Abstract
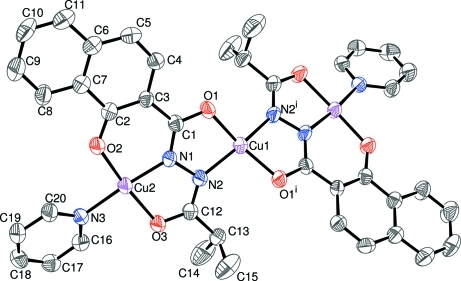
The complete molecule of the title complex, [Cu3(C15H13N2O3)2(C5H5N)2], is generated by crystallographic twofold symmetry, with the central Cu atom lying on the rotation axis: it is coordinated by two N,O-bidentate ligands in a trans-CuN2O2 distorted square-planar arrangement. The other Cu atom is coordinated by an N,O,O′-tridentate ligand and a pyridine molecule in a distorted trans-CuN2O2 arrangement. In the crystal structure, a C—H⋯π interaction occurs.
Related literature
For related structures, see: Patole et al. (2003 ▶); Pouralimardan et al. (2007 ▶). For background on C—H⋯π interactions, see: Nishio (2004 ▶); Saalfrank & Bernt (1998 ▶).
Experimental
Crystal data
[Cu3(C15H13N2O3)2(C5H5N)2]
M r = 887.37
Monoclinic,

a = 23.661 (2) Å
b = 13.0521 (18) Å
c = 13.3142 (15) Å
β = 113.684 (2)°
V = 3765.5 (7) Å3
Z = 4
Mo Kα radiation
μ = 1.74 mm−1
T = 298 K
0.37 × 0.35 × 0.31 mm
Data collection
Siemens SMART CCD diffractometer
Absorption correction: multi-scan (SADABS; Siemens, 1996 ▶) T min = 0.566, T max = 0.615 (expected range = 0.537–0.584)
9477 measured reflections
3310 independent reflections
2319 reflections with I > 2σ(I)
R int = 0.050
Refinement
R[F 2 > 2σ(F 2)] = 0.039
wR(F 2) = 0.135
S = 1.00
3310 reflections
251 parameters
H-atom parameters constrained
Δρmax = 0.82 e Å−3
Δρmin = −0.33 e Å−3
Data collection: SMART (Siemens, 1996 ▶); cell refinement: SAINT (Siemens, 1996 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809009003/hb2917sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809009003/hb2917Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected bond lengths (Å).
| Cu1—O1 | 1.920 (3) |
| Cu1—O1i | 1.920 (3) |
| Cu1—N2 | 1.946 (3) |
| Cu1—N2i | 1.946 (3) |
| Cu2—N1 | 1.884 (3) |
| Cu2—O2 | 1.890 (3) |
| Cu2—O3 | 1.953 (3) |
| Cu2—N3 | 1.975 (3) |
Symmetry code: (i) 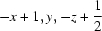 .
.
Table 2. Hydrogen-bond geometry (Å, °).
Cg1 is the centroid of the C2–C7 ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C17—H17⋯Cg1ii | 0.93 | 2.53 | 3.362 (4) | 150 |
Symmetry code: (ii) 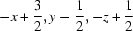 .
.
Acknowledgments
We acknowledge financial support from the National Natural Science Foundation of China (grant No. 20671048).
supplementary crystallographic information
Comment
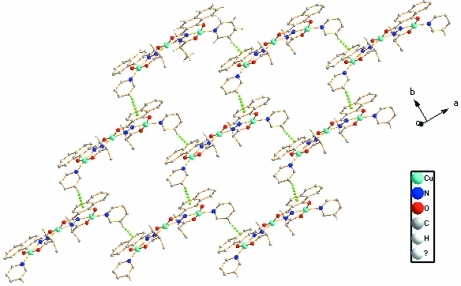
A large number of aroylhydrazine complexes have been prepared and studied due to their diverse molecular architectures and quite interesting chemical properties (Patole et al., 2003; Pouralimardan et al., 2007). However, researches on the copper(II) complexes with N-isobutyryl-1-hydroxy-2-naphthalenecarbohydrazide have not reported. So we have synthesized a new complex, (I), (Fig. 1), which has been characterized by X-ray diffraction and elemental analysis. The molecule of (I) contains three copper(II), two ligand molecules, and two pyridine molecules. Both copper centres adopt distorted square planar trans-CuN2O2 arrangements. The triple-deprotonated N-isobutyryl-1-hydroxy-2-naphthalenecarbohydrazide bridges the metal ions using a hydrazide N—N group and formed the trinuclear copper complex. In the crystal packing, the complex molecules are linked into two-dimensional network by intermolecular C—H···π interactions (Fig. 2) (Saalfrank & Bernt, 1998; Nishio, 2004).
Experimental
Isobutyric anhydride (0.632 g, 4 mmol) and 1-hydroxy-2-naphthalenecarbohydrazide (0.808 g, 4 mmol) were added to 40 ml of chloroform at ice-water bath. The reaction mixture was slowly warmed to room temperature and stirred for 24 h. After overnight refrigeration, the resulting white precipitate was filtered and rinsed with chloroform and diethyl ether (1.02 g, 93.57% yield). A solution of CuNO3(0.04 g,0.2 mmol) in methanol (10 ml) was added to a mixture of N-isobutyryl-1-hydroxy-2-naphthalenecarbohydrazide (0.055 g, 0.2 mmol) and sodium methylate (0.0324 g, 0.6 mmol) in pyridine (10 ml). A green solution was obtained after refluxing for 3 h. After being filtrated, dimethyl ether was slowly diffused into the filtrate, and green blocks of (I) were obtained after two weeks. Elemental analysis calculated for C40H36N6O6Cu3: C, 54.09; H, 4.05; O, 10.78; N, 9.43. Found (%): C, 54.12; H, 4.06; O, 10.82; N, 9.47
Refinement
The C-bound H atoms were positioned with idealized geometry (C—H = 0.93–0.98Å) and refined as riding with Uiso(H) = 1.2 Ueq(C) or 1.5Ueq(methyl C).
Figures
Fig. 1.
The molecular structure of (I) showing 40% probability displacement ellipsoids. H atoms have been omitted for clarity. Symmetry code: (i) 1–x, y, 1/2–z.
Fig. 2.
View of the two-dimensional network structure in (I). Intermolecular C—H···π are shown as dashed lines. Most of H atoms are omitted.
Crystal data
| [Cu3(C15H13N2O3)2(C5H5N)2] | F(000) = 1812 |
| Mr = 887.37 | Dx = 1.565 Mg m−3Dm = 1.565 Mg m−3Dm measured by not measured |
| Monoclinic, C2/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -C 2yc | Cell parameters from 3140 reflections |
| a = 23.661 (2) Å | θ = 2.7–26.3° |
| b = 13.0521 (18) Å | µ = 1.74 mm−1 |
| c = 13.3142 (15) Å | T = 298 K |
| β = 113.684 (2)° | Block, green |
| V = 3765.5 (7) Å3 | 0.37 × 0.35 × 0.31 mm |
| Z = 4 |
Data collection
| Siemens SMART CCD diffractometer | 3310 independent reflections |
| Radiation source: fine-focus sealed tube | 2319 reflections with I > 2σ(I) |
| graphite | Rint = 0.050 |
| ω scans | θmax = 25.0°, θmin = 1.8° |
| Absorption correction: multi-scan (SADABS; Siemens, 1996) | h = −28→27 |
| Tmin = 0.566, Tmax = 0.615 | k = −14→15 |
| 9477 measured reflections | l = −13→15 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.039 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.135 | H-atom parameters constrained |
| S = 1.00 | w = 1/[σ2(Fo2) + (0.079P)2 + 4.0071P] where P = (Fo2 + 2Fc2)/3 |
| 3310 reflections | (Δ/σ)max = 0.001 |
| 251 parameters | Δρmax = 0.82 e Å−3 |
| 0 restraints | Δρmin = −0.33 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cu1 | 0.5000 | 0.86302 (6) | 0.2500 | 0.0410 (2) | |
| Cu2 | 0.68569 (2) | 0.80745 (4) | 0.23216 (4) | 0.0404 (2) | |
| N1 | 0.62147 (15) | 0.8496 (3) | 0.2723 (3) | 0.0401 (8) | |
| N2 | 0.56137 (15) | 0.8380 (3) | 0.1900 (3) | 0.0425 (9) | |
| N3 | 0.74587 (15) | 0.7504 (3) | 0.1787 (3) | 0.0386 (8) | |
| O1 | 0.57183 (12) | 0.8896 (2) | 0.3816 (2) | 0.0446 (7) | |
| O2 | 0.74777 (12) | 0.8431 (2) | 0.3698 (2) | 0.0442 (7) | |
| O3 | 0.61596 (13) | 0.7851 (3) | 0.0920 (2) | 0.0523 (8) | |
| C1 | 0.62281 (18) | 0.8758 (3) | 0.3692 (3) | 0.0363 (9) | |
| C2 | 0.73934 (18) | 0.8742 (3) | 0.4567 (3) | 0.0361 (9) | |
| C3 | 0.68203 (18) | 0.8912 (3) | 0.4623 (3) | 0.0341 (9) | |
| C4 | 0.68036 (19) | 0.9281 (3) | 0.5618 (3) | 0.0399 (10) | |
| H4 | 0.6421 | 0.9386 | 0.5645 | 0.048* | |
| C5 | 0.7314 (2) | 0.9484 (3) | 0.6518 (3) | 0.0440 (11) | |
| H5 | 0.7278 | 0.9733 | 0.7144 | 0.053* | |
| C6 | 0.7909 (2) | 0.9322 (3) | 0.6522 (3) | 0.0421 (10) | |
| C7 | 0.79500 (19) | 0.8936 (3) | 0.5546 (3) | 0.0398 (10) | |
| C8 | 0.85377 (19) | 0.8776 (4) | 0.5545 (4) | 0.0503 (11) | |
| H8 | 0.8570 | 0.8522 | 0.4919 | 0.060* | |
| C9 | 0.9059 (2) | 0.8990 (5) | 0.6448 (4) | 0.0674 (15) | |
| H9 | 0.9443 | 0.8885 | 0.6426 | 0.081* | |
| C10 | 0.9026 (2) | 0.9367 (4) | 0.7415 (4) | 0.0655 (15) | |
| H10 | 0.9385 | 0.9507 | 0.8029 | 0.079* | |
| C11 | 0.8464 (2) | 0.9522 (4) | 0.7439 (4) | 0.0559 (13) | |
| H11 | 0.8443 | 0.9767 | 0.8080 | 0.067* | |
| C12 | 0.56432 (19) | 0.8035 (4) | 0.0990 (4) | 0.0451 (11) | |
| C13 | 0.5056 (2) | 0.7872 (4) | −0.0027 (4) | 0.0548 (13) | |
| H13 | 0.4703 | 0.7944 | 0.0176 | 0.066* | |
| C14 | 0.5046 (3) | 0.6793 (5) | −0.0479 (5) | 0.090 (2) | |
| H14A | 0.5318 | 0.6765 | −0.0854 | 0.135* | |
| H14B | 0.4634 | 0.6627 | −0.0982 | 0.135* | |
| H14C | 0.5180 | 0.6310 | 0.0114 | 0.135* | |
| C15 | 0.5004 (3) | 0.8681 (5) | −0.0879 (5) | 0.0862 (19) | |
| H15A | 0.5084 | 0.9344 | −0.0539 | 0.129* | |
| H15B | 0.4595 | 0.8668 | −0.1449 | 0.129* | |
| H15C | 0.5299 | 0.8541 | −0.1188 | 0.129* | |
| C16 | 0.7284 (2) | 0.6777 (3) | 0.0995 (3) | 0.0414 (10) | |
| H16 | 0.6872 | 0.6577 | 0.0689 | 0.050* | |
| C17 | 0.7684 (2) | 0.6324 (3) | 0.0624 (4) | 0.0468 (11) | |
| H17 | 0.7548 | 0.5822 | 0.0084 | 0.056* | |
| C18 | 0.8288 (2) | 0.6624 (4) | 0.1064 (4) | 0.0585 (13) | |
| H18 | 0.8571 | 0.6324 | 0.0829 | 0.070* | |
| C19 | 0.8476 (2) | 0.7379 (5) | 0.1861 (4) | 0.0604 (13) | |
| H19 | 0.8883 | 0.7605 | 0.2160 | 0.072* | |
| C20 | 0.8046 (2) | 0.7787 (4) | 0.2200 (4) | 0.0489 (11) | |
| H20 | 0.8174 | 0.8285 | 0.2745 | 0.059* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cu1 | 0.0270 (4) | 0.0562 (5) | 0.0400 (4) | 0.000 | 0.0138 (3) | 0.000 |
| Cu2 | 0.0282 (3) | 0.0537 (4) | 0.0391 (3) | −0.0004 (2) | 0.0133 (2) | −0.0070 (2) |
| N1 | 0.0243 (17) | 0.058 (2) | 0.0350 (19) | −0.0019 (16) | 0.0083 (15) | −0.0053 (17) |
| N2 | 0.0269 (18) | 0.062 (2) | 0.0371 (19) | −0.0036 (17) | 0.0110 (16) | −0.0097 (18) |
| N3 | 0.0349 (19) | 0.044 (2) | 0.0392 (19) | 0.0012 (16) | 0.0175 (16) | −0.0024 (17) |
| O1 | 0.0286 (15) | 0.0644 (19) | 0.0435 (17) | −0.0017 (14) | 0.0172 (13) | −0.0100 (15) |
| O2 | 0.0308 (15) | 0.0602 (19) | 0.0417 (17) | −0.0003 (14) | 0.0146 (13) | −0.0083 (15) |
| O3 | 0.0291 (16) | 0.088 (2) | 0.0386 (17) | 0.0002 (16) | 0.0123 (13) | −0.0135 (16) |
| C1 | 0.031 (2) | 0.039 (2) | 0.041 (2) | −0.0013 (18) | 0.0161 (18) | −0.0011 (19) |
| C2 | 0.034 (2) | 0.032 (2) | 0.040 (2) | −0.0022 (17) | 0.0121 (18) | 0.0006 (18) |
| C3 | 0.029 (2) | 0.034 (2) | 0.037 (2) | −0.0010 (17) | 0.0120 (17) | −0.0024 (18) |
| C4 | 0.039 (2) | 0.042 (2) | 0.040 (2) | −0.0004 (19) | 0.016 (2) | −0.0016 (19) |
| C5 | 0.057 (3) | 0.041 (2) | 0.034 (2) | −0.003 (2) | 0.017 (2) | −0.0002 (19) |
| C6 | 0.046 (3) | 0.032 (2) | 0.041 (2) | −0.0017 (19) | 0.011 (2) | 0.0039 (19) |
| C7 | 0.040 (2) | 0.032 (2) | 0.043 (2) | −0.0017 (18) | 0.013 (2) | 0.0008 (19) |
| C8 | 0.034 (2) | 0.059 (3) | 0.053 (3) | 0.002 (2) | 0.012 (2) | −0.008 (2) |
| C9 | 0.037 (3) | 0.089 (4) | 0.066 (3) | 0.001 (3) | 0.009 (3) | −0.006 (3) |
| C10 | 0.041 (3) | 0.072 (4) | 0.058 (3) | −0.002 (3) | −0.007 (2) | −0.004 (3) |
| C11 | 0.057 (3) | 0.054 (3) | 0.043 (3) | −0.003 (2) | 0.006 (2) | −0.002 (2) |
| C12 | 0.031 (2) | 0.064 (3) | 0.040 (2) | −0.001 (2) | 0.0130 (19) | −0.001 (2) |
| C13 | 0.031 (2) | 0.088 (4) | 0.038 (2) | 0.001 (2) | 0.008 (2) | −0.009 (3) |
| C14 | 0.078 (4) | 0.082 (4) | 0.070 (4) | −0.011 (3) | −0.012 (3) | −0.016 (3) |
| C15 | 0.074 (4) | 0.084 (4) | 0.064 (4) | −0.006 (3) | −0.009 (3) | 0.004 (3) |
| C16 | 0.042 (2) | 0.038 (2) | 0.044 (2) | −0.0023 (19) | 0.018 (2) | 0.001 (2) |
| C17 | 0.056 (3) | 0.040 (3) | 0.046 (3) | 0.004 (2) | 0.022 (2) | −0.002 (2) |
| C18 | 0.057 (3) | 0.070 (3) | 0.055 (3) | 0.024 (3) | 0.031 (3) | 0.002 (3) |
| C19 | 0.035 (3) | 0.089 (4) | 0.056 (3) | 0.005 (3) | 0.017 (2) | −0.003 (3) |
| C20 | 0.035 (2) | 0.062 (3) | 0.047 (3) | 0.000 (2) | 0.014 (2) | −0.008 (2) |
Geometric parameters (Å, °)
| Cu1—O1 | 1.920 (3) | C8—C9 | 1.361 (6) |
| Cu1—O1i | 1.920 (3) | C8—H8 | 0.9300 |
| Cu1—N2 | 1.946 (3) | C9—C10 | 1.409 (7) |
| Cu1—N2i | 1.946 (3) | C9—H9 | 0.9300 |
| Cu2—N1 | 1.884 (3) | C10—C11 | 1.360 (7) |
| Cu2—O2 | 1.890 (3) | C10—H10 | 0.9300 |
| Cu2—O3 | 1.953 (3) | C11—H11 | 0.9300 |
| Cu2—N3 | 1.975 (3) | C12—C13 | 1.516 (6) |
| N1—C1 | 1.323 (5) | C13—C15 | 1.518 (8) |
| N1—N2 | 1.412 (5) | C13—C14 | 1.529 (8) |
| N2—C12 | 1.320 (5) | C13—H13 | 0.9800 |
| N3—C20 | 1.326 (5) | C14—H14A | 0.9600 |
| N3—C16 | 1.354 (5) | C14—H14B | 0.9600 |
| O1—C1 | 1.294 (5) | C14—H14C | 0.9600 |
| O2—C2 | 1.315 (5) | C15—H15A | 0.9600 |
| O3—C12 | 1.285 (5) | C15—H15B | 0.9600 |
| C1—C3 | 1.465 (5) | C15—H15C | 0.9600 |
| C2—C3 | 1.405 (5) | C16—C17 | 1.365 (6) |
| C2—C7 | 1.455 (6) | C16—H16 | 0.9300 |
| C3—C4 | 1.425 (6) | C17—C18 | 1.367 (7) |
| C4—C5 | 1.342 (6) | C17—H17 | 0.9300 |
| C4—H4 | 0.9300 | C18—C19 | 1.384 (7) |
| C5—C6 | 1.423 (6) | C18—H18 | 0.9300 |
| C5—H5 | 0.9300 | C19—C20 | 1.374 (6) |
| C6—C11 | 1.412 (6) | C19—H19 | 0.9300 |
| C6—C7 | 1.433 (6) | C20—H20 | 0.9300 |
| C7—C8 | 1.406 (6) | ||
| O1—Cu1—O1i | 159.21 (19) | C7—C8—H8 | 119.5 |
| O1—Cu1—N2 | 82.65 (13) | C8—C9—C10 | 121.0 (5) |
| O1i—Cu1—N2 | 100.87 (13) | C8—C9—H9 | 119.5 |
| O1—Cu1—N2i | 100.87 (13) | C10—C9—H9 | 119.5 |
| O1i—Cu1—N2i | 82.65 (13) | C11—C10—C9 | 119.2 (4) |
| N2—Cu1—N2i | 160.7 (2) | C11—C10—H10 | 120.4 |
| N1—Cu2—O2 | 93.11 (13) | C9—C10—H10 | 120.4 |
| N1—Cu2—O3 | 81.17 (13) | C10—C11—C6 | 122.0 (5) |
| O2—Cu2—O3 | 173.00 (13) | C10—C11—H11 | 119.0 |
| N1—Cu2—N3 | 172.94 (14) | C6—C11—H11 | 119.0 |
| O2—Cu2—N3 | 92.88 (13) | O3—C12—N2 | 122.2 (4) |
| O3—Cu2—N3 | 93.10 (13) | O3—C12—C13 | 117.8 (4) |
| C1—N1—N2 | 114.0 (3) | N2—C12—C13 | 120.0 (4) |
| C1—N1—Cu2 | 130.2 (3) | C12—C13—C15 | 109.9 (4) |
| N2—N1—Cu2 | 115.1 (3) | C12—C13—C14 | 110.1 (4) |
| C12—N2—N1 | 109.9 (3) | C15—C13—C14 | 111.2 (5) |
| C12—N2—Cu1 | 139.2 (3) | C12—C13—H13 | 108.5 |
| N1—N2—Cu1 | 110.4 (2) | C15—C13—H13 | 108.5 |
| C20—N3—C16 | 117.3 (4) | C14—C13—H13 | 108.5 |
| C20—N3—Cu2 | 122.2 (3) | C13—C14—H14A | 109.5 |
| C16—N3—Cu2 | 120.4 (3) | C13—C14—H14B | 109.5 |
| C1—O1—Cu1 | 112.8 (3) | H14A—C14—H14B | 109.5 |
| C2—O2—Cu2 | 126.6 (3) | C13—C14—H14C | 109.5 |
| C12—O3—Cu2 | 111.4 (3) | H14A—C14—H14C | 109.5 |
| O1—C1—N1 | 120.1 (4) | H14B—C14—H14C | 109.5 |
| O1—C1—C3 | 119.8 (4) | C13—C15—H15A | 109.5 |
| N1—C1—C3 | 120.1 (3) | C13—C15—H15B | 109.5 |
| O2—C2—C3 | 125.9 (4) | H15A—C15—H15B | 109.5 |
| O2—C2—C7 | 116.0 (4) | C13—C15—H15C | 109.5 |
| C3—C2—C7 | 118.1 (4) | H15A—C15—H15C | 109.5 |
| C2—C3—C4 | 119.4 (4) | H15B—C15—H15C | 109.5 |
| C2—C3—C1 | 123.3 (4) | N3—C16—C17 | 123.1 (4) |
| C4—C3—C1 | 117.3 (3) | N3—C16—H16 | 118.5 |
| C5—C4—C3 | 123.1 (4) | C17—C16—H16 | 118.5 |
| C5—C4—H4 | 118.5 | C16—C17—C18 | 118.6 (4) |
| C3—C4—H4 | 118.5 | C16—C17—H17 | 120.7 |
| C4—C5—C6 | 120.6 (4) | C18—C17—H17 | 120.7 |
| C4—C5—H5 | 119.7 | C17—C18—C19 | 119.4 (4) |
| C6—C5—H5 | 119.7 | C17—C18—H18 | 120.3 |
| C11—C6—C5 | 123.4 (4) | C19—C18—H18 | 120.3 |
| C11—C6—C7 | 118.2 (4) | C20—C19—C18 | 118.4 (5) |
| C5—C6—C7 | 118.4 (4) | C20—C19—H19 | 120.8 |
| C8—C7—C6 | 118.6 (4) | C18—C19—H19 | 120.8 |
| C8—C7—C2 | 120.9 (4) | N3—C20—C19 | 123.2 (4) |
| C6—C7—C2 | 120.5 (4) | N3—C20—H20 | 118.4 |
| C9—C8—C7 | 121.0 (5) | C19—C20—H20 | 118.4 |
| C9—C8—H8 | 119.5 | ||
| O2—Cu2—N1—C1 | −10.5 (4) | N1—C1—C3—C4 | 174.6 (4) |
| O3—Cu2—N1—C1 | 173.6 (4) | C2—C3—C4—C5 | 0.4 (6) |
| O2—Cu2—N1—N2 | 179.6 (3) | C1—C3—C4—C5 | −177.8 (4) |
| O3—Cu2—N1—N2 | 3.6 (3) | C3—C4—C5—C6 | −0.9 (7) |
| C1—N1—N2—C12 | −174.6 (4) | C4—C5—C6—C11 | 179.9 (4) |
| Cu2—N1—N2—C12 | −3.0 (5) | C4—C5—C6—C7 | −0.1 (6) |
| C1—N1—N2—Cu1 | −0.9 (5) | C11—C6—C7—C8 | −0.1 (6) |
| Cu2—N1—N2—Cu1 | 170.69 (17) | C5—C6—C7—C8 | 179.8 (4) |
| O1—Cu1—N2—C12 | 172.5 (5) | C11—C6—C7—C2 | −178.4 (4) |
| O1i—Cu1—N2—C12 | −28.3 (5) | C5—C6—C7—C2 | 1.6 (6) |
| N2i—Cu1—N2—C12 | 70.6 (5) | O2—C2—C7—C8 | −1.0 (6) |
| O1—Cu1—N2—N1 | 1.6 (3) | C3—C2—C7—C8 | 179.8 (4) |
| O1i—Cu1—N2—N1 | 160.8 (3) | O2—C2—C7—C6 | 177.1 (4) |
| N2i—Cu1—N2—N1 | −100.4 (3) | C3—C2—C7—C6 | −2.1 (6) |
| O2—Cu2—N3—C20 | −23.5 (4) | C6—C7—C8—C9 | −0.4 (7) |
| O3—Cu2—N3—C20 | 152.8 (4) | C2—C7—C8—C9 | 177.8 (5) |
| O2—Cu2—N3—C16 | 154.6 (3) | C7—C8—C9—C10 | 0.7 (8) |
| O3—Cu2—N3—C16 | −29.1 (3) | C8—C9—C10—C11 | −0.3 (9) |
| O1i—Cu1—O1—C1 | −103.3 (3) | C9—C10—C11—C6 | −0.3 (8) |
| N2—Cu1—O1—C1 | −2.0 (3) | C5—C6—C11—C10 | −179.4 (5) |
| N2i—Cu1—O1—C1 | 158.7 (3) | C7—C6—C11—C10 | 0.5 (7) |
| N1—Cu2—O2—C2 | 6.4 (3) | Cu2—O3—C12—N2 | 3.1 (6) |
| N3—Cu2—O2—C2 | −169.9 (3) | Cu2—O3—C12—C13 | −178.6 (3) |
| N1—Cu2—O3—C12 | −3.6 (3) | N1—N2—C12—O3 | −0.2 (6) |
| N3—Cu2—O3—C12 | 172.2 (3) | Cu1—N2—C12—O3 | −171.2 (3) |
| Cu1—O1—C1—N1 | 2.2 (5) | N1—N2—C12—C13 | −178.4 (4) |
| Cu1—O1—C1—C3 | −179.0 (3) | Cu1—N2—C12—C13 | 10.7 (8) |
| N2—N1—C1—O1 | −0.8 (6) | O3—C12—C13—C15 | −69.6 (6) |
| Cu2—N1—C1—O1 | −170.8 (3) | N2—C12—C13—C15 | 108.6 (5) |
| N2—N1—C1—C3 | −179.6 (3) | O3—C12—C13—C14 | 53.2 (6) |
| Cu2—N1—C1—C3 | 10.4 (6) | N2—C12—C13—C14 | −128.5 (5) |
| Cu2—O2—C2—C3 | −3.0 (6) | C20—N3—C16—C17 | 0.9 (6) |
| Cu2—O2—C2—C7 | 177.9 (3) | Cu2—N3—C16—C17 | −177.3 (3) |
| O2—C2—C3—C4 | −178.1 (4) | N3—C16—C17—C18 | −0.7 (7) |
| C7—C2—C3—C4 | 1.1 (6) | C16—C17—C18—C19 | −0.5 (7) |
| O2—C2—C3—C1 | 0.1 (7) | C17—C18—C19—C20 | 1.4 (8) |
| C7—C2—C3—C1 | 179.2 (4) | C16—N3—C20—C19 | 0.0 (7) |
| O1—C1—C3—C2 | 177.6 (4) | Cu2—N3—C20—C19 | 178.2 (4) |
| N1—C1—C3—C2 | −3.6 (6) | C18—C19—C20—N3 | −1.2 (8) |
| O1—C1—C3—C4 | −4.3 (6) |
Symmetry codes: (i) −x+1, y, −z+1/2.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C17—H17···Cg1ii | 0.93 | 2.53 | 3.362 (4) | 150 |
Symmetry codes: (ii) −x+3/2, y−1/2, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HB2917).
References
- Nishio, M. (2004). CrystEngComm, 6, 130–158.
- Patole, J., Sandbhor, U., Padhye, S., Deobagkar, D. N., Anson, C. E. & Powell, A. (2003). Bioorg. Med. Chem. Lett.13, 51–55. [DOI] [PubMed]
- Pouralimardan, O., Chamayou, A. C., Janiak, C. & Hassan, H. M. (2007). Inorg. Chim. Acta, 360, 1599–1608.
- Saalfrank, R. W. & Bernt, I. (1998). Curr. Opin. Solid State Mater. Sci.3, 407–413.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Siemens (1996). SMART, SAINT and SADABS Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809009003/hb2917sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809009003/hb2917Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report