Abstract
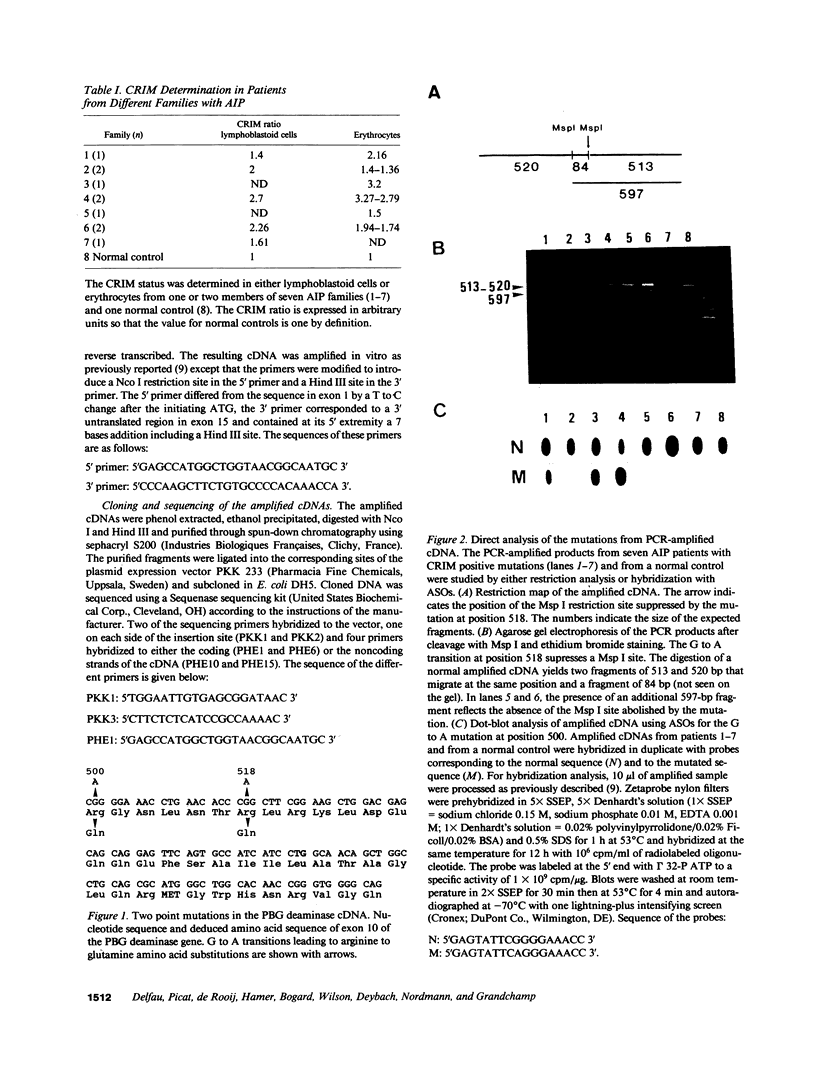
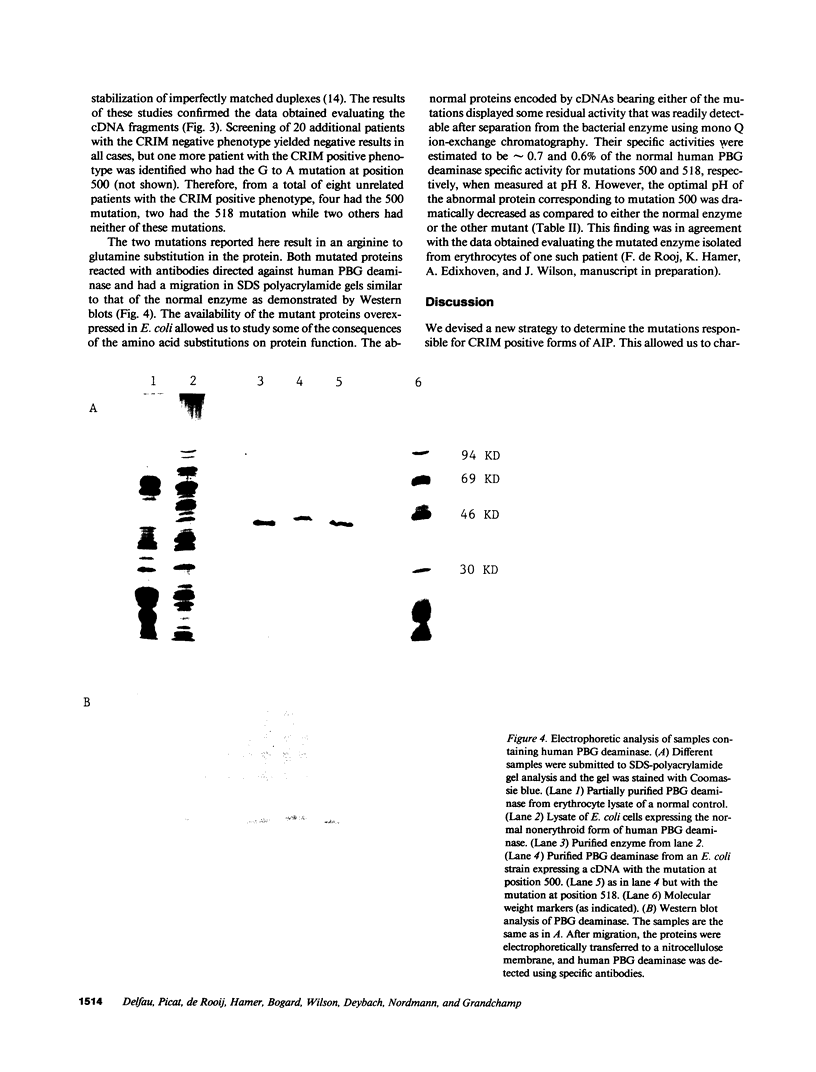
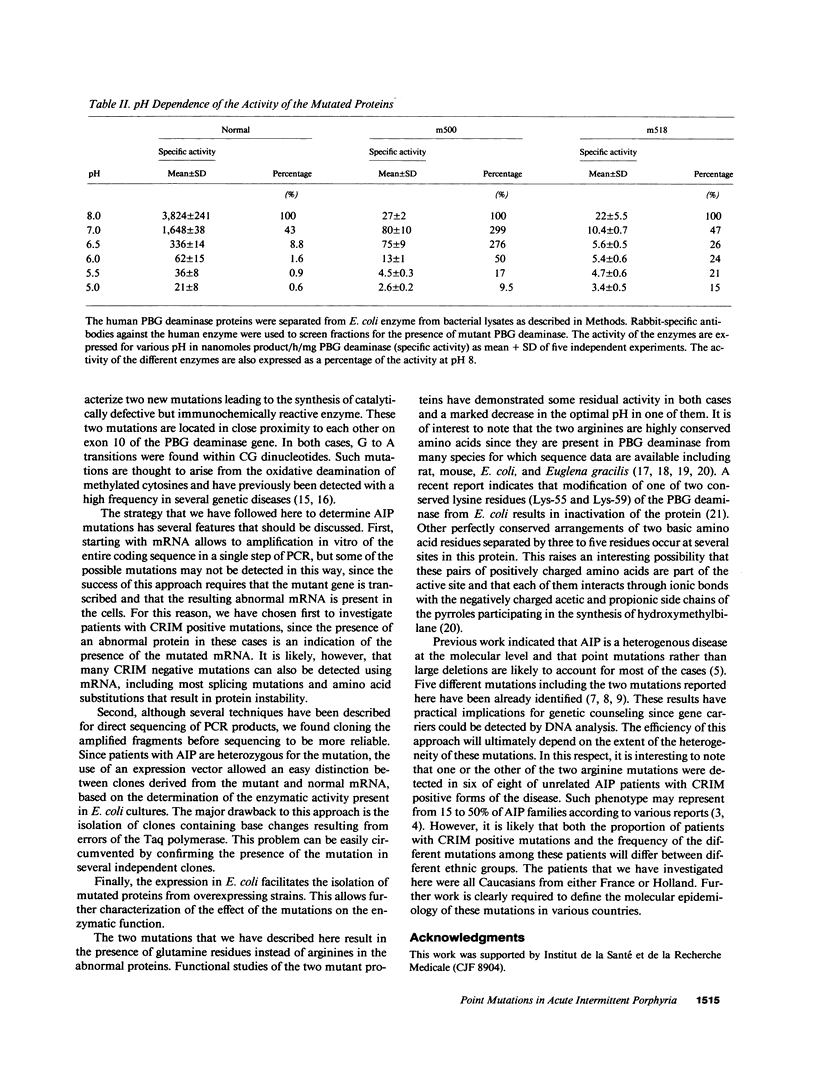
Two mutations of the porphobilinogen (PBG) deaminase gene resulting in cross-reacting immunological material (CRIM) positive forms of acute intermittent porphyria (AIP) have been identified by in vitro amplification of cDNA and cloning of the amplified products in a bacterial expression vector. Both mutations resulted from G to A transitions in exon 10 of the gene and produced arginine to glutamine substitutions in the abnormal protein. Expression of mutant cDNA in Escherichia coli reveals that one but not the other of these amino acid changes results in a striking decrease of the optimal pH of the mutated enzyme. One or the other of these two mutations accounted for the defect causing AIP in six unrelated patients among the eight patients evaluated with the CRIM positive subtype of this disorder.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaumont C., Porcher C., Picat C., Nordmann Y., Grandchamp B. The mouse porphobilinogen deaminase gene. Structural organization, sequence, and transcriptional analysis. J Biol Chem. 1989 Sep 5;264(25):14829–14834. [PubMed] [Google Scholar]
- Grandchamp B., De Verneuil H., Beaumont C., Chretien S., Walter O., Nordmann Y. Tissue-specific expression of porphobilinogen deaminase. Two isoenzymes from a single gene. Eur J Biochem. 1987 Jan 2;162(1):105–110. doi: 10.1111/j.1432-1033.1987.tb10548.x. [DOI] [PubMed] [Google Scholar]
- Grandchamp B., Nordmann Y. Enzymes of the heme biosynthesis pathway: recent advances in molecular genetics. Semin Hematol. 1988 Oct;25(4):303–311. [PubMed] [Google Scholar]
- Grandchamp B., Picat C., Kauppinen R., Mignotte V., Peltonen L., Mustajoki P., Roméo P. H., Goossens M., Nordmann Y. Molecular analysis of acute intermittent porphyria in a Finnish family with normal erythrocyte porphobilinogen deaminase. Eur J Clin Invest. 1989 Oct;19(5):415–418. doi: 10.1111/j.1365-2362.1989.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Grandchamp B., Picat C., Mignotte V., Wilson J. H., Te Velde K., Sandkuyl L., Roméo P. H., Goossens M., Nordmann Y. Tissue-specific splicing mutation in acute intermittent porphyria. Proc Natl Acad Sci U S A. 1989 Jan;86(2):661–664. doi: 10.1073/pnas.86.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandchamp B., Picat C., de Rooij F., Beaumont C., Wilson P., Deybach J. C., Nordmann Y. A point mutation G----A in exon 12 of the porphobilinogen deaminase gene results in exon skipping and is responsible for acute intermittent porphyria. Nucleic Acids Res. 1989 Aug 25;17(16):6637–6649. doi: 10.1093/nar/17.16.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta S., Takagi K., Wallace R. B., Itakura K. Dissociation kinetics of 19 base paired oligonucleotide-DNA duplexes containing different single mismatched base pairs. Nucleic Acids Res. 1987 Jan 26;15(2):797–811. doi: 10.1093/nar/15.2.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keohavong P., Thilly W. G. Fidelity of DNA polymerases in DNA amplification. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9253–9257. doi: 10.1073/pnas.86.23.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Anvret M., Lindsten J., Lannfelt L., Gellerfors P., Wetterberg L., Floderus Y., Thunell S. DNA polymorphisms within the porphobilinogen deaminase gene in two Swedish families with acute intermittent porphyria. Hum Genet. 1988 Aug;79(4):379–381. doi: 10.1007/BF00282182. [DOI] [PubMed] [Google Scholar]
- Lee J. T., Nussbaum R. L. An arginine to glutamine mutation in residue 109 of human ornithine transcarbamylase completely abolishes enzymatic activity in Cos1 cells. J Clin Invest. 1989 Dec;84(6):1762–1766. doi: 10.1172/JCI114360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn D. H., Elder G. H., Kalsheker N. A., Marsh O. W., Harrison P. R., Grandchamp B., Picat C., Nordmann Y., Romeo P. H., Goossens M. DNA polymorphism of human porphobilinogen deaminase gene in acute intermittent porphyria. Lancet. 1987 Sep 26;2(8561):706–708. doi: 10.1016/s0140-6736(87)91073-7. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Packman L. C., Hart G. J., Alefounder P. R., Abell C., Battersby A. R. Evidence that pyridoxal phosphate modification of lysine residues (Lys-55 and Lys-59) causes inactivation of hydroxymethylbilane synthase (porphobilinogen deaminase). Biochem J. 1989 Aug 15;262(1):119–124. doi: 10.1042/bj2620119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sharif A. L., Smith A. G., Abell C. Isolation and characterisation of a cDNA clone for a chlorophyll synthesis enzyme from Euglena gracilis. The chloroplast enzyme hydroxymethylbilane synthase (porphobilinogen deaminase) is synthesised with a very long transit peptide in Euglena. Eur J Biochem. 1989 Sep 15;184(2):353–359. doi: 10.1111/j.1432-1033.1989.tb15026.x. [DOI] [PubMed] [Google Scholar]
- Stubnicer A. C., Picat C., Grandchamp B. Rat porphobilinogen deaminase cDNA: nucleotide sequence of the erythropoietic form. Nucleic Acids Res. 1988 Apr 11;16(7):3102–3102. doi: 10.1093/nar/16.7.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. D., Jordan P. M. Nucleotide sequence of the hemC locus encoding porphobilinogen deaminase of Escherichia coli K12. Nucleic Acids Res. 1986 Aug 11;14(15):6215–6226. doi: 10.1093/nar/14.15.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssoufian H., Antonarakis S. E., Bell W., Griffin A. M., Kazazian H. H., Jr Nonsense and missense mutations in hemophilia A: estimate of the relative mutation rate at CG dinucleotides. Am J Hum Genet. 1988 May;42(5):718–725. [PMC free article] [PubMed] [Google Scholar]
- de Rooij F. W., Hamer C. M., Wilson J. H. Purification of porphobilinogen deaminase from human erythrocytes by fast protein liquid chromatography. Clin Chim Acta. 1987 Jan 15;162(1):61–68. doi: 10.1016/0009-8981(87)90233-6. [DOI] [PubMed] [Google Scholar]