styrene divinylbenzene) matrix is sequence dependent. Separation characteristics of RNA are expected to resemble those of ssDNA under fully denaturing conditions.
In this report the use of IP RP HPLC technology under fully denaturing conditions is extended to RNA analysis. This technology can be applied to qualification, quantification and purification of a wide range of labeled/unlabeled RNA samples, such as discrete transcripts, rRNA, mRNA and total RNA. The technology is shown to offer a significant improvement over current methods of RNA analysis.
MATERIALS AND METHODS
Materials
The WAVE® System with DNASep® cartridges (7.8 × 50 mm) and triethylammonium acetate (TEAA) was provided by Transgenomic Inc. (San Jose, CA). RNA ladders were purchased from Gibco BRL (Rockville, MD). Total RNA and mRNA samples were obtained from Clontech (Palo Alto, CA). In vitro transcription kits for RNA synthesis were purchased from Ambion (Austin, TX). Digoxigenin (DIG)-labeled β-actin RNA, the Titan reverse transcription PCR kit and MS2 bacteriophage RNA were purchased from Roche Molecular Biochemicals (Indianapolis, IN). The β-actin-specific reverse transcription PCR primers used were 5′-GTC GAC AAC GGC TCC GGC ATG-3′ and 5′-GGA TCT TCA TGA GGT AGT CAG-3′, yielding a 550 bp product.
HPLC analysis
All RNA samples were analyzed by IP RP HPLC on the WAVE System using a DNASep cartridge. The stationary phase of the cartridge consists of a non-porous, alkylated poly(styrene divinylbenzene) matrix. Chromatography was performed using a two eluent buffer system. Buffer A consists of an aqueous solution of 0.1 M TEAA, pH 7.0, and buffer B consists of an aqueous solution of 0.1 M TEAA, pH 7.0, with 25% (v/v) acetonitrile. RNA analyses were performed under fully denaturing conditions at 75°C. Sizing of dsDNA was performed under non-denaturing conditions at 50°C. Chromatograms were recorded at a wavelength of 260 nm. Quantification of peaks was performed by integration of peak areas. RNA for downstream uses was purified by peak capture. IP RP HPLC analyses were performed using the following gradient conditions: condition 1, flow rate 0.9 ml/min, 38–40% B over 1.0 min, to 60% B over 15 min, to 66% B over 6.0 min, to 70% B over 0.5 min, to 100% B over 0.5 min, held at 100% B for 1 min, to 38% B over 1 min, held at 38% B for 2 min; condition 2, flow rate 0.9 ml/min, 38–60% B over 30 min, to 100% B over 2 min, to 38% B over 3 min; condition 3, flow rate 0.9 ml/min, 38–40% B over 1 min, to 60% B over 3 min, to 100% B over 1 min, held at 100% B for 6 min, to 38% B over 1 min, held at 38% B for 1 min.
RNA purification
RNA was purified by manual peak collection. Fractions were collected for 1 min, corresponding to a fraction volume of 0.9 ml at a flow rate of 0.9 ml/min. Collected RNA samples may be precipitated or used directly in the collection buffer for downstream uses. For reverse transcription PCR studies 5 µl aliquots of collected fractions were used directly. Precipitation of eluted RNA samples was by addition of 10% (v/v) precipitation buffer (10 mM Tris–HCl, pH 7.0, 1 mM EDTA, 3.0 M NaCl), 1% (v/v) glycogen (10 mg/ml) and 2.5 vol ethanol. Samples were kept at –70°C for 10 min or at –20°C for 2 h before centrifugation at 13 000 r.p.m. for 15 min at 4°C. All precipitated RNA fractions were reconstituted in DEPC-treated water.
RESULTS AND DISCUSSION
Resolution of discrete RNA fragments and determination of RNA integrity
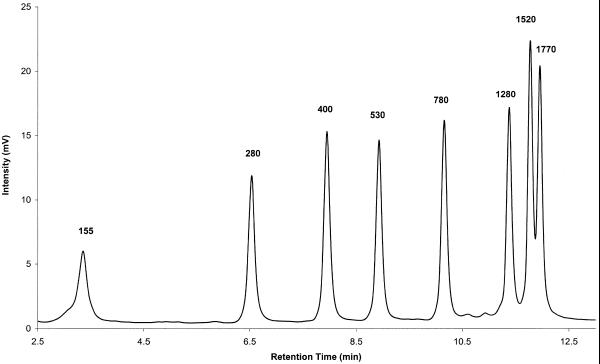
IP RP HPLC under fully denaturing conditions with on-line UV detection offers a sensitive and reliable method for the detection and analysis of RNA transcripts and size markers. The integrity of RNA is not compromised under the analysis conditions used, 75°C and elution buffers containing TEAA and acetonitrile. Figure 1 shows the chromatogram of an RNA ladder containing fragments ranging in size from 155 to 1770 nt. The integrity of the individual fragments is apparent from their well-defined peak shape. Degradation of RNA, which would result in the appearance of spurious peaks in the chromatogram, is not observed.
Figure 1.
Separation of the components of an RNA ladder by IP RP HPLC. An RNA ladder (1 µg) containing fragments ranging in size from 155 to 1770 nt was analyzed by IP RP HPLC using gradient condition 1.
Recently, Georgopoulos and Leibowitz (14) reported the fractionation of ssRNA molecules ranging in size from 200 to 1000 nt by IP RP HPLC. The authors claim that in the size range 400–1000 nt retention times are strictly size dependent. However, they do note that chromatographic mobility of ssRNA molecules may be determined by other factors in addition to size. This concession is prompted by the observation of aberrant retention times of some of the RNA molecules. Oefner reported a clear sequence dependence of retention time for ssDNA molecules (<100 nt in length) analyzed by IP RP HPLC under fully denaturing conditions (7). Based on the reports by Georgopoulos and Leibowitz (14) as well as Oefner (7), retention times for ssRNA fragments were expected to be sequence dependent. We analyzed various RNA size markers and transcripts of known sequence and were able to confirm the sequence dependence of retention time for RNA fragments (data not shown).
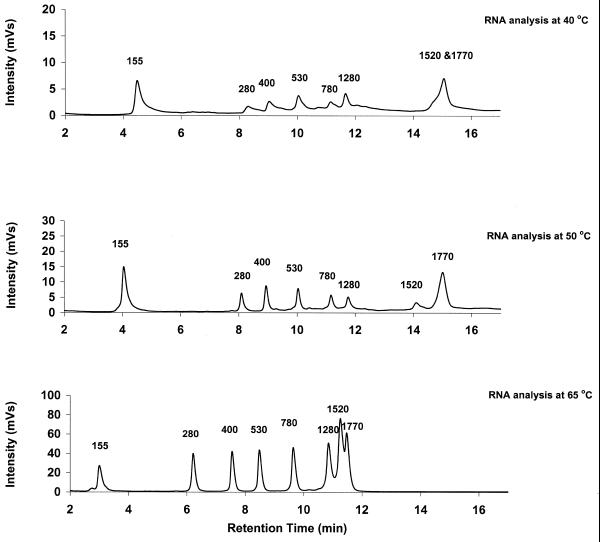
Oefner (13) recently reported a strong effect of analysis temperature on resolution for ssDNA fragments. Resolution generally improved with increasing temperature. Thus, the effect of temperature on resolution was attributed to the disruption of intra- and intermolecular interactions. In agreement with Oefner’s observations for ssDNA, we noticed a decrease in resolution for RNA at lower analysis temperatures (Fig. 2). Consequently, all RNA analyses were performed at 75°C.
Figure 2.
Effect of analysis temperature on resolution of RNA fragments. The RNA ladder shown in Figure 1 was analyzed by IP RP HPLC at three different temperatures using gradient condition 1. Analysis of the ladder at 75°C is shown in Figure 1.
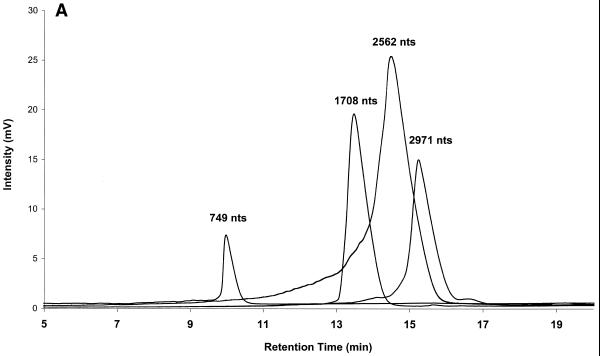
The ability to separate RNA fragments by IP RP HPLC under fully denaturing conditions can be used to determine the integrity and purity of RNA transcripts. In order to demonstrate this, different plasmid vectors were digested with restriction endonucleases and transcribed to generate specific RNA transcripts of known length and sequence. Each individual transcript was analyzed by IP RP HPLC under fully denaturing conditions to determine its quality and purity. Several superimposed chromatograms of individual transcripts are shown in Figure 3A. The purity and integrity of each transcript in Figure 3A is apparent from the presence of a single peak.
Figure 3.
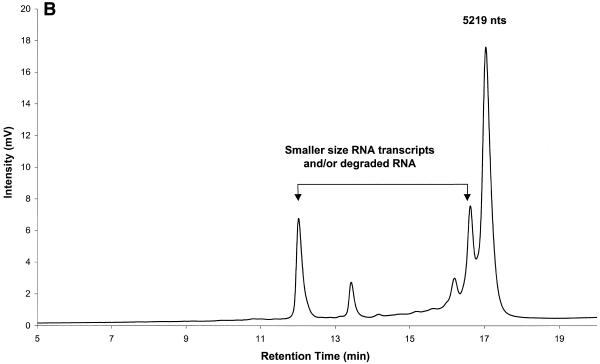
Analysis of RNA transcript integrity by IP RP HPLC. (A) RNA transcripts ranging in size from 749 to 2971 nt were analyzed individually by IP RP HPLC using gradient condition 2. The superimposed chromatograms of four individual transcripts are shown. (B) A crude transcription reaction product is shown. The full-length transcription product has a length of 5219 nt. Analysis was performed under elution condition 2.
RNA undergoing degradation is broken down into smaller fragments. These breakdown products are retained less strongly on the cartridge and result in peaks that elute at shorter retention times. Early termination products of transcription also result in shorter RNA fragments, which likewise elute prior to the full-length RNA transcript. The detection of RNA degradation and spurious transcription products is shown in Figure 3B, which shows the analysis of a crude 5219 nt RNA transcript. Peaks preceding the full-length 5219 nt transcript indicate the presence of spurious transcription and/or degradation products in this crude transcription reaction. Therefore, IP RP HPLC may be used to assess the quality and integrity of transcripts. Furthermore, chromatography has the advantage over other technologies that samples may be recovered by peak capture. Thus, IP RP HPLC can also be used for transcript purification.
RNA quantification
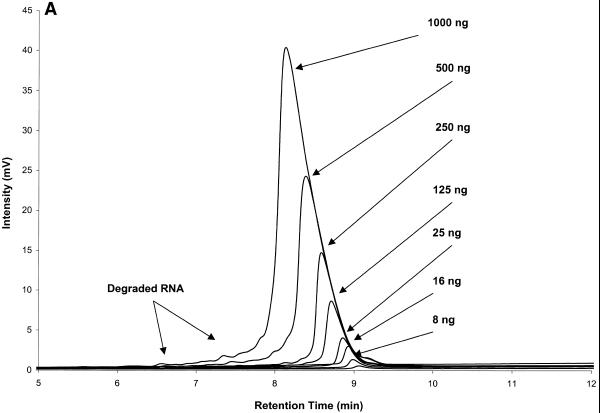
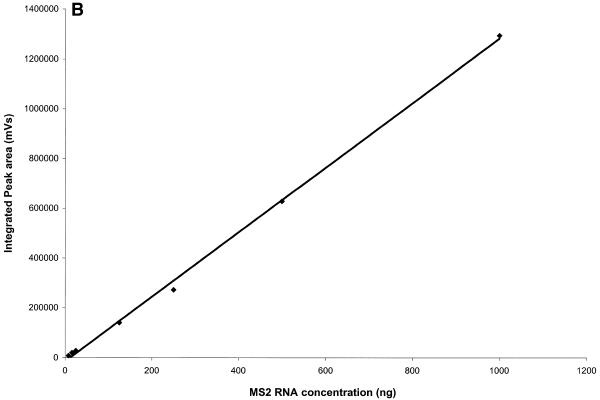
IP RP HPLC provides a reliable and accurate method for RNA quantification by peak integration. RNA quantities per peak in the low nanogram range can be detected. The largest amount of RNA loaded for an individual peak in Figure 4A is 1000 ng. Amounts of total RNA of up to 100 µg have been analyzed and fractionated on the cartridge used here (see below). Analysis and quantification of MS2 RNA, which is 3569 nt long, is shown in Figure 4. Amounts loaded ranged from 8 to 1000 ng. RNA quantification was performed by peak integration. Results are shown in Table 1 and graphed in Figure 4B. A correlation factor of 1250 mVs/ng RNA was used for the determination of RNA quantities. This factor is dependent on flow rate and may also vary between instruments. The procedure for determining the correlation factor is described in Transgenomic Application Note 115 (http://www.transgenomic.com/pdf/AN115.pdf ) for DNA. Figure 4 shows that peak area of an individual fragment increases linearly with the amount of RNA analyzed from the low nanogram range to at least 1000 ng.
Figure 4.
RNA quantification after IP RP HPLC by peak integration. (A) Superimposed chromatograms of known amounts of MS2 RNA analyzed by IP RP HPLC under gradient condition 2. (B) Standard curve for MS2 RNA quantification, plot of integrated peak area from (A) versus injected amount of MS2 RNA (Table 1). The square of the Pearson product moment correlation coefficient (R2) through the given data points is 0.9985.
Table 1. MS2 RNA quantification results.
| MS2 RNA injected (ng) |
Integrated peak area (mVs) |
Calculated RNA amount (ng) |
| 8 | 8062 | 6.4 |
| 16 | 20 380 | 16.3 |
| 25 | 28 245 | 22.6 |
| 125 | 140 173 | 112.1 |
| 250 | 272 300 | 217.8 |
| 500 | 628 035 | 502.4 |
| 1000 | 1 292 338 | 1033.8 |
Purification of unlabeled and DIG-labeled RNA transcripts
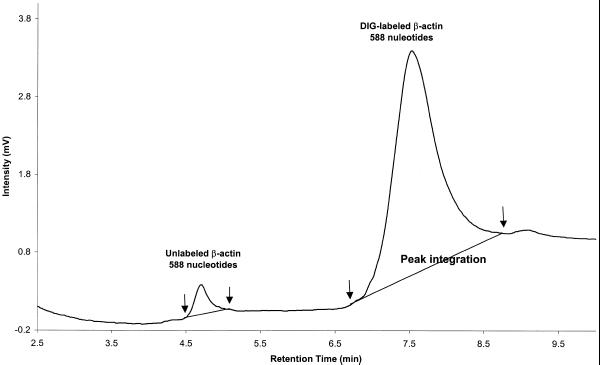
RNA fragments separated by IP RP HPLC can be recovered after analysis. Figure 5 shows the separation of DIG-labeled β-actin RNA standard (588 nt) from unlabeled β-actin RNA transcript, which was present in small amounts. Two well-resolved peaks corresponding to unlabeled (retention time 4.7 min) and DIG-labeled (retention time 7.8 min) β-actin RNA are observed. Retention times of nucleic acids on the DNASep cartridge are determined by the hydrophobicity of the molecules analyzed (9,10). The β-actin RNA labeled with the hydrophobic DIG tag shows a greater retention time than unlabeled β-actin RNA. Individual peaks were captured by manual collection. The presence and integrity of captured β-actin RNA, both unlabeled and labeled, was confirmed by reverse transcription–PCR using 5 µl aliquots of each collected sample. A 550 bp PCR product was successfully amplified from the labeled and unlabeled β-actin RNA fractions. Fractions collected before 4 or after 9 min during the run served as negative controls and did not produce a 550 bp PCR product (data not shown).
Figure 5.
Separation and purification of unlabeled and labeled β-actin RNA transcripts. DIG-labeled β-actin RNA transcript (1 µg) was analyzed by IP RP HPLC under elution condition 3. Unlabeled β-actin RNA elutes with a retention time of ∼4.7 min. DIG-labeled β-actin RNA elutes at the end of the gradient. Each peak was captured by manual collection.
RNA samples collected during IP RP HPLC were more stable in the collection buffer than expected. RNA purified by peak capture was stable for days when kept at room temperature and for months when kept at –20 or –70°C. Stability of RNA after collection was assessed by gene-specific reverse transcription–PCR on mRNA template after purification by IP RP HPLC (manuscript in preparation). Our explanation for this observation is that since proteins have a very low affinity for the alkylated poly(styrene divinylbenzene) matrix under the elution conditions used for RNA analysis, they are hardly retained during IP RP HPLC. RNases and other proteins elute at an early stage in the gradient and are removed from RNA samples during analysis. Thus, RNA purified by IP RP HPLC exhibits improved stability.
Purification of mRNA from total RNA by IP RP HPLC
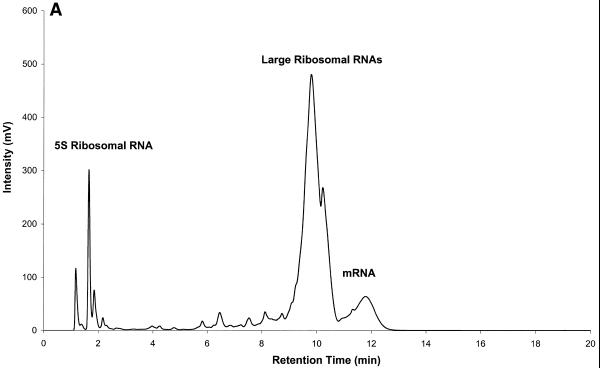
IP RP HPLC can be used for the separation and purification of rRNA and mRNA. An analysis of mouse brain total RNA (Clontech, Palo Alto, CA) on a DNASep cartridge is shown in Figure 6A. The 5S, 18S (1900 nt) and 28S (4700 nt) rRNA fragments elute before the bulk of mRNA fragments. It was pointed out above that retention times of RNA fragments are sequence dependent. However, the reasons for this apparent separation of rRNA and mRNA require some explanation. The alkylated, hydrophobic column matrix interacts directly with the hydrophobic alkyl chains of the TEAA contained in the elution buffer. The positively charged ammonium ions in turn interact with the negatively charged phosphate backbone of nucleic acids, thereby mediating the interaction between nucleic acids and column matrix. In addition to the latter interactions, the exposed bases of single-stranded nucleic acids themselves interact with the column matrix due to their hydrophobic character. Different bases exhibit different degrees of hydrophobicity. This is the basis for the sequence dependence of retention time in IP RP HPLC under the analysis conditions described here. Hydrophobicity increases in the order C < G < T < A (7). Thus, it is expected that single-stranded nucleic acid molecules with a high adenine content exhibit longer retention times than those with high guanine or cytosine content. Polyadenylated mRNA is therefore retained more strongly on the DNASep cartridge than rRNA, which is not polyadenylated.
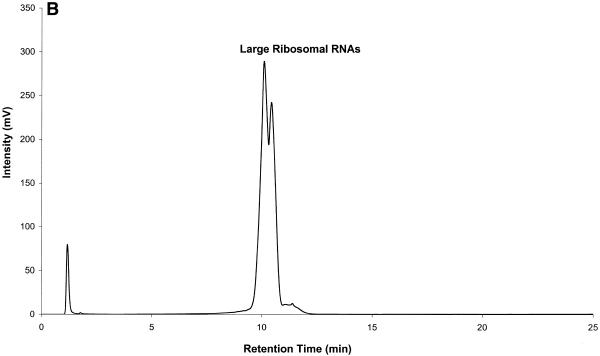
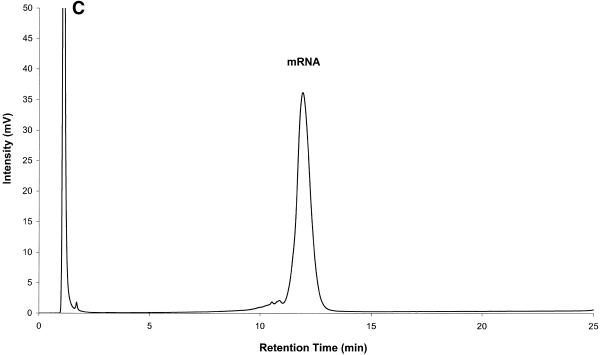
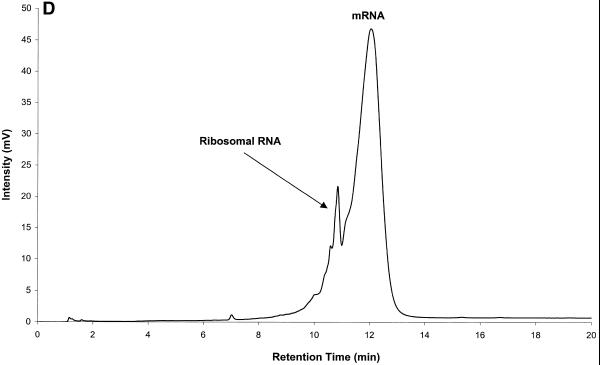
Figure 6.
Mouse brain total RNA analyzed by IP RP HPLC. All RNA samples were analyzed by IP RP HPLC using gradient condition 1. (A) Mouse brain total RNA (20 µg). (B) Analysis of large rRNAs (a mixture of co-eluting 28S and 18S rRNA, respectively) purified by peak capture from total RNA. (C) Analysis of mRNA purified by peak capture from total RNA. (D) Analysis of mouse brain poly(A)+ mRNA (5 µg) obtained after two rounds of oligo(dT)–cellulose purification.
Figure 6B and C shows rRNA and mRNA purified by peak capture. Fractions containing rRNA and mRNA were collected. RNA was precipitated, resuspended in 20 µl of DEPC-treated water and re-analyzed by IP RP HPLC. Using primers specific for 18S rRNA (Ambion, Austin, TX) a 495 bp product was successfully amplified from rRNA fractions. Using primers specific for β-actin message a 550 bp PCR product was amplified from mRNA fractions (data not shown). rRNA purified by IP RP HPLC contains only traces of mRNA and purified mRNA contains only trace amounts of rRNA. We concede that some mRNA species, mainly shorter mRNA species, are lost in this purification procedure due to co-elution with rRNA. However, we point out that the bulk of the mRNA, particularly longer mRNA species, is captured.
The hydrophobicity ratios calculated for both 18S and 28S rRNA were 0.8 and 0.5, respectively. From these ratios it was expected that 28S should elute before 18S rRNA in Figure 6A. Performing reverse transcription–PCR studies on the collected rRNA fractions (using gene-specific primers to 18S and 28S RNA) it was concluded that this is indeed the case. However, these studies also indicated that under the gradient conditions used here the two rRNA peaks are not well resolved, i.e. the peaks for the 28S and 18S rRNA show significant overlap. Thus, the rRNA peaks in Figure 6A and B are labeled as large rRNAs.
For comparative purposes mouse brain mRNA (Clontech), which was isolated by two rounds of poly(A)+ RNA selection, was analyzed by IP RP HPLC. As shown in Figure 6D, this sample still contained some rRNA contamination (confirmed by reverse transcription–PCR analysis) after two rounds of poly(A)+ selection. Significantly less rRNA contamination is observed after only a single round of purification by IP RP HPLC (Fig. 7C). Recovery of RNA after IP RP HPLC is generally higher than that after oligo(dT)–cellulose purification. Purification of RNA samples by IP RP HPLC can be performed in a matter of minutes. Classical methods of mRNA purification require several hours of labor intensive manipulation.
In conclusion, the new technology presented here for RNA analysis and purification combines the high resolution capability of IP RP HPLC, the high sensitivity of on-line UV detection and ease of sample recovery to provide a reliable and fast alternative to current, conventional methods of RNA analysis.
References
- 1. Promega Corp. (1996) Protocols and Applications Guide, 3rd Edn. Promega Corp., Madison, WI, Chap. 6.
- 2.Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, Chap. 7.
- 3.Alwine J.C., Kemp,D.J. and Stark,G.R. (1977) Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc. Natl Acad. Sci. USA, 74, 5350–5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bach H.J., Hartmann,A., Trevors,J.T. and Munch,J.C. (1999) Magnetic capture-hybridization method for purification and probing of mRNA for neutral protease of Bacillus cereus. J. Microbiol. Methods, 37, 187–192. [DOI] [PubMed] [Google Scholar]
- 5.Prescott A.M. and Fricker,C.R. (1999) Use of PNA oligonucleotides for the in situ detection of Escherichia coli in water. Mol. Cell. Probes, 13, 261–268. [DOI] [PubMed] [Google Scholar]
- 6.Putz J., Wientges,J., Sissler,M., Giege,R., Florentz,C. and Schwienhorst,A. (1997) Rapid selection of aminoacyl-tRNAs based on biotinylation of alpha-NH2 group of charged amino acids. Nucleic Acids Res., 25, 1862–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huber C.G., Oefner,P.J. and Bonn,G.K. (1995) Rapid and accurate sizing of DNA fragments by ion-pair chromatography on alkylated nonporous poly(styrene-divinylbenzene). Anal. Chem., 67, 578–585. [Google Scholar]
- 8.Hecker K.H., Turpie,B. and Kuklin,A. (1999) Optimization of cloning efficacy by pre-cloning DNA fragment analysis. Biotechniques, 26, 216–218. [DOI] [PubMed] [Google Scholar]
- 9.Hoogendoorn B., Owen,M.J., Oefner,P.J., Williams,N., Austin,J. and O’Donovan,M. (1999) Genotyping single nucleotide polymorphisms by primer extension and high performance liquid chromatography. Hum. Genet., 104, 89–93. [DOI] [PubMed] [Google Scholar]
- 10.O’Donovan M.C., Oefner,P.J., Roberts,S.C., Austin,J., Hoogendoorn,B., Guy,C., Speight,G., Upadhyaya,M., Sommer,S.S. and McGuffin,P. (1998) Blind analysis of denaturing high-performance liquid chromatography as a tool for mutation detection. Genomics, 52, 44–49. [DOI] [PubMed] [Google Scholar]
- 11.Huber C.G., Oefner,P.J. and Bonn,G.K. (1993) High-resolution liquid chromatography of oligonucleotides on non-porous alkylated styrene-divinylbenzene copolymers. Anal. Biochem., 212, 351–358. [DOI] [PubMed] [Google Scholar]
- 12.Huber C.G., Stimpfl,E., Ofener,P.J. and Bonn,G.K. (1996) A comparison of micropellicular anion exchange and reversed-phase stationary phases for HPLC analysis of oligonucleotides. Liq. Chromatog-Gas Chromatogr., 14, 114–127. [Google Scholar]
- 13.Oefner P.J. (2000) Allelic discrimination by denaturing high-performance liquid chromatography. J. Chromatogr., 739, 345–355. [DOI] [PubMed] [Google Scholar]
- 14.Georgopoulos D.E. and Leibowitz,M.J. (1999) Use of high-performance liquid chromatographic fractionation of large RNA molecules in the assay of group I intron ribozyme activity. J. Chromatogr., 868, 109–114. [DOI] [PubMed] [Google Scholar]