Abstract
The title compound, C13H9BrF3N5O, crystallizes with two independent molecules in the asymmetric unit. The pyrimidine rings of the molecules are planar [maximum deviations 0.053 (3) and 0.012 (3) Å], while the triazine rings adopt flattened half-boat conformations with the p-bromophenyl rings in the flagpole positions. The crystal packing is stabilized by a three-dimensional network of intermolecular N—H⋯N, N—H⋯O and N—H⋯F hydrogen bonds.
Related literature
For the crystal structure of 7,7-dimethyl-2-phenyl-6,7-dihydro-1,2,4-triazolo[1,5-a][1,3,5]triazin-5-amine, see: Dolzhenko et al. (2007 ▶). For the preparation of benzo-fused analogues, see: Dolzhenko et al. (2008a
▶). For the previous report in this series, see: Dolzhenko et al. (2008b
▶).
Experimental
Crystal data
C13H9BrF3N5O
M r = 388.16
Orthorhombic,

a = 10.0531 (4) Å
b = 29.9108 (13) Å
c = 10.1945 (4) Å
V = 3065.4 (2) Å3
Z = 8
Mo Kα radiation
μ = 2.73 mm−1
T = 223 K
0.46 × 0.34 × 0.20 mm
Data collection
Bruker SMART APEX CCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2001 ▶) T min = 0.367, T max = 0.612 (expected range = 0.348–0.580)
20728 measured reflections
6287 independent reflections
4979 reflections with I > 2σ(I)
R int = 0.037
Refinement
R[F 2 > 2σ(F 2)] = 0.052
wR(F 2) = 0.138
S = 1.04
6287 reflections
432 parameters
16 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 1.50 e Å−3
Δρmin = −0.74 e Å−3
Absolute structure: Flack (1983 ▶), 2044 Friedel pairs
Flack parameter: 0.011 (10)
Data collection: SMART (Bruker, 2001 ▶); cell refinement: SAINT (Bruker, 2001 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, New_Global_Publ_Block. DOI: 10.1107/S1600536809007612/ng2543sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809007612/ng2543Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1N⋯N2i | 0.87 (2) | 2.15 (2) | 3.005 (5) | 171 (5) |
| N5—H5A⋯O2ii | 0.895 (14) | 2.09 (3) | 2.905 (5) | 152 (5) |
| N5—H5B⋯N4i | 0.892 (14) | 2.25 (2) | 3.095 (6) | 159 (5) |
| N5—H5B⋯F1i | 0.892 (14) | 2.46 (4) | 3.054 (5) | 124 (4) |
| N6—H6N⋯N7iii | 0.902 (19) | 2.10 (3) | 2.967 (5) | 160 (5) |
| N10—H10A⋯O1 | 0.89 (2) | 2.03 (3) | 2.885 (5) | 162 (5) |
| N10—H10B⋯N9iii | 0.90 (2) | 2.15 (2) | 3.041 (5) | 171 (5) |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
Acknowledgments
This work was supported by the National Medical Research Council, Singapore (grant Nos. NMRC/NIG/0019/2008 and NMRC/NIG/0020/2008).
supplementary crystallographic information
Comment
The title compound was synthesized via thermal cyclocondensation of 6-oxo-4-trifluoromethyl-1,6-dihydropyrimidin-2-yl guanidine with p-bromobenzaldehyde (Fig.1) using the methodology that we successfully applied previously for the preparation of its benzofused analogues (Dolzhenko et al., 2008a). In general, the synthesized compound might be involved in tautomerism with four possible tautomers (Fig. 2). However, only one tautomeric form viz. 2-amino-4-(4-bromophenyl)-8-trifluoromethyl-3,4-dihydro-6H-pyrimido[1,2-a][1,3,5]triazin-6-one was found in the crystal.
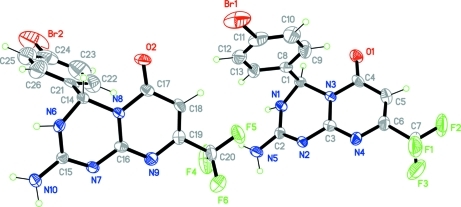
The compound crystallized with two independent molecules (A and B) in the asymmetric unit (Fig. 3). The pyrimidine rings in the molecules are planar with maximum deviations 0.0525 (26) Å (C3) and 0.0118 (32) Å (C18) from the planes C3/N3/C4—C6/N4 and C16/N8/C17—C19/N9 of molecules A and B, respectively. Similarly to structurally related 7,7-dimethyl-2-phenyl-6,7-dyhydro-1,2,4-triazolo[1,5-a][1,3,5]triazin-5-amine (Dolzhenko et al., 2007), the triazine rings in the molecules adopt flattened half-boat conformations with atoms N2 and N7 at the sterns and sp3-hybridized atoms C1 and C14 at the bows with p-bromophenyl rings as flagpoles. However, the molecules are significantly different in the geometry at bridgehead nitrogen atoms (N3 and N8) and sp3-hybridized atoms C1 and C14 of the triazine rings.The torsion angles C4—N3—C1—N1 and C17—N8—C14—N6 are 136.7 (4)° and 150.4 (3)°, respectively. In molecule B, the bond N8—C14 is located almost in the phenyl ring (C21—C26) plane: the torsion angle N8—C14—C21—C26 is 2.6 (5)°. The corresponding torsion angle N3—C1—C8—C9 of molecule A is 42.5 (6)°. The N1—C2, N2—C2, N5—C2 bond distances in A and N6—C15, N7—C15, N10—C15 in B are similar that suggests guanidine-like electron delocalization in N1/N2/N5/C2 and N6/N7/N10/C15 fragments of the molecules.
The crystal packing is stabilized by three-dimensional network of intramolecular N—H···N, N—H···O and N—H···F hydrogen bonds (Table 1, Fig. 4).
Experimental
2-Amino-4-(4-bromophenyl)-8-trifluoromethyl-3,4-dihydro-6H-pyrimido[1,2-a][1,3,5]triazin-6-one was synthesized from 6-oxo-4-trifluoromethyl-1,6-dihydropyrimidin-2-yl guanidine and p-bromobenzaldehyde according to general method reported by Dolzhenko et al. (2008a). Single crystals suitable for crystallographic analysis were grown by slow evaporation of the solution in ethyl acetate / methanol.
Refinement
N-bound H atoms were located in a difference map and refined freely. C-bound H atoms were positioned geometrically (C—H = 0.94 or 0.99 Å) and were constrained in a riding motion approximation with Uiso(H) = 1.2Ueq(C). One of the BrC6H4 parts is disordered into two parts at 89:11 ratio.
Figures
Fig. 1.
The synthesis of 2-amino-4-(4-bromophenyl)-8-trifluoromethyl-3,4-dihydro-6H-pyrimido[1,2-a][1,3,5]triazin-6-one
Fig. 2.
Tautomerism in the title compound
Fig. 3.
The molecular structure of 2-amino-4-(4-bromophenyl)-8-trifluoromethyl-3,4-dihydro-6H-pyrimido[1,2-a][1,3,5]triazin-6-one with the atomic numbering scheme. Displacement ellipsoids are drawn at the 50% probability level.
Fig. 4.
Molecular parking in the crystal, viewed along the a axis. Hydrogen bonds are shown as dashed lines.
Crystal data
| C13H9BrF3N5O | Dx = 1.682 Mg m−3 |
| Mr = 388.16 | Melting point: 516 K |
| Orthorhombic, Pna21 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2c -2n | Cell parameters from 5069 reflections |
| a = 10.0531 (4) Å | θ = 2.4–25.1° |
| b = 29.9108 (13) Å | µ = 2.73 mm−1 |
| c = 10.1945 (4) Å | T = 223 K |
| V = 3065.4 (2) Å3 | Block, colourless |
| Z = 8 | 0.46 × 0.34 × 0.20 mm |
| F(000) = 1536 |
Data collection
| Bruker SMART APEX CCD diffractometer | 6287 independent reflections |
| Radiation source: fine-focus sealed tube | 4979 reflections with I > 2σ(I) |
| graphite | Rint = 0.037 |
| φ and ω scans | θmax = 27.5°, θmin = 1.4° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2001) | h = −12→13 |
| Tmin = 0.367, Tmax = 0.612 | k = −38→38 |
| 20728 measured reflections | l = −13→11 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.052 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.138 | w = 1/[σ2(Fo2) + (0.0646P)2 + 2.1384P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.04 | (Δ/σ)max = 0.001 |
| 6287 reflections | Δρmax = 1.50 e Å−3 |
| 432 parameters | Δρmin = −0.73 e Å−3 |
| 16 restraints | Absolute structure: Flack (1983), 2044 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: 0.011 (10) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| O1 | 1.0316 (3) | 0.34878 (10) | 1.2353 (3) | 0.0346 (7) | |
| F1 | 0.9317 (4) | 0.36022 (11) | 1.7842 (4) | 0.0634 (10) | |
| F2 | 0.8409 (4) | 0.30866 (11) | 1.6709 (4) | 0.0750 (12) | |
| F3 | 0.7371 (4) | 0.37004 (16) | 1.7065 (5) | 0.0873 (14) | |
| N1 | 1.0194 (3) | 0.47967 (11) | 1.2317 (4) | 0.0259 (7) | |
| H1N | 1.012 (5) | 0.4927 (15) | 1.156 (3) | 0.031* | |
| N2 | 0.9826 (3) | 0.48272 (10) | 1.4583 (3) | 0.0237 (7) | |
| N3 | 1.0181 (3) | 0.41352 (10) | 1.3521 (3) | 0.0232 (7) | |
| N4 | 0.9342 (4) | 0.41721 (11) | 1.5665 (4) | 0.0290 (7) | |
| N5 | 0.9597 (4) | 0.54572 (11) | 1.3299 (4) | 0.0335 (8) | |
| H5A | 0.919 (5) | 0.5568 (15) | 1.401 (3) | 0.040* | |
| H5B | 0.982 (5) | 0.5632 (14) | 1.262 (4) | 0.040* | |
| C1 | 1.0910 (4) | 0.43826 (13) | 1.2510 (4) | 0.0250 (8) | |
| H1A | 1.0904 | 0.4209 | 1.1683 | 0.030* | |
| C2 | 0.9892 (4) | 0.50251 (13) | 1.3412 (4) | 0.0249 (8) | |
| C3 | 0.9789 (4) | 0.43766 (13) | 1.4607 (4) | 0.0258 (8) | |
| C4 | 0.9958 (4) | 0.36750 (13) | 1.3367 (5) | 0.0288 (9) | |
| C5 | 0.9359 (5) | 0.34747 (15) | 1.4486 (5) | 0.0387 (10) | |
| H5 | 0.9122 | 0.3171 | 1.4478 | 0.046* | |
| C6 | 0.9137 (5) | 0.37273 (15) | 1.5558 (5) | 0.0358 (10) | |
| C7 | 0.8558 (7) | 0.35237 (18) | 1.6796 (6) | 0.0542 (15) | |
| C8 | 1.2338 (4) | 0.44558 (14) | 1.2947 (5) | 0.0279 (9) | |
| C9 | 1.3053 (5) | 0.41197 (16) | 1.3543 (6) | 0.0454 (13) | |
| H9 | 1.2654 | 0.3838 | 1.3664 | 0.054* | |
| C10 | 1.4348 (5) | 0.41866 (18) | 1.3968 (6) | 0.0519 (14) | |
| H10 | 1.4826 | 0.3954 | 1.4372 | 0.062* | |
| C11 | 1.4917 (4) | 0.45984 (18) | 1.3788 (6) | 0.0441 (12) | |
| C12 | 1.4249 (5) | 0.49351 (18) | 1.3178 (6) | 0.0450 (12) | |
| H12 | 1.4660 | 0.5214 | 1.3044 | 0.054* | |
| C13 | 1.2959 (5) | 0.48627 (15) | 1.2758 (5) | 0.0362 (10) | |
| H13 | 1.2495 | 0.5095 | 1.2336 | 0.043* | |
| Br1 | 1.66612 (4) | 0.47071 (3) | 1.44122 (8) | 0.0697 (2) | |
| O2 | 0.7222 (3) | 0.09824 (9) | 0.9889 (3) | 0.0338 (7) | |
| F4 | 1.2813 (4) | 0.10516 (13) | 1.0040 (5) | 0.0782 (13) | |
| F5 | 1.1837 (3) | 0.05015 (8) | 1.0941 (4) | 0.0578 (10) | |
| F6 | 1.2446 (3) | 0.10589 (11) | 1.2081 (5) | 0.0631 (10) | |
| N6 | 0.7243 (3) | 0.22885 (11) | 1.0553 (4) | 0.0271 (8) | |
| H6N | 0.653 (3) | 0.2463 (13) | 1.073 (5) | 0.032* | |
| N7 | 0.9576 (3) | 0.22901 (10) | 1.0690 (4) | 0.0261 (7) | |
| N8 | 0.8395 (3) | 0.16152 (10) | 1.0287 (4) | 0.0237 (7) | |
| N9 | 1.0675 (3) | 0.16188 (11) | 1.0790 (4) | 0.0288 (8) | |
| N10 | 0.8364 (4) | 0.29270 (11) | 1.1091 (4) | 0.0319 (8) | |
| H10A | 0.909 (3) | 0.3050 (15) | 1.144 (5) | 0.038* | |
| H10B | 0.762 (3) | 0.3090 (15) | 1.104 (6) | 0.038* | |
| C20 | 1.1928 (5) | 0.09342 (14) | 1.0926 (6) | 0.0377 (11) | |
| C14 | 0.7273 (4) | 0.18807 (12) | 0.9806 (5) | 0.0258 (9) | |
| H14 | 0.6443 | 0.1713 | 0.9985 | 0.031* | |
| C15 | 0.8407 (4) | 0.25037 (12) | 1.0759 (4) | 0.0231 (8) | |
| C16 | 0.9555 (4) | 0.18357 (12) | 1.0582 (4) | 0.0246 (8) | |
| C17 | 0.8279 (4) | 0.11509 (13) | 1.0201 (4) | 0.0255 (8) | |
| C18 | 0.9505 (4) | 0.09218 (13) | 1.0451 (5) | 0.0287 (9) | |
| H18 | 0.9540 | 0.0608 | 1.0441 | 0.034* | |
| C19 | 1.0602 (4) | 0.11631 (13) | 1.0699 (4) | 0.0291 (9) | |
| Br2 | 0.74894 (14) | 0.22662 (3) | 0.38715 (10) | 0.0924 (5) | 0.899 (3) |
| C21 | 0.7359 (4) | 0.19636 (19) | 0.8349 (3) | 0.0312 (13) | 0.899 (3) |
| C22 | 0.6379 (4) | 0.22284 (19) | 0.7782 (4) | 0.0475 (14) | 0.899 (3) |
| H22 | 0.5695 | 0.2348 | 0.8304 | 0.057* | 0.899 (3) |
| C23 | 0.6409 (4) | 0.23160 (16) | 0.6443 (4) | 0.0599 (19) | 0.899 (3) |
| H23 | 0.5747 | 0.2495 | 0.6060 | 0.072* | 0.899 (3) |
| C24 | 0.7420 (4) | 0.21389 (14) | 0.5671 (3) | 0.0509 (16) | 0.899 (3) |
| C25 | 0.8400 (4) | 0.18741 (14) | 0.6238 (3) | 0.0621 (19) | 0.899 (3) |
| H25 | 0.9084 | 0.1754 | 0.5716 | 0.075* | 0.899 (3) |
| C26 | 0.8370 (4) | 0.17865 (16) | 0.7577 (3) | 0.0463 (16) | 0.899 (3) |
| H26 | 0.9033 | 0.1607 | 0.7960 | 0.056* | 0.899 (3) |
| Br3 | 0.8608 (7) | 0.21715 (19) | 0.3838 (6) | 0.054 (2)* | 0.101 (3) |
| C21A | 0.756 (6) | 0.197 (3) | 0.829 (2) | 0.045* | 0.101 (3) |
| C22A | 0.668 (5) | 0.219 (3) | 0.747 (3) | 0.062* | 0.101 (3) |
| H22A | 0.5867 | 0.2298 | 0.7806 | 0.075* | 0.101 (3) |
| C23A | 0.699 (4) | 0.225 (2) | 0.616 (2) | 0.062* | 0.101 (3) |
| H23A | 0.6392 | 0.2402 | 0.5601 | 0.075* | 0.101 (3) |
| C24A | 0.818 (3) | 0.2090 (17) | 0.5660 (15) | 0.062* | 0.101 (3) |
| C25A | 0.907 (4) | 0.1867 (17) | 0.6480 (18) | 0.062* | 0.101 (3) |
| H25A | 0.9876 | 0.1758 | 0.6145 | 0.075* | 0.101 (3) |
| C26A | 0.875 (5) | 0.181 (2) | 0.7796 (19) | 0.062* | 0.101 (3) |
| H26A | 0.9351 | 0.1654 | 0.8350 | 0.075* | 0.101 (3) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0404 (17) | 0.0260 (14) | 0.0375 (18) | −0.0012 (12) | −0.0011 (14) | −0.0105 (13) |
| F1 | 0.103 (3) | 0.0442 (17) | 0.0428 (19) | −0.0128 (17) | 0.0068 (19) | 0.0091 (14) |
| F2 | 0.119 (3) | 0.0427 (17) | 0.063 (2) | −0.039 (2) | 0.021 (2) | 0.0051 (17) |
| F3 | 0.071 (3) | 0.101 (3) | 0.090 (3) | 0.004 (2) | 0.044 (2) | 0.032 (3) |
| N1 | 0.0288 (17) | 0.0241 (16) | 0.0247 (18) | 0.0042 (13) | −0.0028 (14) | −0.0010 (14) |
| N2 | 0.0241 (15) | 0.0214 (15) | 0.0255 (19) | 0.0018 (12) | 0.0019 (14) | 0.0007 (14) |
| N3 | 0.0235 (16) | 0.0197 (14) | 0.0265 (19) | 0.0033 (12) | −0.0003 (13) | −0.0010 (13) |
| N4 | 0.0333 (18) | 0.0281 (16) | 0.0258 (18) | −0.0022 (14) | 0.0025 (15) | 0.0023 (15) |
| N5 | 0.047 (2) | 0.0229 (17) | 0.031 (2) | 0.0074 (15) | 0.0066 (18) | 0.0020 (16) |
| C1 | 0.0256 (19) | 0.0257 (19) | 0.024 (2) | 0.0051 (15) | 0.0029 (15) | −0.0009 (16) |
| C2 | 0.0203 (18) | 0.0254 (18) | 0.029 (2) | 0.0001 (14) | −0.0008 (16) | 0.0024 (16) |
| C3 | 0.0210 (17) | 0.0277 (19) | 0.029 (2) | 0.0016 (14) | −0.0037 (16) | −0.0034 (17) |
| C4 | 0.027 (2) | 0.0196 (18) | 0.040 (2) | 0.0005 (15) | −0.0060 (18) | −0.0055 (18) |
| C5 | 0.047 (2) | 0.028 (2) | 0.041 (3) | −0.0088 (18) | −0.003 (2) | 0.002 (2) |
| C6 | 0.034 (2) | 0.032 (2) | 0.041 (3) | −0.0076 (18) | 0.000 (2) | 0.005 (2) |
| C7 | 0.071 (4) | 0.041 (3) | 0.050 (4) | −0.013 (3) | 0.016 (3) | 0.005 (3) |
| C8 | 0.027 (2) | 0.031 (2) | 0.026 (2) | 0.0038 (16) | 0.0029 (17) | −0.0034 (17) |
| C9 | 0.034 (2) | 0.037 (2) | 0.065 (4) | −0.0027 (19) | −0.006 (2) | 0.019 (2) |
| C10 | 0.035 (2) | 0.059 (3) | 0.062 (4) | 0.012 (2) | −0.008 (2) | 0.018 (3) |
| C11 | 0.020 (2) | 0.067 (3) | 0.045 (3) | 0.000 (2) | −0.009 (2) | −0.015 (3) |
| C12 | 0.033 (2) | 0.044 (3) | 0.058 (3) | −0.009 (2) | 0.006 (2) | −0.012 (2) |
| C13 | 0.029 (2) | 0.033 (2) | 0.047 (3) | −0.0071 (18) | −0.001 (2) | 0.000 (2) |
| Br1 | 0.0269 (2) | 0.1078 (5) | 0.0743 (4) | 0.0028 (3) | −0.0111 (3) | −0.0396 (4) |
| O2 | 0.0328 (16) | 0.0275 (14) | 0.0410 (19) | −0.0115 (12) | −0.0019 (13) | −0.0060 (13) |
| F4 | 0.054 (2) | 0.075 (2) | 0.105 (3) | 0.0289 (17) | 0.040 (2) | 0.035 (2) |
| F5 | 0.0513 (18) | 0.0253 (13) | 0.097 (3) | 0.0075 (12) | −0.0090 (18) | −0.0024 (16) |
| F6 | 0.0541 (19) | 0.0514 (17) | 0.084 (3) | 0.0087 (15) | −0.0318 (19) | −0.0024 (19) |
| N6 | 0.0182 (16) | 0.0241 (16) | 0.039 (2) | 0.0027 (12) | −0.0038 (14) | −0.0013 (15) |
| N7 | 0.0238 (16) | 0.0228 (15) | 0.0317 (19) | −0.0017 (12) | −0.0012 (14) | −0.0055 (15) |
| N8 | 0.0236 (16) | 0.0170 (14) | 0.0306 (19) | −0.0037 (13) | 0.0000 (13) | −0.0022 (13) |
| N9 | 0.0234 (16) | 0.0271 (16) | 0.036 (2) | −0.0004 (13) | −0.0010 (15) | −0.0021 (16) |
| N10 | 0.0282 (18) | 0.0223 (17) | 0.045 (2) | −0.0005 (13) | −0.0071 (16) | −0.0066 (16) |
| C20 | 0.036 (2) | 0.024 (2) | 0.053 (3) | 0.0041 (17) | 0.004 (2) | 0.006 (2) |
| C14 | 0.0235 (19) | 0.0188 (18) | 0.035 (2) | −0.0034 (14) | −0.0021 (16) | 0.0006 (16) |
| C15 | 0.0263 (19) | 0.0203 (17) | 0.0227 (19) | 0.0001 (14) | −0.0019 (16) | 0.0032 (16) |
| C16 | 0.0223 (18) | 0.0272 (19) | 0.024 (2) | −0.0066 (15) | 0.0011 (16) | −0.0003 (16) |
| C17 | 0.033 (2) | 0.0238 (18) | 0.0199 (19) | −0.0006 (16) | 0.0032 (16) | −0.0034 (16) |
| C18 | 0.032 (2) | 0.0179 (17) | 0.036 (2) | −0.0016 (16) | −0.0008 (18) | 0.0034 (17) |
| C19 | 0.031 (2) | 0.028 (2) | 0.028 (2) | 0.0018 (16) | 0.0023 (18) | 0.0035 (18) |
| Br2 | 0.1629 (13) | 0.0842 (6) | 0.0300 (3) | −0.0488 (7) | −0.0104 (5) | 0.0130 (4) |
| C21 | 0.044 (3) | 0.019 (2) | 0.030 (3) | −0.017 (2) | −0.012 (2) | −0.0023 (19) |
| C22 | 0.053 (4) | 0.051 (4) | 0.039 (3) | 0.004 (3) | −0.011 (3) | 0.008 (3) |
| C23 | 0.084 (5) | 0.051 (4) | 0.045 (4) | −0.008 (4) | −0.024 (4) | 0.012 (3) |
| C24 | 0.087 (5) | 0.043 (3) | 0.023 (3) | −0.027 (3) | −0.007 (3) | 0.000 (3) |
| C25 | 0.092 (6) | 0.063 (4) | 0.031 (3) | −0.010 (4) | 0.006 (3) | −0.011 (3) |
| C26 | 0.065 (4) | 0.046 (3) | 0.028 (3) | 0.001 (3) | −0.003 (3) | −0.004 (2) |
Geometric parameters (Å, °)
| O1—C4 | 1.229 (5) | N6—H6N | 0.902 (19) |
| F1—C7 | 1.331 (8) | N7—C15 | 1.339 (5) |
| F2—C7 | 1.319 (6) | N7—C16 | 1.364 (5) |
| F3—C7 | 1.334 (7) | N8—C16 | 1.373 (5) |
| N1—C2 | 1.344 (6) | N8—C17 | 1.396 (5) |
| N1—C1 | 1.446 (5) | N8—C14 | 1.464 (5) |
| N1—H1N | 0.87 (2) | N9—C16 | 1.317 (5) |
| N2—C2 | 1.334 (6) | N9—C19 | 1.368 (5) |
| N2—C3 | 1.348 (5) | N10—C15 | 1.311 (5) |
| N3—C3 | 1.379 (5) | N10—H10A | 0.89 (2) |
| N3—C4 | 1.403 (5) | N10—H10B | 0.90 (2) |
| N3—C1 | 1.465 (5) | C20—C19 | 1.516 (6) |
| N4—C3 | 1.318 (6) | C14—C21 | 1.509 (5) |
| N4—C6 | 1.351 (6) | C14—C21A | 1.591 (9) |
| N5—C2 | 1.331 (5) | C14—H14 | 0.9900 |
| N5—H5A | 0.895 (14) | C17—C18 | 1.433 (6) |
| N5—H5B | 0.892 (14) | C18—C19 | 1.343 (6) |
| C1—C8 | 1.519 (6) | C18—H18 | 0.9400 |
| C1—H1A | 0.9900 | Br2—C24 | 1.875 (3) |
| C4—C5 | 1.423 (7) | C21—C22 | 1.3900 |
| C5—C6 | 1.347 (8) | C21—C26 | 1.3900 |
| C5—H5 | 0.9400 | C22—C23 | 1.3900 |
| C6—C7 | 1.517 (7) | C22—H22 | 0.9400 |
| C8—C9 | 1.377 (6) | C23—C24 | 1.3900 |
| C8—C13 | 1.381 (6) | C23—H23 | 0.9400 |
| C9—C10 | 1.387 (7) | C24—C25 | 1.3900 |
| C9—H9 | 0.9400 | C25—C26 | 1.3900 |
| C10—C11 | 1.371 (8) | C25—H25 | 0.9400 |
| C10—H10 | 0.9400 | C26—H26 | 0.9400 |
| C11—C12 | 1.361 (8) | Br3—C24A | 1.921 (7) |
| C11—Br1 | 1.893 (4) | C21A—C22A | 1.3900 |
| C12—C13 | 1.383 (7) | C21A—C26A | 1.3900 |
| C12—H12 | 0.9400 | C22A—C23A | 1.3900 |
| C13—H13 | 0.9400 | C22A—H22A | 0.9400 |
| O2—C17 | 1.219 (5) | C23A—C24A | 1.3900 |
| F4—C20 | 1.316 (6) | C23A—H23A | 0.9400 |
| F5—C20 | 1.298 (5) | C24A—C25A | 1.3900 |
| F6—C20 | 1.341 (7) | C25A—C26A | 1.3900 |
| N6—C15 | 1.352 (5) | C25A—H25A | 0.9400 |
| N6—C14 | 1.439 (5) | C26A—H26A | 0.9400 |
| C2—N1—C1 | 115.8 (4) | F5—C20—F4 | 108.7 (4) |
| C2—N1—H1N | 119 (3) | F5—C20—F6 | 107.1 (4) |
| C1—N1—H1N | 123 (3) | F4—C20—F6 | 105.4 (4) |
| C2—N2—C3 | 117.5 (3) | F5—C20—C19 | 113.0 (4) |
| C3—N3—C4 | 123.9 (4) | F4—C20—C19 | 111.7 (4) |
| C3—N3—C1 | 116.3 (3) | F6—C20—C19 | 110.5 (4) |
| C4—N3—C1 | 119.7 (3) | N6—C14—N8 | 107.3 (3) |
| C3—N4—C6 | 116.3 (4) | N6—C14—C21 | 112.5 (4) |
| C2—N5—H5A | 113 (3) | N8—C14—C21 | 112.0 (4) |
| C2—N5—H5B | 126 (4) | N6—C14—C21A | 112 (3) |
| H5A—N5—H5B | 121 (5) | N8—C14—C21A | 106 (2) |
| N1—C1—N3 | 106.2 (3) | C21—C14—C21A | 7(2) |
| N1—C1—C8 | 112.7 (3) | N6—C14—H14 | 108.3 |
| N3—C1—C8 | 109.8 (3) | N8—C14—H14 | 108.3 |
| N1—C1—H1A | 109.3 | C21—C14—H14 | 108.3 |
| N3—C1—H1A | 109.3 | C21A—C14—H14 | 114.4 |
| C8—C1—H1A | 109.3 | N10—C15—N7 | 120.2 (3) |
| N5—C2—N2 | 119.8 (4) | N10—C15—N6 | 118.1 (3) |
| N5—C2—N1 | 118.1 (4) | N7—C15—N6 | 121.6 (3) |
| N2—C2—N1 | 122.0 (4) | N9—C16—N7 | 117.7 (3) |
| N4—C3—N2 | 119.2 (4) | N9—C16—N8 | 121.7 (3) |
| N4—C3—N3 | 120.7 (3) | N7—C16—N8 | 120.6 (3) |
| N2—C3—N3 | 120.0 (4) | O2—C17—N8 | 120.0 (4) |
| O1—C4—N3 | 119.7 (4) | O2—C17—C18 | 126.8 (4) |
| O1—C4—C5 | 127.3 (4) | N8—C17—C18 | 113.1 (3) |
| N3—C4—C5 | 113.0 (4) | C19—C18—C17 | 118.9 (4) |
| C6—C5—C4 | 119.0 (4) | C19—C18—H18 | 120.6 |
| C6—C5—H5 | 120.5 | C17—C18—H18 | 120.6 |
| C4—C5—H5 | 120.5 | C18—C19—N9 | 126.3 (4) |
| C5—C6—N4 | 126.3 (4) | C18—C19—C20 | 120.6 (4) |
| C5—C6—C7 | 120.9 (4) | N9—C19—C20 | 113.1 (4) |
| N4—C6—C7 | 112.8 (4) | C22—C21—C26 | 120.0 |
| F2—C7—F1 | 107.1 (5) | C22—C21—C14 | 117.5 (3) |
| F2—C7—F3 | 107.7 (5) | C26—C21—C14 | 122.5 (3) |
| F1—C7—F3 | 106.2 (5) | C21—C22—C23 | 120.0 |
| F2—C7—C6 | 112.7 (5) | C21—C22—H22 | 120.0 |
| F1—C7—C6 | 112.1 (4) | C23—C22—H22 | 120.0 |
| F3—C7—C6 | 110.8 (5) | C24—C23—C22 | 120.0 |
| C9—C8—C13 | 118.0 (4) | C24—C23—H23 | 120.0 |
| C9—C8—C1 | 121.2 (4) | C22—C23—H23 | 120.0 |
| C13—C8—C1 | 120.9 (4) | C23—C24—C25 | 120.0 |
| C8—C9—C10 | 121.5 (4) | C23—C24—Br2 | 120.2 (2) |
| C8—C9—H9 | 119.2 | C25—C24—Br2 | 119.7 (2) |
| C10—C9—H9 | 119.2 | C24—C25—C26 | 120.0 |
| C11—C10—C9 | 118.7 (5) | C24—C25—H25 | 120.0 |
| C11—C10—H10 | 120.7 | C26—C25—H25 | 120.0 |
| C9—C10—H10 | 120.7 | C25—C26—C21 | 120.0 |
| C12—C11—C10 | 121.3 (4) | C25—C26—H26 | 120.0 |
| C12—C11—Br1 | 118.9 (4) | C21—C26—H26 | 120.0 |
| C10—C11—Br1 | 119.7 (4) | C22A—C21A—C26A | 120.0 |
| C11—C12—C13 | 119.3 (5) | C22A—C21A—C14 | 123 (3) |
| C11—C12—H12 | 120.4 | C26A—C21A—C14 | 117 (3) |
| C13—C12—H12 | 120.4 | C21A—C22A—C23A | 120.0 |
| C8—C13—C12 | 121.2 (5) | C21A—C22A—H22A | 120.0 |
| C8—C13—H13 | 119.4 | C23A—C22A—H22A | 120.0 |
| C12—C13—H13 | 119.4 | C24A—C23A—C22A | 120.0 |
| C15—N6—C14 | 117.9 (3) | C24A—C23A—H23A | 120.0 |
| C15—N6—H6N | 112 (3) | C22A—C23A—H23A | 120.0 |
| C14—N6—H6N | 128 (3) | C23A—C24A—C25A | 120.0 |
| C15—N7—C16 | 117.8 (3) | C23A—C24A—Br3 | 119.9 (4) |
| C16—N8—C17 | 124.2 (3) | C25A—C24A—Br3 | 120.0 (4) |
| C16—N8—C14 | 117.9 (3) | C26A—C25A—C24A | 120.0 |
| C17—N8—C14 | 117.0 (3) | C26A—C25A—H25A | 120.0 |
| C16—N9—C19 | 115.7 (3) | C24A—C25A—H25A | 120.0 |
| C15—N10—H10A | 118 (3) | C25A—C26A—C21A | 120.0 |
| C15—N10—H10B | 122 (3) | C25A—C26A—H26A | 120.0 |
| H10A—N10—H10B | 119 (5) | C21A—C26A—H26A | 120.0 |
| C2—N1—C1—N3 | 49.4 (5) | C14—N6—C15—N10 | 160.7 (4) |
| C2—N1—C1—C8 | −70.9 (5) | C14—N6—C15—N7 | −22.5 (6) |
| C3—N3—C1—N1 | −45.3 (4) | C19—N9—C16—N7 | 178.7 (4) |
| C4—N3—C1—N1 | 136.7 (4) | C19—N9—C16—N8 | −0.3 (6) |
| C3—N3—C1—C8 | 76.9 (4) | C15—N7—C16—N9 | −166.1 (4) |
| C4—N3—C1—C8 | −101.0 (4) | C15—N7—C16—N8 | 12.9 (6) |
| C3—N2—C2—N5 | 163.1 (4) | C17—N8—C16—N9 | 1.2 (6) |
| C3—N2—C2—N1 | −13.9 (6) | C14—N8—C16—N9 | −167.5 (4) |
| C1—N1—C2—N5 | 160.7 (4) | C17—N8—C16—N7 | −177.8 (4) |
| C1—N1—C2—N2 | −22.3 (6) | C14—N8—C16—N7 | 13.5 (6) |
| C6—N4—C3—N2 | 170.1 (4) | C16—N8—C17—O2 | −177.5 (4) |
| C6—N4—C3—N3 | −8.0 (6) | C14—N8—C17—O2 | −8.7 (6) |
| C2—N2—C3—N4 | −160.4 (4) | C16—N8—C17—C18 | −0.2 (6) |
| C2—N2—C3—N3 | 17.8 (5) | C14—N8—C17—C18 | 168.6 (4) |
| C4—N3—C3—N4 | 9.9 (6) | O2—C17—C18—C19 | 175.5 (4) |
| C1—N3—C3—N4 | −167.9 (4) | N8—C17—C18—C19 | −1.6 (6) |
| C4—N3—C3—N2 | −168.2 (3) | C17—C18—C19—N9 | 2.6 (7) |
| C1—N3—C3—N2 | 13.9 (5) | C17—C18—C19—C20 | −178.1 (4) |
| C3—N3—C4—O1 | 178.5 (4) | C16—N9—C19—C18 | −1.6 (7) |
| C1—N3—C4—O1 | −3.8 (6) | C16—N9—C19—C20 | 179.1 (4) |
| C3—N3—C4—C5 | −3.8 (5) | F5—C20—C19—C18 | −4.4 (7) |
| C1—N3—C4—C5 | 174.0 (4) | F4—C20—C19—C18 | 118.5 (5) |
| O1—C4—C5—C6 | 174.3 (4) | F6—C20—C19—C18 | −124.5 (5) |
| N3—C4—C5—C6 | −3.2 (6) | F5—C20—C19—N9 | 175.0 (4) |
| C4—C5—C6—N4 | 5.0 (8) | F4—C20—C19—N9 | −62.1 (6) |
| C4—C5—C6—C7 | −177.2 (5) | F6—C20—C19—N9 | 54.9 (5) |
| C3—N4—C6—C5 | 0.8 (7) | N6—C14—C21—C22 | −56.3 (4) |
| C3—N4—C6—C7 | −177.1 (4) | N8—C14—C21—C22 | −177.3 (3) |
| C5—C6—C7—F2 | 4.5 (8) | C21A—C14—C21—C22 | −147 (25) |
| N4—C6—C7—F2 | −177.4 (5) | N6—C14—C21—C26 | 123.7 (4) |
| C5—C6—C7—F1 | 125.4 (5) | N8—C14—C21—C26 | 2.6 (5) |
| N4—C6—C7—F1 | −56.6 (6) | C21A—C14—C21—C26 | 33 (24) |
| C5—C6—C7—F3 | −116.3 (6) | C26—C21—C22—C23 | 0.0 |
| N4—C6—C7—F3 | 61.8 (6) | C14—C21—C22—C23 | 180.0 (5) |
| N1—C1—C8—C9 | 160.8 (5) | C21—C22—C23—C24 | 0.0 |
| N3—C1—C8—C9 | 42.5 (6) | C22—C23—C24—C25 | 0.0 |
| N1—C1—C8—C13 | −19.2 (6) | C22—C23—C24—Br2 | −178.6 (3) |
| N3—C1—C8—C13 | −137.5 (4) | C23—C24—C25—C26 | 0.0 |
| C13—C8—C9—C10 | 1.3 (8) | Br2—C24—C25—C26 | 178.6 (3) |
| C1—C8—C9—C10 | −178.7 (5) | C24—C25—C26—C21 | 0.0 |
| C8—C9—C10—C11 | 0.1 (9) | C22—C21—C26—C25 | 0.0 |
| C9—C10—C11—C12 | −1.5 (9) | C14—C21—C26—C25 | −180.0 (5) |
| C9—C10—C11—Br1 | 177.8 (5) | N6—C14—C21A—C22A | −66 (4) |
| C10—C11—C12—C13 | 1.4 (9) | N8—C14—C21A—C22A | 177 (3) |
| Br1—C11—C12—C13 | −177.9 (4) | C21—C14—C21A—C22A | 26 (22) |
| C9—C8—C13—C12 | −1.4 (8) | N6—C14—C21A—C26A | 114 (3) |
| C1—C8—C13—C12 | 178.6 (5) | N8—C14—C21A—C26A | −3(4) |
| C11—C12—C13—C8 | 0.1 (8) | C21—C14—C21A—C26A | −154 (27) |
| C15—N6—C14—N8 | 44.6 (5) | C26A—C21A—C22A—C23A | 0.0 |
| C15—N6—C14—C21 | −79.0 (5) | C14—C21A—C22A—C23A | −180 (6) |
| C15—N6—C14—C21A | −71 (2) | C21A—C22A—C23A—C24A | 0.0 |
| C16—N8—C14—N6 | −40.1 (5) | C22A—C23A—C24A—C25A | 0.0 |
| C17—N8—C14—N6 | 150.4 (3) | C22A—C23A—C24A—Br3 | 179 (4) |
| C16—N8—C14—C21 | 83.9 (5) | C23A—C24A—C25A—C26A | 0.0 |
| C17—N8—C14—C21 | −85.6 (5) | Br3—C24A—C25A—C26A | −179 (4) |
| C16—N8—C14—C21A | 80 (3) | C24A—C25A—C26A—C21A | 0.0 |
| C17—N8—C14—C21A | −89 (3) | C22A—C21A—C26A—C25A | 0.0 |
| C16—N7—C15—N10 | 168.1 (4) | C14—C21A—C26A—C25A | 180 (6) |
| C16—N7—C15—N6 | −8.7 (6) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1N···N2i | 0.87 (2) | 2.15 (2) | 3.005 (5) | 171 (5) |
| N5—H5A···O2ii | 0.90 (1) | 2.09 (3) | 2.905 (5) | 152 (5) |
| N5—H5B···N4i | 0.89 (1) | 2.25 (2) | 3.095 (6) | 159 (5) |
| N5—H5B···F1i | 0.89 (1) | 2.46 (4) | 3.054 (5) | 124 (4) |
| N6—H6N···N7iii | 0.90 (2) | 2.10 (3) | 2.967 (5) | 160 (5) |
| N10—H10A···O1 | 0.89 (2) | 2.03 (3) | 2.885 (5) | 162 (5) |
| N10—H10B···N9iii | 0.90 (2) | 2.15 (2) | 3.041 (5) | 171 (5) |
Symmetry codes: (i) −x+2, −y+1, z−1/2; (ii) −x+3/2, y+1/2, z+1/2; (iii) x−1/2, −y+1/2, z.
Footnotes
Part 13 in the series ‘Fused heterocyclic systems with an s-triazine ring’. For Part 12, see Dolzhenko et al. (2008b).
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: NG2543).
References
- Bruker (2001). SMART and SAINT Bruker AXS GmbH, Karlsruhe, Germany.
- Dolzhenko, A. V., Dolzhenko, A. V. & Chui, W. K. (2008a). J. Heterocycl. Chem.45, 173-176.
- Dolzhenko, A. V., Pastorin, G., Dolzhenko, A. V. & Chui, W. K. (2008b). Tetrahedron Lett. 49, 7180-7183.
- Dolzhenko, A. V., Tan, G. K., Koh, L. L., Dolzhenko, A. V. & Chui, W. K. (2007). Acta Cryst. E63, o2796.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Sheldrick, G. M. (2001). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, New_Global_Publ_Block. DOI: 10.1107/S1600536809007612/ng2543sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809007612/ng2543Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report