Abstract
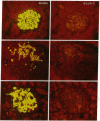
Previous studies from our laboratories have suggested a defect in glucose transport in islets isolated from BB rats on the first day of overt diabetes. To quantitate by immunostaining the glucose transporter of beta-cells (GLUT-2) before and at the onset of autoimmune diabetes we employed an antibody to its COOH-terminal octapeptide. On the first day of overt diabetes, defined as the day the daily blood glucose first reached 200 mg/dl, the volume density ratio of GLUT-2-positive to insulin-positive beta-cells was only 0.48 +/- 0.06, compared to 0.91 +/- 0.02 in age-matched nondiabetic diabetes-resistant controls (P less than 0.001). In age-matched nondiabetic diabetes-prone rats, most of which would have become diabetic, the ratio was 0.85 +/- 0.02, also less than the controls (P less than 0.05). Protein A-gold labeling of GLUT-2 in beta-cells of day 1 diabetic rats revealed 2.17 +/- 0.16 gold particles per micrometer length of microvillar plasma membranes compared to 3.91 +/- 0.14 in controls (P less than 0.001) and 2.87 +/- 0.24 in the nondiabetic diabetes-prone rats (P less than 0.02). Reduction in GLUT-2 correlates temporally with and may contribute to the loss of glucose-stimulated insulin secretion that precedes profound beta-cell depletion of autoimmune diabetes.
Full text
PDF

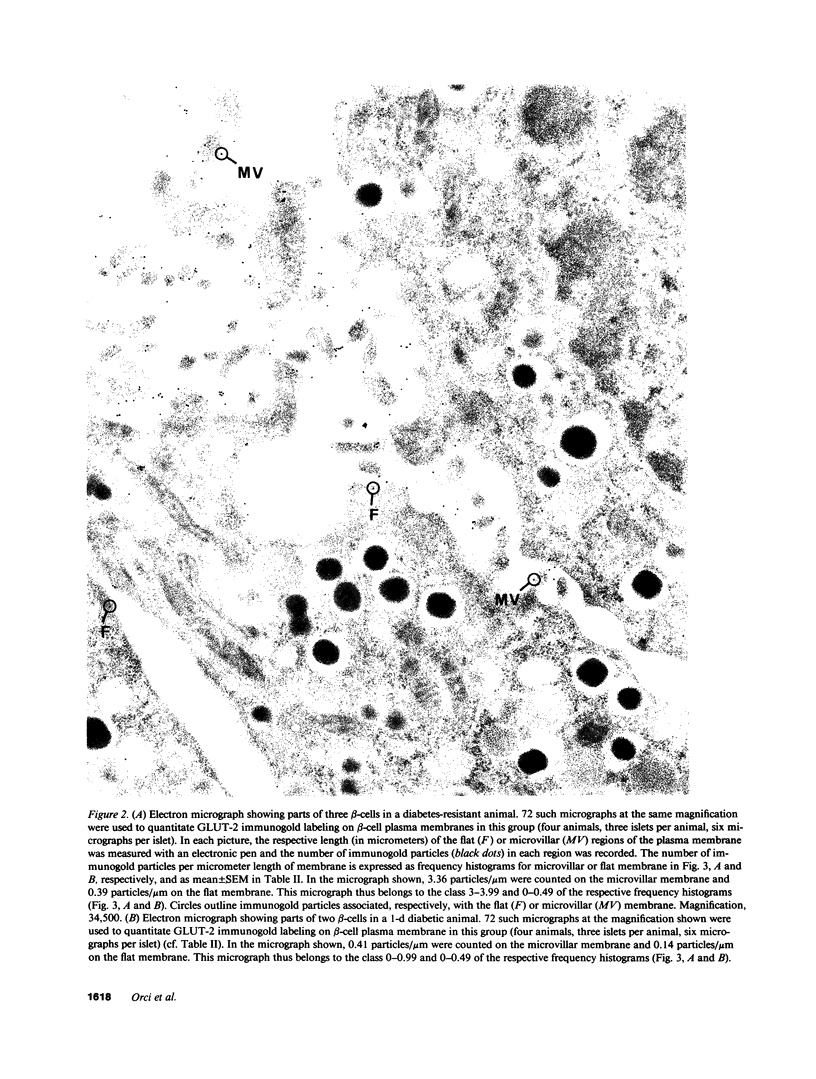


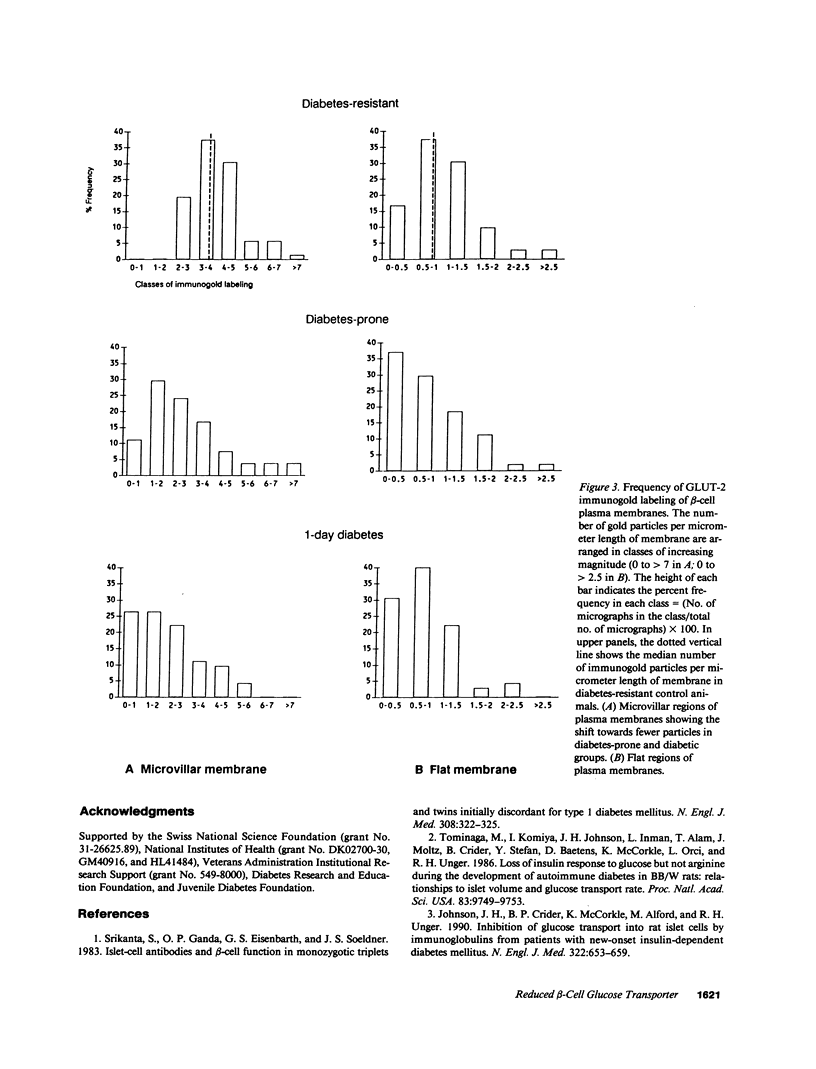

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armbruster B. L., Carlemalm E., Chiovetti R., Garavito R. M., Hobot J. A., Kellenberger E., Villiger W. Specimen preparation for electron microscopy using low temperature embedding resins. J Microsc. 1982 Apr;126(Pt 1):77–85. doi: 10.1111/j.1365-2818.1982.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Baetens D., Stefan Y., Ravazzola M., Malaisse-Lagae F., Coleman D. L., Orci L. Alteration of islet cell populations in spontaneously diabetic mice. Diabetes. 1978 Jan;27(1):1–7. doi: 10.2337/diab.27.1.1. [DOI] [PubMed] [Google Scholar]
- Fukumoto H., Seino S., Imura H., Seino Y., Eddy R. L., Fukushima Y., Byers M. G., Shows T. B., Bell G. I. Sequence, tissue distribution, and chromosomal localization of mRNA encoding a human glucose transporter-like protein. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5434–5438. doi: 10.1073/pnas.85.15.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodsky G. M., Fanska R. E. The in vitro perfused pancreas. Methods Enzymol. 1975;39:364–372. doi: 10.1016/s0076-6879(75)39033-2. [DOI] [PubMed] [Google Scholar]
- Heaton D. A., Lazarus N. R., Pyke D. A., Leslie R. D. B-cell responses to intravenous glucose and glucagon in non-diabetic twins of patients with type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1989 Nov;32(11):814–817. doi: 10.1007/BF00264913. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hisatomi A., Maruyama H., Orci L., Vasko M., Unger R. H. Adrenergically mediated intrapancreatic control of the glucagon response to glucopenia in the isolated rat pancreas. J Clin Invest. 1985 Feb;75(2):420–426. doi: 10.1172/JCI111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. H., Crider B. P., McCorkle K., Alford M., Unger R. H. Inhibition of glucose transport into rat islet cells by immunoglobulins from patients with new-onset insulin-dependent diabetes mellitus. N Engl J Med. 1990 Mar 8;322(10):653–659. doi: 10.1056/NEJM199003083221003. [DOI] [PubMed] [Google Scholar]
- Johnson J. H., Newgard C. B., Milburn J. L., Lodish H. F., Thorens B. The high Km glucose transporter of islets of Langerhans is functionally similar to the low affinity transporter of liver and has an identical primary sequence. J Biol Chem. 1990 Apr 25;265(12):6548–6551. [PubMed] [Google Scholar]
- Komiya I., Baetens D., Kuwajima M., Orci L., Unger R. H. Compensatory capabilities of islets of BB/Wor rats exposed to sustained hyperglycemia. Metabolism. 1990 Jun;39(6):614–618. doi: 10.1016/0026-0495(90)90028-b. [DOI] [PubMed] [Google Scholar]
- Orci L. Macro- and micro-domains in the endocrine pancreas. Diabetes. 1982 Jun;31(6 Pt 1):538–565. doi: 10.2337/diab.31.6.538. [DOI] [PubMed] [Google Scholar]
- Orci L., Thorens B., Ravazzola M., Lodish H. F. Localization of the pancreatic beta cell glucose transporter to specific plasma membrane domains. Science. 1989 Jul 21;245(4915):295–297. doi: 10.1126/science.2665080. [DOI] [PubMed] [Google Scholar]
- Pilch P. F. Glucose transporters: what's in a name? Endocrinology. 1990 Jan;126(1):3–5. doi: 10.1210/endo-126-1-3. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Srikanta S., Ganda O. P., Eisenbarth G. S., Soeldner J. S. Islet-cell antibodies and beta-cell function in monozygotic triplets and twins initially discordant for Type I diabetes mellitus. N Engl J Med. 1983 Feb 10;308(6):322–325. doi: 10.1056/NEJM198302103080607. [DOI] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Tominaga M., Komiya I., Johnson J. H., Inman L., Alam T., Moltz J., Crider B., Stefan Y., Baetens D., McCorkle K. Loss of insulin response to glucose but not arginine during the development of autoimmune diabetes in BB/W rats: relationships to islet volume and glucose transport rate. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9749–9753. doi: 10.1073/pnas.83.24.9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler T. J., Simpson I. A., Sogin D. C., Hinkle P. C., Cushman S. W. Detection of the rat adipose cell glucose transporter with antibody against the human red cell glucose transporter. Biochem Biophys Res Commun. 1982 Mar 15;105(1):89–95. doi: 10.1016/s0006-291x(82)80014-4. [DOI] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]