Abstract
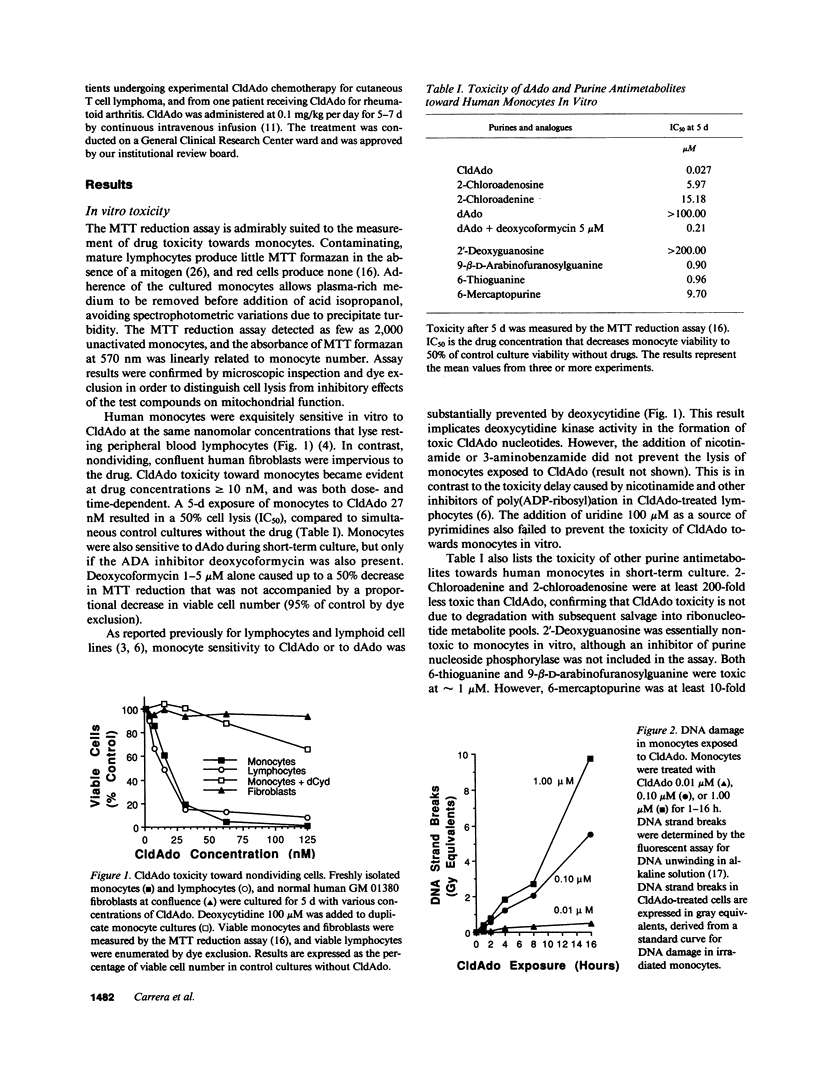
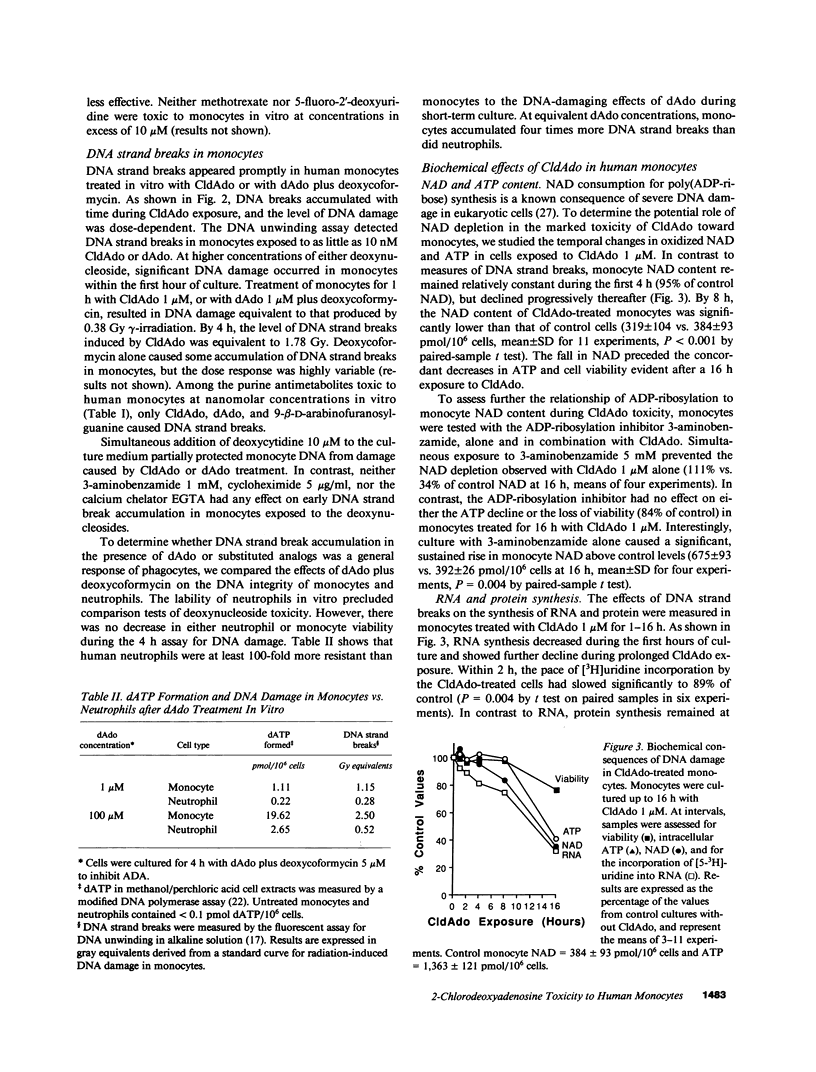
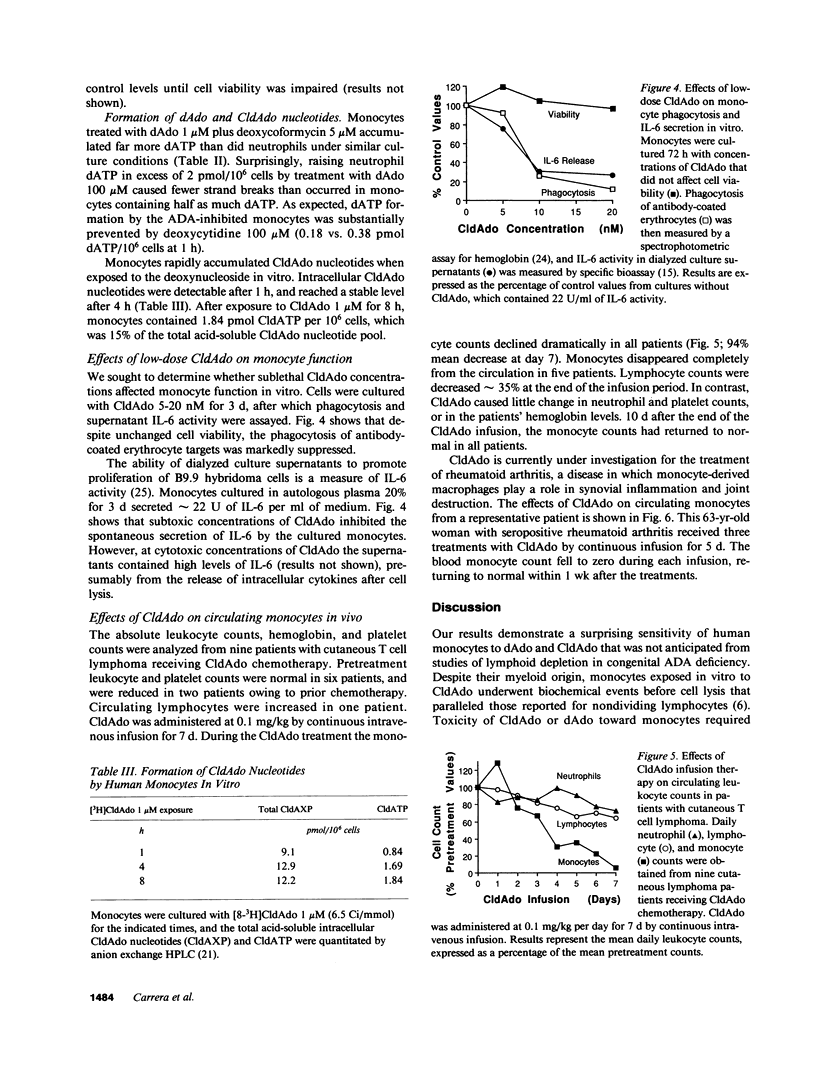
Lymphoid cells were thought to be uniquely susceptible to excess 2'-deoxyadenosine (dAdo), when exposed to inhibitors of adenosine deaminase (ADA). However, we now find that human monocytes are as sensitive as lymphocytes to dAdo or to the ADA-resistant congener 2-chloro-2'-deoxyadenosine (CldAdo). Monocytes exposed in vitro to CldAdo, or to dAdo plus deoxycoformycin rapidly developed DNA strand breaks. Both the DNA damage and the toxicity of CldAdo or dAdo toward monocytes were blocked by deoxycytidine, but not by inhibitors of poly(ADP-ribose) polymerase. A partial decrease in RNA synthesis and a gradual decline of cellular NAD were early biochemical events associated with monocyte DNA damage. Low CldAdo concentrations (5-20 nM) inhibited monocyte phagocytosis and reduced the release of interleukin 6. Higher CldAdo concentrations led to a dose- and time-dependent loss of monocyte viability. Circulating monocytes disappeared within 1 wk in patients with cutaneous T cell lymphoma or with rheumatoid arthritis during continuous CldAdo infusion. The marked sensitivity of human monocyte function and survival to CldAdo in vitro, together with the monocyte depletion in patients receiving CldAdo chemotherapy, suggests that CldAdo or other dAdo analogues offer a novel therapeutic strategy for chronic inflammatory and autoimmune diseases characterized by inappropriate monocyte deployment or function.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Andrews B. S., Friou G. J., Berman M. A., Sandborg C. I., Mirick G. R., Cesario T. C. Changes in circulating monocytes in patients with progressive systemic sclerosis. J Rheumatol. 1987 Oct;14(5):930–935. [PubMed] [Google Scholar]
- Begleiter A., Pugh L., Israels L. G., Johnston J. B. Enhanced cytotoxicity and inhibition of DNA damage repair in irradiated murine L5178Y lymphoblasts and human chronic lymphocytic leukemia cells treated with 2'-deoxycoformycin and deoxyadenosine in vitro. Cancer Res. 1988 Jul 15;48(14):3981–3986. [PubMed] [Google Scholar]
- Benjamin R. C., Gill D. M. Poly(ADP-ribose) synthesis in vitro programmed by damaged DNA. A comparison of DNA molecules containing different types of strand breaks. J Biol Chem. 1980 Nov 10;255(21):10502–10508. [PubMed] [Google Scholar]
- Birnboim H. C., Jevcak J. J. Fluorometric method for rapid detection of DNA strand breaks in human white blood cells produced by low doses of radiation. Cancer Res. 1981 May;41(5):1889–1892. [PubMed] [Google Scholar]
- Boldt D. H., Von Hoff D. D., Kuhn J. G., Hersh M. Effects on human peripheral lymphocytes of in vivo administration of 9-beta-D-arabinofuranosyl-2-fluoroadenine-5'-monophosphate (NSC 312887), a new purine antimetabolite. Cancer Res. 1984 Oct;44(10):4661–4666. [PubMed] [Google Scholar]
- Brox L., Ng A., Pollock E., Belch A. DNA strand breaks induced in human T-lymphocytes by the combination of deoxyadenosine and deoxycoformycin. Cancer Res. 1984 Mar;44(3):934–937. [PubMed] [Google Scholar]
- Carson D. A., Seto S., Wasson D. B. Pyridine nucleotide cycling and poly(ADP-ribose) synthesis in resting human lymphocytes. J Immunol. 1987 Mar 15;138(6):1904–1907. [PubMed] [Google Scholar]
- Carson D. A., Wasson D. B., Beutler E. Antileukemic and immunosuppressive activity of 2-chloro-2'-deoxyadenosine. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2232–2236. doi: 10.1073/pnas.81.7.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D. A., Wasson D. B., Kaye J., Ullman B., Martin D. W., Jr, Robins R. K., Montgomery J. A. Deoxycytidine kinase-mediated toxicity of deoxyadenosine analogs toward malignant human lymphoblasts in vitro and toward murine L1210 leukemia in vivo. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6865–6869. doi: 10.1073/pnas.77.11.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D. A., Wasson D. B., Taetle R., Yu A. Specific toxicity of 2-chlorodeoxyadenosine toward resting and proliferating human lymphocytes. Blood. 1983 Oct;62(4):737–743. [PubMed] [Google Scholar]
- Chan T. S. Purine excretion by mouse peritoneal macrophages lacking adenosine deaminase activity. Proc Natl Acad Sci U S A. 1979 Feb;76(2):925–929. doi: 10.1073/pnas.76.2.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen L. F., Broom A. D., Robins M. J., Bloch A. Synthesis and biological activity of selected 2,6-disubstituted-(2-deoxy- -and- -D-erythro-pentofuranosyl)purines. J Med Chem. 1972 Jul;15(7):735–739. doi: 10.1021/jm00277a010. [DOI] [PubMed] [Google Scholar]
- Cohen A., Thompson E. DNA repair in nondividing human lymphocytes: inhibition by deoxyadenosine. Cancer Res. 1986 Apr;46(4 Pt 1):1585–1588. [PubMed] [Google Scholar]
- Dalal B. I., Freier L., Johnston J. B., Merry C. C., Israels L. G. Peripheral blood and bone marrow changes following 2'-deoxycoformycin therapy in hairy cell leukemia. Results of 200 weeks' follow-up. Cancer. 1989 Jan 1;63(1):14–22. doi: 10.1002/1097-0142(19890101)63:1<14::aid-cncr2820630103>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Dillman R. O., Mick R., McIntyre O. R. Pentostatin in chronic lymphocytic leukemia: a phase II trial of Cancer and Leukemia group B. J Clin Oncol. 1989 Apr;7(4):433–438. doi: 10.1200/JCO.1989.7.4.433. [DOI] [PubMed] [Google Scholar]
- Donofrio J., Coleman M. S., Hutton J. J., Daoud A., Lampkin B., Dyminski J. Overproduction of adenine deoxynucleosides and deoxynucletides in adenosine deaminase deficiency with severe combined immunodeficiency disease. J Clin Invest. 1978 Oct;62(4):884–887. doi: 10.1172/JCI109201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaneh F., Shall S., Johnstone A. P. The dynamic nature of DNA-strand breaks present in differentiating muscle cells and quiescent lymphocytes. FEBS Lett. 1985 Sep 9;189(1):62–66. doi: 10.1016/0014-5793(85)80842-5. [DOI] [PubMed] [Google Scholar]
- Fischer D., Van der Weyden M. B., Snyderman R., Kelley W. N. A role for adenosine deaminase in human monocyte maturation. J Clin Invest. 1976 Aug;58(2):399–407. doi: 10.1172/JCI108484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundlich B., Avdalovic N. Use of gelatin/plasma coated flasks for isolating human peripheral blood monocytes. J Immunol Methods. 1983 Aug 12;62(1):31–37. doi: 10.1016/0022-1759(83)90107-2. [DOI] [PubMed] [Google Scholar]
- Green H., Chan T. Pyrimidine starvation induced by adenosine in fibroblasts and lymphoid cells: role of adenosine deaminase. Science. 1973 Nov 23;182(4114):836–837. doi: 10.1126/science.182.4114.836. [DOI] [PubMed] [Google Scholar]
- Guerne P. A., Zuraw B. L., Vaughan J. H., Carson D. A., Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989 Feb;83(2):585–592. doi: 10.1172/JCI113921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Yoshioka A., Tanaka S., Watanabe K., Otani T., Minowada J., Matsuda A., Ueda T., Wataya Y. Imbalance of deoxyribonucleoside triphosphates, DNA double-strand breaks, and cell death caused by 2-chlorodeoxyadenosine in mouse FM3A cells. Cancer Res. 1989 Feb 15;49(4):915–919. [PubMed] [Google Scholar]
- Houssiau F. A., Devogelaer J. P., Van Damme J., de Deuxchaisnes C. N., Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988 Jun;31(6):784–788. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- Ince C., Thio B., van Duijn B., van Dissel J. T., Ypey D. L., Leijh P. C. Intracellular K+, Na+ and Cl- concentrations and membrane potential in human monocytes. Biochim Biophys Acta. 1987 Nov 27;905(1):195–204. doi: 10.1016/0005-2736(87)90023-x. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Kimura H., Shikama Y., Uchida T., Kariyone S., Hirano T., Kishimoto T., Takatsuki F., Akiyama Y. Interleukin-6 is a potent thrombopoietic factor in vivo in mice. Blood. 1989 Sep;74(4):1241–1244. [PubMed] [Google Scholar]
- Jacobson E. L., Jacobson M. K. Pyridine nucleotide levels as a function of growth in normal and transformed 3T3 cells. Arch Biochem Biophys. 1976 Aug;175(2):627–634. doi: 10.1016/0003-9861(76)90553-1. [DOI] [PubMed] [Google Scholar]
- Johnson W. D., Jr, Mei B., Cohn Z. A. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med. 1977 Dec 1;146(6):1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J. B., Eisenhauer E., Corbett W. E., Scott J. G., Zaentz S. D. Efficacy of 2'-deoxycoformycin in hairy-cell leukemia: a study of the National Cancer Institute of Canada Clinical Trials Group. J Natl Cancer Inst. 1988 Jul 20;80(10):765–769. doi: 10.1093/jnci/80.10.765. [DOI] [PubMed] [Google Scholar]
- Jungi T. W. A rapid and sensitive method allowing photometric determination of erythrophagocytosis by mononuclear phagocytes. J Immunol Methods. 1985 Sep 3;82(1):141–153. doi: 10.1016/0022-1759(85)90233-9. [DOI] [PubMed] [Google Scholar]
- Kaplan A. M., Gerrard T. L., Strawson J., Squire C., Aultman M. Role of adenosine deaminase in human monocyte differentiation and tumor cell cytotoxicity. Ann N Y Acad Sci. 1985;451:264–278. doi: 10.1111/j.1749-6632.1985.tb27118.x. [DOI] [PubMed] [Google Scholar]
- Khym J. X. An analytical system for rapid separation of tissue nucleotides at low pressures on conventional anion exchangers. Clin Chem. 1975 Aug;21(9):1245–1252. [PubMed] [Google Scholar]
- Kinsella T. D., Baum J., Ziff M. Studies of isolated synovial living cells of rheumatoid and nonrheumatoid synovial membranes. Arthritis Rheum. 1970 Nov-Dec;13(6):734–753. doi: 10.1002/art.1780130603. [DOI] [PubMed] [Google Scholar]
- Lamballe F., Le Prise P. Y., Le Gall E., David J. C. dATP-mediated inhibition of DNA ligase by 2'-deoxycoformycin in T and B cell leukemia. Leukemia. 1989 Feb;3(2):97–103. [PubMed] [Google Scholar]
- Lindberg U., Skoog L. A method for the determination of dATP and dTTP in picomole amounts. Anal Biochem. 1970 Mar;34:152–160. doi: 10.1016/0003-2697(70)90096-5. [DOI] [PubMed] [Google Scholar]
- Major P. P., Agarwal R. P., Kufe D. W. Clinical pharmacology of deoxycoformycin. Blood. 1981 Jul;58(1):91–96. [PubMed] [Google Scholar]
- Matsumoto S. S., Yu J., Yu A. L. Inhibition of RNA synthesis by deoxyadenosine plus deoxycoformycin in resting lymphocytes. J Immunol. 1983 Dec;131(6):2762–2766. [PubMed] [Google Scholar]
- Matsumoto S. S., Yu J., Yu A. L. The effect of deoxyadenosine plus deoxycoformycin on replicative and repair synthesis of DNA in human lymphoblasts and isolated nuclei. J Biol Chem. 1988 May 25;263(15):7153–7158. [PubMed] [Google Scholar]
- Melinn M., McLaughlin H. Nitroblue tetrazolium reduction in lymphocytes. J Leukoc Biol. 1987 Apr;41(4):325–329. doi: 10.1002/jlb.41.4.325. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North T. W., Bestwick R. K., Mathews C. K. Detection of activities that interfere with the enzymatic assay of deoxyribonucleoside 5'-triphosphates. J Biol Chem. 1980 Jul 25;255(14):6640–6645. [PubMed] [Google Scholar]
- Parker W. B., Bapat A. R., Shen J. X., Townsend A. J., Cheng Y. C. Interaction of 2-halogenated dATP analogs (F, Cl, and Br) with human DNA polymerases, DNA primase, and ribonucleotide reductase. Mol Pharmacol. 1988 Oct;34(4):485–491. [PubMed] [Google Scholar]
- Piro L. D., Carrera C. J., Beutler E., Carson D. A. 2-Chlorodeoxyadenosine: an effective new agent for the treatment of chronic lymphocytic leukemia. Blood. 1988 Sep;72(3):1069–1073. [PubMed] [Google Scholar]
- Piro L. D., Carrera C. J., Carson D. A., Beutler E. Lasting remissions in hairy-cell leukemia induced by a single infusion of 2-chlorodeoxyadenosine. N Engl J Med. 1990 Apr 19;322(16):1117–1121. doi: 10.1056/NEJM199004193221605. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Kornbluth R. S., Carson D. A. Failure of dideoxynucleosides to inhibit human immunodeficiency virus replication in cultured human macrophages. J Exp Med. 1987 Oct 1;166(4):1144–1149. doi: 10.1084/jem.166.4.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart J. J., Sagone A. L., Balcerzak S. P., Ackerman G. A., LoBuglio A. F. Effects of corticosteroid therapy on human monocyte function. N Engl J Med. 1975 Jan 30;292(5):236–241. doi: 10.1056/NEJM197501302920504. [DOI] [PubMed] [Google Scholar]
- Seto S., Carrera C. J., Kubota M., Wasson D. B., Carson D. A. Mechanism of deoxyadenosine and 2-chlorodeoxyadenosine toxicity to nondividing human lymphocytes. J Clin Invest. 1985 Feb;75(2):377–383. doi: 10.1172/JCI111710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto S., Carrera C. J., Wasson D. B., Carson D. A. Inhibition of DNA repair by deoxyadenosine in resting human lymphocytes. J Immunol. 1986 Apr 15;136(8):2839–2843. [PubMed] [Google Scholar]
- Smulson M. E., Schein P., Mullins D. W., Jr, Sudhakar S. A putative role for nicotinamide adenine dinucleotide-promoted nuclear protein modification in the antitumor activity of N-methyl-N-nitrosourea. Cancer Res. 1977 Sep;37(9):3006–3012. [PubMed] [Google Scholar]
- Tanaka K., Yoshioka A., Tanaka S., Wataya Y. An improved method for the quantitative determination of deoxyribonucleoside triphosphates in cell extracts. Anal Biochem. 1984 May 15;139(1):35–41. doi: 10.1016/0003-2697(84)90386-5. [DOI] [PubMed] [Google Scholar]
- Ueda K., Hayaishi O. ADP-ribosylation. Annu Rev Biochem. 1985;54:73–100. doi: 10.1146/annurev.bi.54.070185.000445. [DOI] [PubMed] [Google Scholar]
- Urba W. J., Baseler M. W., Kopp W. C., Steis R. G., Clark J. W., Smith J. W., 2nd, Coggin D. L., Longo D. L. Deoxycoformycin-induced immunosuppression in patients with hairy cell leukemia. Blood. 1989 Jan;73(1):38–46. [PubMed] [Google Scholar]
- Yoshioka A., Tanaka S., Hiraoka O., Koyama Y., Hirota Y., Wataya Y. Deoxyribonucleoside-triphosphate imbalance death: deoxyadenosine-induced dNTP imbalance and DNA double strand breaks in mouse FM3A cells and the mechanism of cell death. Biochem Biophys Res Commun. 1987 Jul 15;146(1):258–264. doi: 10.1016/0006-291x(87)90719-4. [DOI] [PubMed] [Google Scholar]
- Zuckerman S. H., Ackerman S. K., Douglas S. D. Long-term human peripheral blood monocyte cultures: establishment, metabolism and morphology of primary human monocyte-macrophage cell cultures. Immunology. 1979 Oct;38(2):401–411. [PMC free article] [PubMed] [Google Scholar]
- Zvaifler N. J. New perspectives on the pathogenesis of rheumatoid arthritis. Am J Med. 1988 Oct 14;85(4A):12–17. doi: 10.1016/0002-9343(88)90356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Korte D., Haverkort W. A., Roos D., van Gennip A. H. Anion-exchange high performance liquid chromatography method for the quantitation of nucleotides in human blood cells. Clin Chim Acta. 1985 Jun 14;148(3):185–196. doi: 10.1016/0009-8981(85)90145-7. [DOI] [PubMed] [Google Scholar]
- van Furth R., Raeburn J. A., van Zwet T. L. Characteristics of human mononuclear phagocytes. Blood. 1979 Aug;54(2):485–500. [PubMed] [Google Scholar]



