Abstract
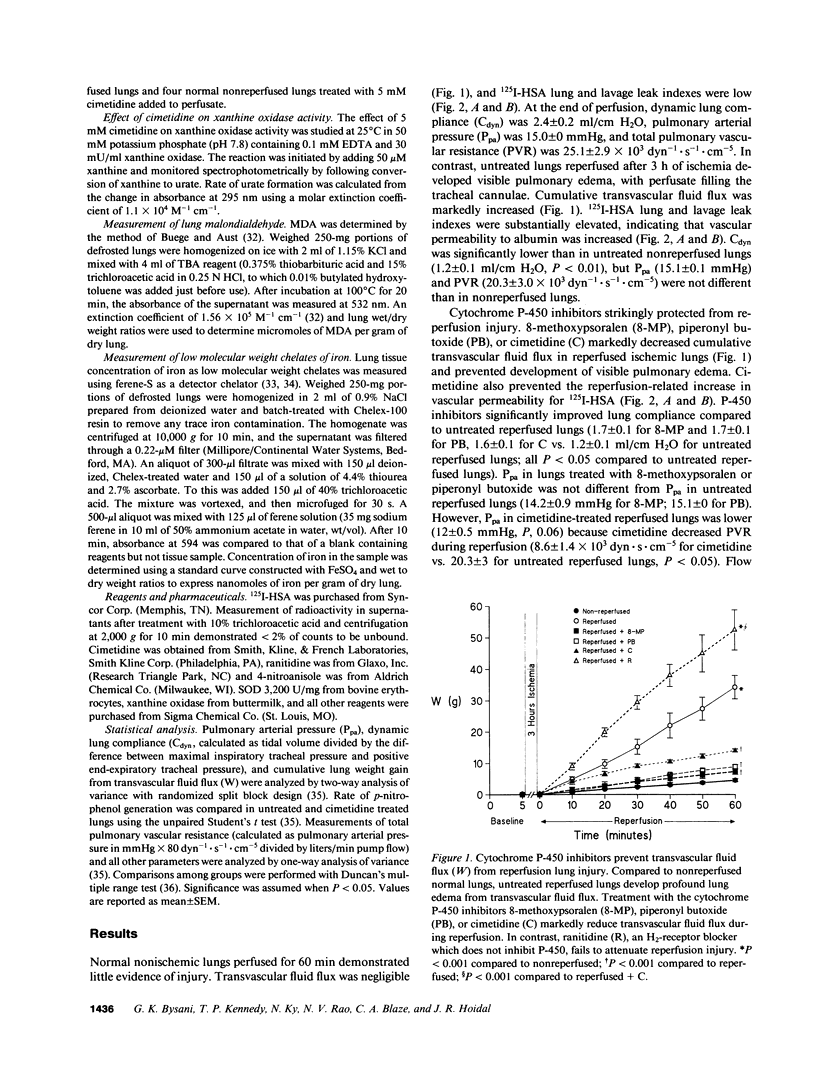
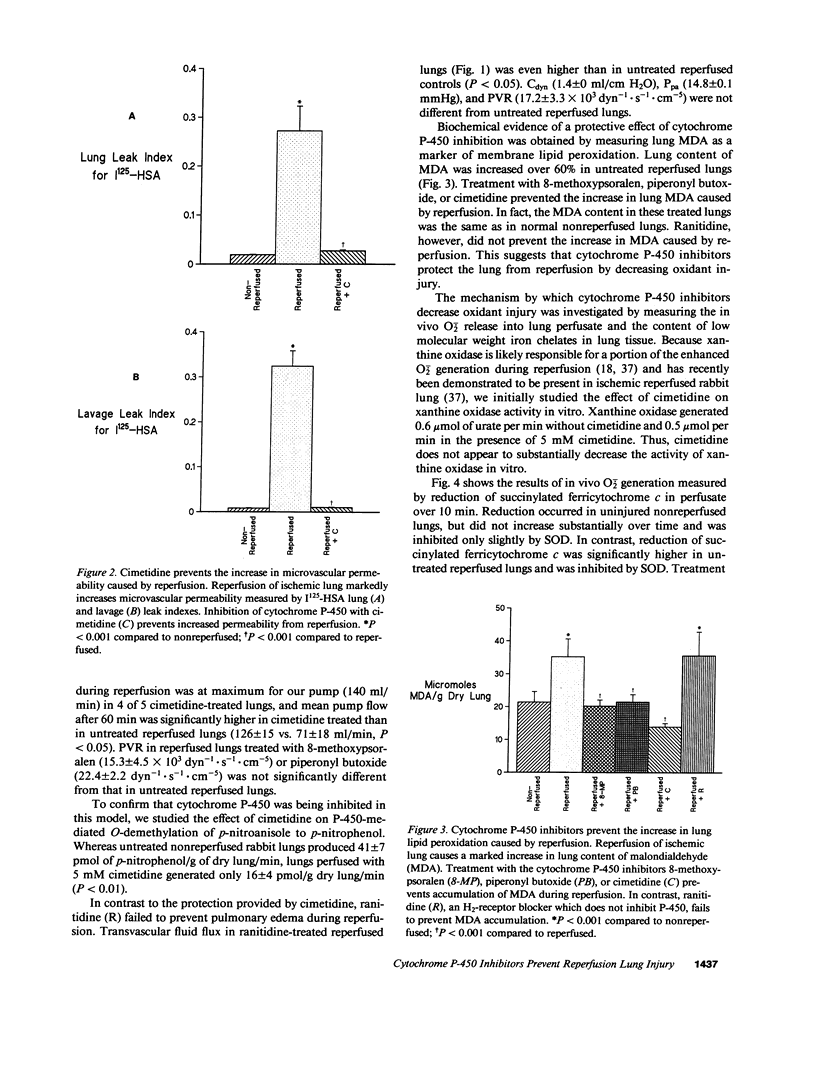
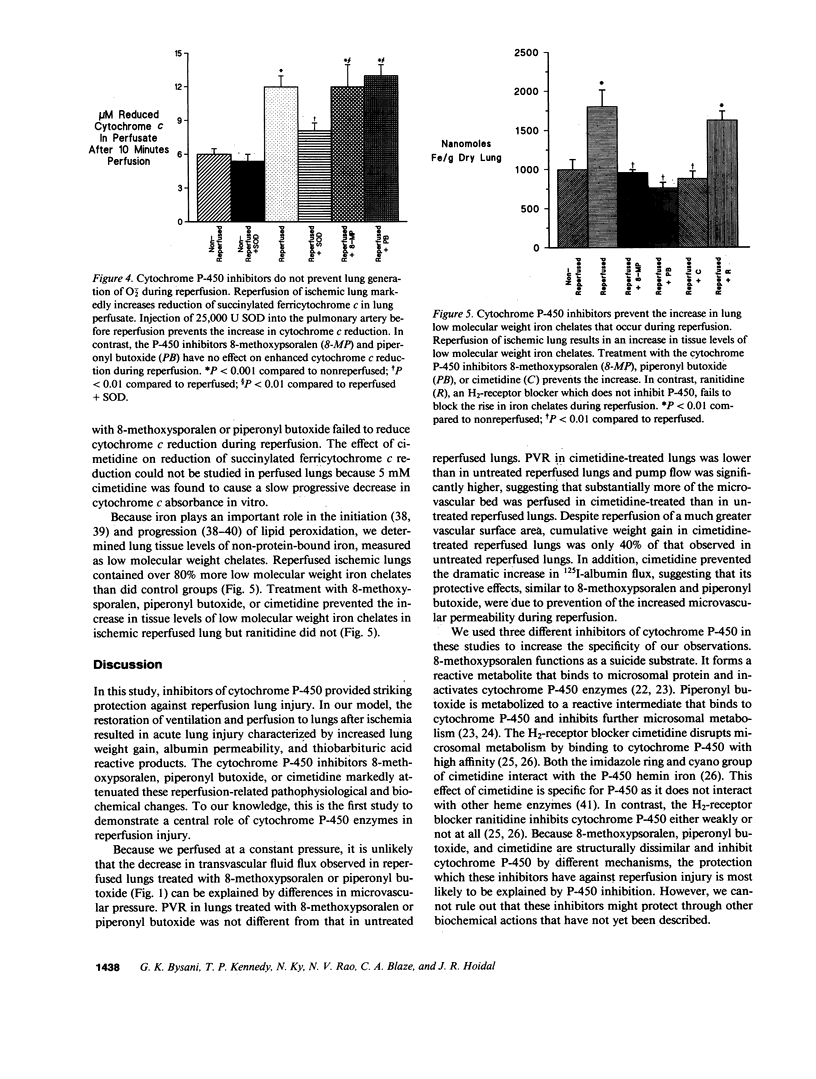
Reactive oxygen species are a major cause of damage occurring in ischemic tissue after reperfusion. During reperfusion transitional metals such as iron are required for reactive oxygen species to mediate their major toxic effects. Xanthine oxidase is an important source of reactive oxygen species during ischemia-reperfusion injury, but not in all organs or species. Because cytochrome P-450 enzymes are an important pulmonary source of superoxide anion (O2-.) generation under basal conditions and during hyperoxia, and provide iron catalysts necessary for hydroxyl radical (.OH) formation and propagation of lipid peroxidation, we postulated that cytochrome P-450 might have a potential role in mediating ischemia-reperfusion injury. In this report, we explored the role of cytochrome P-450 enzymes in a rabbit model of reperfusion lung injury. The P-450 inhibitors 8-methoxypsoralen, piperonyl butoxide, and cimetidine markedly decreased lung edema from transvascular fluid flux. Cimetidine prevented the reperfusion-related increase in lung microvascular permeability, as measured by movement of 125I-albumin from the vascular space into lung water and alveolar fluid. P-450 inhibitors also prevented the increase in lung tissue levels of thiobarbituric acid reactive products in the model. P-450 inhibitors did not block enhanced O2-. generation by ischemic reperfused lungs, measured by in vivo reduction of succinylated ferricytochrome c in lung perfusate, but did prevent the increase in non-protein-bound low molecular weight chelates of iron after reperfusion. Thus, cytochrome P-450 enzymes are not likely a major source of enhanced O2-. generation, but serve as an important source of iron in mediating oxidant injury to the rabbit lung during reperfusion. These results suggest an important role of cytochrome P-450 in reperfusion injury to the lung and suggest potential new therapies for the disorder.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrosio G., Zweier J. L., Jacobus W. E., Weisfeldt M. L., Flaherty J. T. Improvement of postischemic myocardial function and metabolism induced by administration of deferoxamine at the time of reflow: the role of iron in the pathogenesis of reperfusion injury. Circulation. 1987 Oct;76(4):906–915. doi: 10.1161/01.cir.76.4.906. [DOI] [PubMed] [Google Scholar]
- Artiss J. D., Vinogradov S., Zak B. Spectrophotometric study of several sensitive reagents for serum iron. Clin Biochem. 1981 Dec;14(6):311–315. doi: 10.1016/s0009-9120(81)91065-1. [DOI] [PubMed] [Google Scholar]
- Aust S. D., Roerig D. L., Pederson T. C. Evidence for superoxide generation by NADPH-cytochrome c reductase of rat liver microsomes. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1133–1137. doi: 10.1016/0006-291x(72)90952-7. [DOI] [PubMed] [Google Scholar]
- Baird M. B., Sfeir G. T., Slade-Pacini C. D. Lack of inhibition of mouse catalase activity by cimetidine: an argument against a relevant general effect of cimetidine upon heme metabolic pathways. Biochem Pharmacol. 1987 Dec 15;36(24):4366–4369. doi: 10.1016/0006-2952(87)90687-3. [DOI] [PubMed] [Google Scholar]
- Bolann B. J., Ulvik R. J. Release of iron from ferritin by xanthine oxidase. Role of the superoxide radical. Biochem J. 1987 Apr 1;243(1):55–59. doi: 10.1042/bj2430055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolann B. J., Ulvik R. J. Release of iron from ferritin by xanthine oxidase. Role of the superoxide radical. Biochem J. 1987 Apr 1;243(1):55–59. doi: 10.1042/bj2430055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braughler J. M., Duncan L. A., Chase R. L. The involvement of iron in lipid peroxidation. Importance of ferric to ferrous ratios in initiation. J Biol Chem. 1986 Aug 5;261(22):10282–10289. [PubMed] [Google Scholar]
- Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Chevion M. A site-specific mechanism for free radical induced biological damage: the essential role of redox-active transition metals. Free Radic Biol Med. 1988;5(1):27–37. doi: 10.1016/0891-5849(88)90059-7. [DOI] [PubMed] [Google Scholar]
- Downey J. M., Miura T., Eddy L. J., Chambers D. E., Mellert T., Hearse D. J., Yellon D. M. Xanthine oxidase is not a source of free radicals in the ischemic rabbit heart. J Mol Cell Cardiol. 1987 Nov;19(11):1053–1060. doi: 10.1016/s0022-2828(87)80350-4. [DOI] [PubMed] [Google Scholar]
- Eddy L. J., Stewart J. R., Jones H. P., Engerson T. D., McCord J. M., Downey J. M. Free radical-producing enzyme, xanthine oxidase, is undetectable in human hearts. Am J Physiol. 1987 Sep;253(3 Pt 2):H709–H711. doi: 10.1152/ajpheart.1987.253.3.H709. [DOI] [PubMed] [Google Scholar]
- Fisher A. B., Itakura N., Dodia C., Thurman R. G. Relationship between alveolar PO2 and the rate of p-nitroanisole O-demethylation by the cytochrome P-450 pathway in isolated rabbit lungs. J Clin Invest. 1979 Sep;64(3):770–774. doi: 10.1172/JCI109522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouin-Fortunet H., Tinel M., Descatoire V., Letteron P., Larrey D., Geneve J., Pessayre D. Inactivation of cytochrome P-450 by the drug methoxsalen. J Pharmacol Exp Ther. 1986 Jan;236(1):237–247. [PubMed] [Google Scholar]
- Ginsberg M. D., Reivich M., Frinak S., Harbig K. Pyridine nucleotide redox state and blood flow of the cerebral cortex following middle cerebral artery occlusion in the cat. Stroke. 1976 Mar-Apr;7(2):125–131. doi: 10.1161/01.str.7.2.125. [DOI] [PubMed] [Google Scholar]
- Graf E., Mahoney J. R., Bryant R. G., Eaton J. W. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J Biol Chem. 1984 Mar 25;259(6):3620–3624. [PubMed] [Google Scholar]
- Grisham M. B., Granger D. N. Metabolic sources of reactive oxygen metabolites during oxidant stress and ischemia with reperfusion. Clin Chest Med. 1989 Mar;10(1):71–81. [PubMed] [Google Scholar]
- Grover T. A., Piette L. H. Influence of flavin addition and removal on the formation of superoxide by NADPH-Cytochrome P-450 reductase: a spin-trap study. Arch Biochem Biophys. 1981 Nov;212(1):105–114. doi: 10.1016/0003-9861(81)90348-9. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986 Jun 9;201(2):291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S., Gunderson M., Joyce K., Nayini N. R., Eyster G. F., Garitano A. M., Zonia C., Krause G. S., Aust S. D., White B. C. Myocardial tissue iron delocalization and evidence for lipid peroxidation after two hours of ischemia. Ann Emerg Med. 1986 Oct;15(10):1155–1159. doi: 10.1016/s0196-0644(86)80857-5. [DOI] [PubMed] [Google Scholar]
- Horgan M. J., Lum H., Malik A. B. Pulmonary edema after pulmonary artery occlusion and reperfusion. Am Rev Respir Dis. 1989 Nov;140(5):1421–1428. doi: 10.1164/ajrccm/140.5.1421. [DOI] [PubMed] [Google Scholar]
- Itakura N., Fisher A. B., Thurman R. G. Cytochrome P450-linked p-nitroanisole O-demethylation in the perfused lung. J Appl Physiol Respir Environ Exerc Physiol. 1977 Aug;43(2):238–245. doi: 10.1152/jappl.1977.43.2.238. [DOI] [PubMed] [Google Scholar]
- James R. C., Harbison R. D. Hepatic glutathione and hepatotoxicity: effects of cytochrome P-450 complexing compounds SKF 525-A, L-alpha acetylmethadol (LAAM), norLAAM, and piperonyl butoxide. Biochem Pharmacol. 1982 May 15;31(10):1829–1835. doi: 10.1016/0006-2952(82)90484-1. [DOI] [PubMed] [Google Scholar]
- Kennedy T. P., Rao N. V., Hopkins C., Pennington L., Tolley E., Hoidal J. R. Role of reactive oxygen species in reperfusion injury of the rabbit lung. J Clin Invest. 1989 Apr;83(4):1326–1335. doi: 10.1172/JCI114019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch A., Sybert A., Brennan N. J., Sylvester J. T., Gurtner G. H. Effect of hypoxia and CO on a cytochrome P-450-mediated reaction in rabbit lungs. J Appl Physiol Respir Environ Exerc Physiol. 1981 Dec;51(6):1635–1642. doi: 10.1152/jappl.1981.51.6.1635. [DOI] [PubMed] [Google Scholar]
- Kuthan H., Tsuji H., Graf H., Ullrich V. Generation of superoxide anion as a source of hydrogen peroxide in a reconstituted monooxygenase system. FEBS Lett. 1978 Jul 15;91(2):343–345. doi: 10.1016/0014-5793(78)81206-x. [DOI] [PubMed] [Google Scholar]
- Kuthan H., Ullrich V., Estabrook R. W. A quantitative test for superoxide radicals produced in biological systems. Biochem J. 1982 Jun 1;203(3):551–558. doi: 10.1042/bj2030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays D. C., Hilliard J. B., Wong D. D., Gerber N. Activation of 8-methoxypsoralen by cytochrome P-450. Enzyme kinetics of covalent binding and influence of inhibitors and inducers of drug metabolism. Biochem Pharmacol. 1989 May 15;38(10):1647–1655. doi: 10.1016/0006-2952(89)90313-4. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Minotti G., Aust S. D. The requirement for iron (III) in the initiation of lipid peroxidation by iron (II) and hydrogen peroxide. J Biol Chem. 1987 Jan 25;262(3):1098–1104. [PubMed] [Google Scholar]
- Minotti G., Aust S. D. The requirement for iron (III) in the initiation of lipid peroxidation by iron (II) and hydrogen peroxide. J Biol Chem. 1987 Jan 25;262(3):1098–1104. [PubMed] [Google Scholar]
- Nayini N. R., White B. C., Aust S. D., Huang R. R., Indrieri R. J., Evans A. T., Bialek H., Jacobs W. A., Komara J. Post resuscitation iron delocalization and malondialdehyde production in the brain following prolonged cardiac arrest. J Free Radic Biol Med. 1985;1(2):111–116. doi: 10.1016/0748-5514(85)90014-5. [DOI] [PubMed] [Google Scholar]
- Podzuweit T., Braun W., Müller A., Schaper W. Arrhythmias and infarction in the ischemic pig heart are not mediated by xanthine oxidase-derived free oxygen radicals. Basic Res Cardiol. 1987 Sep-Oct;82(5):493–505. doi: 10.1007/BF01907097. [DOI] [PubMed] [Google Scholar]
- Rendić S., Kajfez F., Ruf H. H. Characterization of cimetidine, ranitidine, and related structures' interaction with cytochrome P-450. Drug Metab Dispos. 1983 Mar-Apr;11(2):137–142. [PubMed] [Google Scholar]
- Sadrzadeh S. M., Eaton J. W. Hemoglobin-mediated oxidant damage to the central nervous system requires endogenous ascorbate. J Clin Invest. 1988 Nov;82(5):1510–1515. doi: 10.1172/JCI113759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speeg K. V., Jr, Patwardhan R. V., Avant G. R., Mitchell M. C., Schenker S. Inhibition of microsomal drug metabolism by histamine H2-receptor antagonists studied in vivo and in vitro in rodents. Gastroenterology. 1982 Jan;82(1):89–96. [PubMed] [Google Scholar]
- Svingen B. A., Buege J. A., O'Neal F. O., Aust S. D. The mechanism of NADPH-dependent lipid peroxidation. The propagation of lipid peroxidation. J Biol Chem. 1979 Jul 10;254(13):5892–5899. [PubMed] [Google Scholar]
- Svingen B. A., Buege J. A., O'Neal F. O., Aust S. D. The mechanism of NADPH-dependent lipid peroxidation. The propagation of lipid peroxidation. J Biol Chem. 1979 Jul 10;254(13):5892–5899. [PubMed] [Google Scholar]
- Thomas C. E., Morehouse L. A., Aust S. D. Ferritin and superoxide-dependent lipid peroxidation. J Biol Chem. 1985 Mar 25;260(6):3275–3280. [PubMed] [Google Scholar]
- Thomas C. E., Morehouse L. A., Aust S. D. Ferritin and superoxide-dependent lipid peroxidation. J Biol Chem. 1985 Mar 25;260(6):3275–3280. [PubMed] [Google Scholar]
- Tindberg N., Ingelman-Sundberg M. Cytochrome P-450 and oxygen toxicity. Oxygen-dependent induction of ethanol-inducible cytochrome P-450 (IIE1) in rat liver and lung. Biochemistry. 1989 May 16;28(10):4499–4504. doi: 10.1021/bi00436a056. [DOI] [PubMed] [Google Scholar]
- Turrens J. F., Freeman B. A., Crapo J. D. Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Arch Biochem Biophys. 1982 Sep;217(2):411–421. doi: 10.1016/0003-9861(82)90519-7. [DOI] [PubMed] [Google Scholar]
- White R. E., Coon M. J. Oxygen activation by cytochrome P-450. Annu Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]
- Yoon J. O., Im M. J., Manson P. N., Bulkley G. B., Hoopes J. E. The role of metal ions in ischemia/reperfusion injury in skin flaps. J Surg Res. 1989 Feb;46(2):163–165. doi: 10.1016/0022-4804(89)90221-7. [DOI] [PubMed] [Google Scholar]
- de Groot H., Littauer A. Reoxygenation injury in isolated hepatocytes: cell death precedes conversion of xanthine dehydrogenase to xanthine oxidase. Biochem Biophys Res Commun. 1988 Aug 30;155(1):278–282. doi: 10.1016/s0006-291x(88)81080-5. [DOI] [PubMed] [Google Scholar]



