Abstract
The title molecule, C17H13NO3, adopts a Z configuration about the central olefinic bond. The 2-phenyl ring is almost coplanar with the plane of the oxazolone ring system, making a dihedral angle of 2.03 (11)°. The crystal structure is stabilized by π–π interactions between the oxazolone ring and phenyl ring of a neighbouring molecule [centroid–centroid distance = 3.550 (3)Å], and by two weak intermolecular C—H⋯π interactions. In addition, the crystal structure exhibits one weak intramolecular C—H⋯N hydrogen bond.
Related literature
For general background to azalactones and their biological and pharmaceutical properties, see: Cannella et al. (1996 ▶); Cavelier & Verducci (1995 ▶); Gelmi et al. (1997 ▶); Gonzalez-Martinez, Puchades, Maquieira, Ferrer, Marco & Barcelo (1999 ▶); Gottwald & Seebach (1999 ▶); Mesaik et al. (2004 ▶). For bond-length data, see: Allen et al. (1987 ▶).
Experimental
Crystal data
C17H13NO3
M r = 279.28
Triclinic,
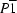
a = 8.8073 (6) Å
b = 9.6140 (6) Å
c = 9.8272 (6) Å
α = 66.503 (4)°
β = 67.248 (4)°
γ = 71.734 (4)°
V = 691.14 (8) Å3
Z = 2
Mo Kα radiation
μ = 0.09 mm−1
T = 296 K
0.28 × 0.08 × 0.05 mm
Data collection
Bruker Kappa APEXII CCD diffractometer
Absorption correction: none
14582 measured reflections
3457 independent reflections
1464 reflections with I > 2σ(I)
R int = 0.048
Refinement
R[F 2 > 2σ(F 2)] = 0.045
wR(F 2) = 0.133
S = 0.93
3457 reflections
192 parameters
H-atom parameters constrained
Δρmax = 0.16 e Å−3
Δρmin = −0.13 e Å−3
Data collection: APEX2 (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SIR97 (Altomare et al., 1999 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶) and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809010216/lx2096sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809010216/lx2096Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C6—H6⋯N1 | 0.93 | 2.43 | 3.087 (3) | 127 |
| C17—H17A⋯Cg3i | 0.96 | 2.81 | 3.682 (3) | 151 |
| C17—H17C⋯Cg2ii | 0.96 | 2.96 | 3.832 (3) | 151 |
Symmetry codes: (i) 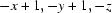 ; (ii)
; (ii) 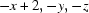 . Cg2 is the centroid of the C1–C6 benzene ring and Cg3 is the centroid of the C11—C16 phenyl ring.
. Cg2 is the centroid of the C1–C6 benzene ring and Cg3 is the centroid of the C11—C16 phenyl ring.
Acknowledgments
AMA acknowledges the Chemistry Department, Faculty of Science, King Abdul-Aziz University, for providing the laboratories and facilities.
supplementary crystallographic information
Comment
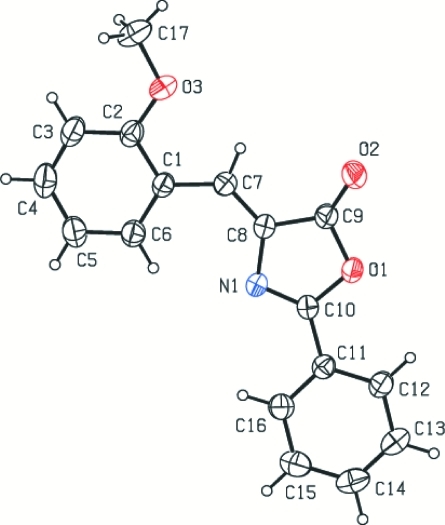
Azalactones are a class of important heterocyclic compounds and exhibit a variety of biological and pharmaceutical properties (Mesaik et al., 2004) They are also useful precursors for the synthesis of amino acids (Gottwald & Seebach, 1999), peptides (Cavelier & Verducci, 1995), heterocycles (Cannella et al., 1996), biosensors (Gonzalez-Martinez et al., 1999), and antitumoror antimicrobial compounds (Gelmi et al., 1997). Here we report the crystal structure of the title compound, 4-(2-methoxybenzylidene)-2-phenyl-1,3-oxazol-5(4H)-one (Fig. 1).
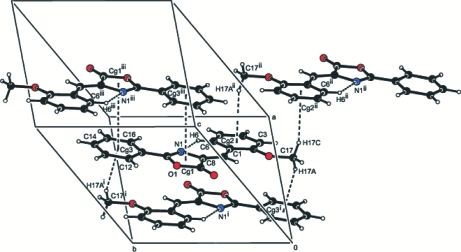
The title molecule (Fig. 1) possesses normal geometric parameters (Allen et al., 1987) and adopts a Z configuration about the central olefinic bond. The C11–C16 phenyl ring makes a dihedral angle of 2.03 (11) ° with the plane of the oxazolone ring system. The molecular packing (Fig. 2) is stabilized by intermolecular π—π interactions between the oxazolone ring and phenyl ring of neighbouring molecules, with a Cg1···Cg3iii distance of 3.550 (3) Å (Cg1 and Cg3 are the centroids of the O1/C10/N1/C8/C9 oxazolone ring and the C11—C16 phenyl ring; symmetry code as in Fig, 2). The crystal packing is further stabilized by two intermolecular C—H···π interactions; one between the H atom of methoxy group and the phenyl ring of a neighbouring molecule, a second between the H atom of methoxy group and the methoxyphenyl ring of an adjacent molecule, respectively (Fig. 2 and Table 1; Cg2 is the centroid of the C1–C6 benzene ring, symmetry code as in Fig, 2). Additionally, there is one intramolecular C—H···N hydrogen bond between a benzene—H atom and the N atom of oxazolone ring (Table 1 and Fig. 2).
Experimental
Anhydrous sodium acetate (2.1 g, 25.3 mmol) was added to a solution of 2-methoxybenzaldehyde (3.5 g, 25.7 mmol) and hippuric acid (7.7 g, 31.1 mmol) in acetic anhydride (2.1 g, 20.6 mmol). The reaction mixture was heated to 353 K and stirred under reflux conditions for the appropriate time 2 h. The reaction mixture was cooled to room temperature and ethanol (10 ml) was added. The mixture was stirred for 10 min until a yellow solid precipitated. The mixture was allowed to stand overnight, and then it was cooled in an ice bath. The crude azalactones were obtained after filtration and washing with hot water. Recrystallization from acetone/water afforded the pure azalactones as yellow crystals. [Yield (5.79 g, 91%), m.p. 440–441 K]. IR (cm-1) 1769 (C═O),1648 (C═C).
Refinement
All H atoms were positioned geometrically with C—H = 0.93 and 0.96 Å and refined using a riding approximation model with Uiso(H) = 1.2 or 1.5Ueq(C).
Figures
Fig. 1.
The molecular structure of the title compound with the atom numbering scheme. Displacement ellipsoids are drawn at the 30% probability level. H atoms are presented as a small spheres of arbitrary radius.
Fig. 2.
π—π, C—H···π and C—H···N interactions (dotted lines) in the title compound. Cg denotes the ring centroids. [Symmetry codes: (i) -x+1, -y+1, -z; (ii) -x+2, -y, -z; (iii) -x+1, -y+1, -z+1].
Crystal data
| C17H13NO3 | Z = 2 |
| Mr = 279.28 | F(000) = 292 |
| Triclinic, P1 | Dx = 1.342 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.8073 (6) Å | Cell parameters from 1969 reflections |
| b = 9.6140 (6) Å | θ = 2.4–22.4° |
| c = 9.8272 (6) Å | µ = 0.09 mm−1 |
| α = 66.503 (4)° | T = 296 K |
| β = 67.248 (4)° | Prism, yellow |
| γ = 71.734 (4)° | 0.28 × 0.08 × 0.05 mm |
| V = 691.14 (8) Å3 |
Data collection
| Bruker Kappa APEXII CCD diffractometer | 1464 reflections with I > 2σ(I) |
| Radiation source: sealed tube | Rint = 0.048 |
| graphite | θmax = 28.5°, θmin = 2.4° |
| Detector resolution: 10.0 pixels mm-1 | h = −11→11 |
| φ and ω scans | k = −12→12 |
| 14582 measured reflections | l = −13→13 |
| 3457 independent reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.045 | H-atom parameters constrained |
| wR(F2) = 0.133 | w = 1/[σ2(Fo2) + (0.0583P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.93 | (Δ/σ)max = 0.001 |
| 3457 reflections | Δρmax = 0.16 e Å−3 |
| 192 parameters | Δρmin = −0.13 e Å−3 |
| 0 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), FC*=KFC[1+0.001XFC2Λ3/SIN(2Θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.008 (3) |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell e.s.d.'s are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement on F2 for ALL reflections except those flagged by the user for potential systematic errors. Weighted R-factors wR and all goodnesses of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The observed criterion of F2 > σ(F2) is used only for calculating -R-factor-obs etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.25166 (15) | 0.44719 (14) | 0.39300 (14) | 0.0569 (5) | |
| O2 | 0.21889 (18) | 0.24891 (17) | 0.34504 (18) | 0.0822 (6) | |
| O3 | 0.71016 (18) | 0.05676 (17) | −0.02406 (16) | 0.0758 (6) | |
| N1 | 0.51111 (18) | 0.48138 (17) | 0.23057 (16) | 0.0496 (6) | |
| C1 | 0.7332 (2) | 0.2733 (2) | 0.0149 (2) | 0.0506 (7) | |
| C2 | 0.8036 (2) | 0.1640 (2) | −0.0653 (2) | 0.0585 (8) | |
| C3 | 0.9578 (3) | 0.1700 (3) | −0.1783 (2) | 0.0728 (9) | |
| C4 | 1.0418 (3) | 0.2832 (3) | −0.2108 (3) | 0.0812 (9) | |
| C5 | 0.9784 (3) | 0.3890 (3) | −0.1317 (2) | 0.0741 (9) | |
| C6 | 0.8245 (3) | 0.3836 (2) | −0.0200 (2) | 0.0613 (8) | |
| C7 | 0.5675 (2) | 0.2690 (2) | 0.1242 (2) | 0.0534 (7) | |
| C8 | 0.4736 (2) | 0.3592 (2) | 0.2139 (2) | 0.0494 (7) | |
| C9 | 0.3054 (3) | 0.3362 (2) | 0.3179 (2) | 0.0570 (8) | |
| C10 | 0.3815 (2) | 0.5263 (2) | 0.3330 (2) | 0.0469 (6) | |
| C11 | 0.3562 (2) | 0.6487 (2) | 0.3944 (2) | 0.0496 (7) | |
| C12 | 0.2070 (3) | 0.6867 (2) | 0.5026 (2) | 0.0623 (8) | |
| C13 | 0.1867 (3) | 0.8024 (3) | 0.5607 (3) | 0.0762 (9) | |
| C14 | 0.3129 (4) | 0.8808 (3) | 0.5107 (3) | 0.0779 (10) | |
| C15 | 0.4617 (3) | 0.8459 (3) | 0.4015 (3) | 0.0760 (10) | |
| C16 | 0.4826 (3) | 0.7300 (2) | 0.3435 (2) | 0.0627 (8) | |
| C17 | 0.7702 (3) | −0.0552 (3) | −0.1038 (3) | 0.0876 (10) | |
| H3 | 1.00400 | 0.09810 | −0.23150 | 0.0870* | |
| H4 | 1.14440 | 0.28850 | −0.28830 | 0.0970* | |
| H5 | 1.03870 | 0.46320 | −0.15340 | 0.0890* | |
| H6 | 0.78080 | 0.45550 | 0.03320 | 0.0740* | |
| H7 | 0.51770 | 0.19180 | 0.13430 | 0.0640* | |
| H12 | 0.12020 | 0.63380 | 0.53610 | 0.0750* | |
| H13 | 0.08670 | 0.82700 | 0.63400 | 0.0910* | |
| H14 | 0.29880 | 0.95860 | 0.55060 | 0.0940* | |
| H15 | 0.54730 | 0.90020 | 0.36740 | 0.0910* | |
| H16 | 0.58250 | 0.70640 | 0.26970 | 0.0750* | |
| H17A | 0.78080 | −0.00370 | −0.21230 | 0.1310* | |
| H17B | 0.69250 | −0.12400 | −0.06060 | 0.1310* | |
| H17C | 0.87760 | −0.11310 | −0.09190 | 0.1310* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0465 (8) | 0.0591 (8) | 0.0597 (8) | −0.0112 (7) | −0.0015 (6) | −0.0266 (7) |
| O2 | 0.0612 (10) | 0.0754 (11) | 0.1086 (12) | −0.0262 (9) | 0.0020 (8) | −0.0443 (9) |
| O3 | 0.0732 (10) | 0.0836 (11) | 0.0839 (10) | −0.0087 (9) | −0.0127 (8) | −0.0548 (9) |
| N1 | 0.0455 (10) | 0.0547 (10) | 0.0470 (9) | −0.0084 (8) | −0.0074 (8) | −0.0216 (8) |
| C1 | 0.0443 (12) | 0.0614 (13) | 0.0448 (10) | −0.0030 (10) | −0.0130 (9) | −0.0211 (10) |
| C2 | 0.0491 (13) | 0.0736 (15) | 0.0529 (12) | 0.0025 (11) | −0.0192 (10) | −0.0271 (11) |
| C3 | 0.0546 (14) | 0.1005 (19) | 0.0614 (13) | 0.0044 (14) | −0.0130 (11) | −0.0424 (13) |
| C4 | 0.0519 (14) | 0.116 (2) | 0.0599 (14) | −0.0092 (15) | −0.0056 (11) | −0.0282 (15) |
| C5 | 0.0538 (14) | 0.0970 (18) | 0.0667 (14) | −0.0201 (13) | −0.0109 (12) | −0.0236 (13) |
| C6 | 0.0533 (13) | 0.0743 (15) | 0.0558 (12) | −0.0103 (12) | −0.0134 (10) | −0.0243 (11) |
| C7 | 0.0508 (12) | 0.0569 (13) | 0.0542 (11) | −0.0078 (10) | −0.0138 (10) | −0.0230 (10) |
| C8 | 0.0442 (12) | 0.0517 (12) | 0.0486 (11) | −0.0064 (10) | −0.0102 (9) | −0.0179 (10) |
| C9 | 0.0504 (13) | 0.0538 (13) | 0.0639 (13) | −0.0091 (11) | −0.0088 (10) | −0.0244 (11) |
| C10 | 0.0417 (11) | 0.0501 (12) | 0.0449 (10) | −0.0090 (10) | −0.0112 (9) | −0.0126 (9) |
| C11 | 0.0476 (12) | 0.0492 (12) | 0.0504 (11) | −0.0018 (10) | −0.0184 (9) | −0.0162 (10) |
| C12 | 0.0587 (14) | 0.0646 (14) | 0.0605 (12) | −0.0086 (11) | −0.0091 (10) | −0.0275 (11) |
| C13 | 0.0818 (18) | 0.0753 (16) | 0.0744 (15) | −0.0038 (14) | −0.0150 (13) | −0.0430 (13) |
| C14 | 0.100 (2) | 0.0622 (15) | 0.0858 (17) | 0.0001 (15) | −0.0409 (15) | −0.0367 (13) |
| C15 | 0.0810 (18) | 0.0670 (15) | 0.0942 (18) | −0.0148 (13) | −0.0392 (15) | −0.0266 (14) |
| C16 | 0.0569 (14) | 0.0641 (14) | 0.0705 (13) | −0.0072 (11) | −0.0207 (11) | −0.0264 (12) |
| C17 | 0.107 (2) | 0.0841 (17) | 0.0864 (16) | 0.0101 (15) | −0.0377 (15) | −0.0545 (15) |
Geometric parameters (Å, °)
| O1—C9 | 1.396 (2) | C11—C16 | 1.380 (3) |
| O1—C10 | 1.378 (2) | C12—C13 | 1.378 (3) |
| O2—C9 | 1.192 (3) | C13—C14 | 1.361 (5) |
| O3—C2 | 1.358 (3) | C14—C15 | 1.380 (4) |
| O3—C17 | 1.431 (3) | C15—C16 | 1.377 (3) |
| N1—C8 | 1.398 (3) | C3—H3 | 0.9300 |
| N1—C10 | 1.285 (2) | C4—H4 | 0.9300 |
| C1—C2 | 1.409 (3) | C5—H5 | 0.9300 |
| C1—C6 | 1.386 (3) | C6—H6 | 0.9300 |
| C1—C7 | 1.441 (3) | C7—H7 | 0.9300 |
| C2—C3 | 1.383 (3) | C12—H12 | 0.9300 |
| C3—C4 | 1.371 (4) | C13—H13 | 0.9300 |
| C4—C5 | 1.375 (4) | C14—H14 | 0.9300 |
| C5—C6 | 1.376 (3) | C15—H15 | 0.9300 |
| C7—C8 | 1.345 (3) | C16—H16 | 0.9300 |
| C8—C9 | 1.464 (3) | C17—H17A | 0.9600 |
| C10—C11 | 1.448 (3) | C17—H17B | 0.9600 |
| C11—C12 | 1.385 (3) | C17—H17C | 0.9600 |
| O1···N1 | 2.260 (2) | C4···H17Cvii | 3.0100 |
| O2···C14i | 3.236 (3) | C5···H17Cvii | 2.9800 |
| O2···C12ii | 3.411 (3) | C8···H6 | 2.8100 |
| O1···H4iii | 2.8000 | C14···H17Aiv | 3.0300 |
| O1···H12 | 2.4500 | C15···H17Aiv | 3.0200 |
| O2···H7 | 2.7000 | C16···H4viii | 3.0700 |
| O2···H14i | 2.7800 | C17···H3 | 2.5300 |
| O2···H12ii | 2.7900 | H3···C17 | 2.5300 |
| O2···H13ii | 2.9200 | H3···H17A | 2.3700 |
| O3···H7 | 2.2700 | H3···H17C | 2.2900 |
| N1···O1 | 2.260 (2) | H4···O1ix | 2.8000 |
| N1···C6 | 3.087 (3) | H4···C16viii | 3.0700 |
| N1···H6 | 2.4300 | H4···H16viii | 2.5000 |
| N1···H16 | 2.6300 | H6···N1 | 2.4300 |
| C1···C10iv | 3.553 (3) | H6···C8 | 2.8100 |
| C2···C10iv | 3.532 (3) | H7···O2 | 2.7000 |
| C5···C9iv | 3.481 (4) | H7···O3 | 2.2700 |
| C6···N1 | 3.087 (3) | H7···H17Bx | 2.5600 |
| C6···C9iv | 3.392 (3) | H12···O1 | 2.4500 |
| C6···C8iv | 3.561 (3) | H12···O2ii | 2.7900 |
| C8···C14v | 3.524 (4) | H13···O2ii | 2.9200 |
| C8···C6iv | 3.561 (3) | H14···O2vi | 2.7800 |
| C9···C5iv | 3.481 (4) | H16···N1 | 2.6300 |
| C9···C6iv | 3.392 (3) | H16···H4viii | 2.5000 |
| C10···C16v | 3.530 (3) | H17A···C3 | 2.7900 |
| C10···C2iv | 3.532 (3) | H17A···H3 | 2.3700 |
| C10···C1iv | 3.553 (3) | H17A···C14iv | 3.0300 |
| C12···O2ii | 3.411 (3) | H17A···C15iv | 3.0200 |
| C14···C8v | 3.524 (4) | H17B···H7x | 2.5600 |
| C14···O2vi | 3.236 (3) | H17C···C3 | 2.7400 |
| C16···C10v | 3.530 (3) | H17C···H3 | 2.2900 |
| C3···H17A | 2.7900 | H17C···C4vii | 3.0100 |
| C3···H17C | 2.7400 | H17C···C5vii | 2.9800 |
| C9—O1—C10 | 105.44 (16) | C14—C15—C16 | 119.5 (3) |
| C2—O3—C17 | 119.06 (18) | C11—C16—C15 | 120.4 (2) |
| C8—N1—C10 | 105.35 (16) | C2—C3—H3 | 120.00 |
| C2—C1—C6 | 118.16 (17) | C4—C3—H3 | 120.00 |
| C2—C1—C7 | 118.56 (17) | C3—C4—H4 | 119.00 |
| C6—C1—C7 | 123.23 (18) | C5—C4—H4 | 119.00 |
| O3—C2—C1 | 115.60 (17) | C4—C5—H5 | 120.00 |
| O3—C2—C3 | 123.94 (19) | C6—C5—H5 | 120.00 |
| C1—C2—C3 | 120.5 (2) | C1—C6—H6 | 119.00 |
| C2—C3—C4 | 119.2 (2) | C5—C6—H6 | 119.00 |
| C3—C4—C5 | 121.7 (3) | C1—C7—H7 | 115.00 |
| C4—C5—C6 | 119.1 (3) | C8—C7—H7 | 115.00 |
| C1—C6—C5 | 121.38 (19) | C11—C12—H12 | 120.00 |
| C1—C7—C8 | 130.09 (18) | C13—C12—H12 | 120.00 |
| N1—C8—C7 | 129.85 (18) | C12—C13—H13 | 120.00 |
| N1—C8—C9 | 108.50 (16) | C14—C13—H13 | 120.00 |
| C7—C8—C9 | 121.64 (18) | C13—C14—H14 | 120.00 |
| O1—C9—O2 | 121.5 (2) | C15—C14—H14 | 120.00 |
| O1—C9—C8 | 104.65 (18) | C14—C15—H15 | 120.00 |
| O2—C9—C8 | 133.90 (18) | C16—C15—H15 | 120.00 |
| O1—C10—N1 | 116.07 (17) | C11—C16—H16 | 120.00 |
| O1—C10—C11 | 116.07 (16) | C15—C16—H16 | 120.00 |
| N1—C10—C11 | 127.85 (18) | O3—C17—H17A | 110.00 |
| C10—C11—C12 | 121.32 (19) | O3—C17—H17B | 109.00 |
| C10—C11—C16 | 119.45 (17) | O3—C17—H17C | 109.00 |
| C12—C11—C16 | 119.23 (18) | H17A—C17—H17B | 109.00 |
| C11—C12—C13 | 120.2 (2) | H17A—C17—H17C | 109.00 |
| C12—C13—C14 | 120.0 (3) | H17B—C17—H17C | 109.00 |
| C13—C14—C15 | 120.6 (3) | ||
| C10—O1—C9—O2 | −179.7 (2) | C3—C4—C5—C6 | −1.8 (4) |
| C10—O1—C9—C8 | 0.43 (19) | C4—C5—C6—C1 | 0.5 (4) |
| C9—O1—C10—N1 | −0.3 (2) | C1—C7—C8—N1 | −2.0 (3) |
| C9—O1—C10—C11 | −179.54 (16) | C1—C7—C8—C9 | 179.55 (19) |
| C17—O3—C2—C1 | 177.88 (19) | N1—C8—C9—O1 | −0.5 (2) |
| C17—O3—C2—C3 | −1.9 (3) | N1—C8—C9—O2 | 179.7 (2) |
| C10—N1—C8—C7 | −178.4 (2) | C7—C8—C9—O1 | 178.32 (17) |
| C10—N1—C8—C9 | 0.3 (2) | C7—C8—C9—O2 | −1.5 (4) |
| C8—N1—C10—O1 | 0.0 (2) | O1—C10—C11—C12 | −2.4 (3) |
| C8—N1—C10—C11 | 179.13 (18) | O1—C10—C11—C16 | 178.04 (17) |
| C6—C1—C2—O3 | 178.68 (18) | N1—C10—C11—C12 | 178.42 (19) |
| C6—C1—C2—C3 | −1.6 (3) | N1—C10—C11—C16 | −1.1 (3) |
| C7—C1—C2—O3 | −3.8 (3) | C10—C11—C12—C13 | 179.2 (2) |
| C7—C1—C2—C3 | 175.91 (19) | C16—C11—C12—C13 | −1.2 (3) |
| C2—C1—C6—C5 | 1.2 (3) | C10—C11—C16—C15 | −179.4 (2) |
| C7—C1—C6—C5 | −176.2 (2) | C12—C11—C16—C15 | 1.1 (3) |
| C2—C1—C7—C8 | −179.85 (19) | C11—C12—C13—C14 | 0.6 (4) |
| C6—C1—C7—C8 | −2.5 (3) | C12—C13—C14—C15 | 0.2 (4) |
| O3—C2—C3—C4 | −180.0 (2) | C13—C14—C15—C16 | −0.4 (4) |
| C1—C2—C3—C4 | 0.3 (3) | C14—C15—C16—C11 | −0.3 (4) |
| C2—C3—C4—C5 | 1.4 (4) |
Symmetry codes: (i) x, y−1, z; (ii) −x, −y+1, −z+1; (iii) x−1, y, z+1; (iv) −x+1, −y+1, −z; (v) −x+1, −y+1, −z+1; (vi) x, y+1, z; (vii) −x+2, −y, −z; (viii) −x+2, −y+1, −z; (ix) x+1, y, z−1; (x) −x+1, −y, −z.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C6—H6···N1 | 0.93 | 2.43 | 3.087 (3) | 127 |
| C17—H17A···Cg3iv | 0.96 | 2.81 | 3.682 (3) | 151 |
| C17—H17C···Cg2vii | 0.96 | 2.96 | 3.832 (3) | 151 |
Symmetry codes: (iv) −x+1, −y+1, −z; (vii) −x+2, −y, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LX2096).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst.32, 115–119.
- Bruker (2007). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Cannella, R., Clerici, F., Gelmi, M. L., Penso, M. & &Pocar, D. (1996). J. Org. Chem 61,1854-1856. [DOI] [PubMed]
- Cavelier, F. & Verducci, J. (1995). Tetrahedron Lett 36, 4425-4428.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Gelmi, M. L., Clerici, F. & Melis, A. (1997). Tetrahedron, 53, 1843-1854.
- Gonzalez-Martinez, M. A., Puchades, R., Maquieira, A., Ferrer, I., Marco, M. P. & Barcelo, D. (1999). Anal. Chim. Acta, 386, 201-210.
- Gottwald, K. & Seebach, D. (1999). Tetrahedron, 55 , 723-738.
- Mesaik, M. A., Rahat, S., Khan, M. K., Ullah, Z., Choudhary, M. I., Murad, S., Ismail, Z. & Atta-ur-Rahman, A. A. (2004). Bioorg. Med. Chem 12, 2049-2057. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809010216/lx2096sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809010216/lx2096Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report