Abstract
Summary
Background
The Mirasol® pathogen reduction technology (PRT) for platelet concentrates (PC) uses riboflavin and UV light (270-360 nm). We evaluated the impact of PRT on platelets in comparison to standard single-donor PC.
Material and Methods
Platelets were resuspended in autologous plasma. After 2 h rest without agitation, PC were split into an untreated control unit (C-PC) and an immediately treated unit (T-PC) (series I). In series IV, split PC were stored under agitation over night before PRT was carried out. Platelet quality was assessed by pH, glucose consumption, lactate production rate, LDH, soluble sCD62p and CD62p expression with and without TRAP (thrombin receptor-activating peptide) over 7 days.
Results
Series I: On day 5, pH values were lower for T-PC (6.8 ± 0.2 vs. 7.4 ± 0.1, C-PC), accompanied by a higher glucose consumption rate of 0.069 ± 0.016 vs. 0.035 ± 0.006 mmol/1012 platelets/h and lactate production rate of 0.126 ± 0.031 vs. 0.063 ± 0.011 mmol/1012 platelets/h. CD62p using TRAP was lower for T-PC (50 ± 11 vs. 62 ± 14%). Baseline activation was higher in T-PC (35 ± 12 vs. 28 ± 15%). Longer initial rest time had no impact on these results (series II/III/IV).
Conclusion
PRT leads to an increase of platelet metabolism and activation independent of the length of the initial rest times. PC resuspended in autologous plasma should be stored at maximum up to day 5.
Key Words: Pathogen inactivation, Pathogen reduction, Platelet concentrates, Mirasol®, Riboflavin
Abstract
Zusammenfassung
Hintergrund
Das Mirasol®-Verfahren (PRT) unter Verwendung von Riboflavin und UV-Licht (270-360 nm) wurde für die Pathogeninaktivierung von Thrombozy-tenkonzentraten entwickelt. Wir haben den Einfluss des Verfahrens auf die Thrombozytenqualität im Vergleich zu Standard-Apherese-Thrombozytenkonzentraten untersucht.
Material und Methoden
Thrombozyten wurden in autologem Plasma resuspendiert. Nach zweistündiger Lagerung ohne Agitation wurde das Thrombozytenkonzentrat in Serie I in ein unbehandeltes Kontrollprodukt (C-PC) und ein unmittelbar behandeltes Testprodukt (T-PC) aufgeteilt. In Serie IV wurden die geteilten Produkte vor Behandlung über Nacht unter Agitation gelagert. pH-Wert, Glukoseverbrauch, Laktatproduktion, LDH, lösliches sCD62p sowie die CD62p-Expression mit und ohne TRAP (Thrombinrezeptor aktivierende Peptide) wurden über 7 Tage Lagerung untersucht.
Ergebnisse
Serie I: PRT führt zu einem pH-Abfall auf 6,8 ± 0,2 vs. 7,4 ± 0,1 (C-PC) an Tag 5. Parallel dazu kam es zu einem erhöhten Glukoseverbrauch von 0,069 ± 0,016 vs. 0,035 ± 0,006 mmol/1012 Thrombozyten/h sowie einer erhöhten Laktatproduktion von 0,126 ± 0,031 vs. 0,063 ± 0,011 mmol/1012 Thrombozyten/h (C-PC). Die maximale Thrombozytenak-tivierbarkeit unter Verwendung von TRAP lag für T-PC niedriger (50 ± 11 vs. 62 ± 14%). Die Basisaktivierung bei diesen Präparaten war höher (35 ± 12 vs. 28 ± 15%). Eine verlängerte initiale Lagerungszeit hat keinen Einfluss auf die Ergebnisse (Serie II/III/IV).
Schlussfolgerung
PRT führt zu gesteigertem Thrombozytenmetabolismus und Zellaktivierung unabhängig von der initialen Lagerungszeit. Thrombozyten, resuspendiert in autologem Plasma, können maximal bis Tag 5 gelagert werden.
Introduction
To prevent transfusion-transmitted infections after transfusion of platelet concentrates (PC), different pathogen reduction strategies have been pursued: the photochemical inactivation procedure using amotosalen in combination with UVA light and the pathogen reduction technology (PRT) utilizing riboflavin (RB) in combination with UV light.
Upon application of UV light and absorption of low wavelength photons, the PRT treatment results in nucleic acid alterations directly. Moreover, UV light in the presence of RB causes the formation of 8-oxodGuo in nucleic acids. Formation of 8-oxodGuo may be mediated by the direct interaction of photoexcited RB with DNA, resulting in one-electron oxidation of the guanine moieties. RB enhances the rate of DNA modification and reduces the likelihood of DNA repair and reactivation of treated pathogens and white blood cells [1,2,3]. It could be demonstrated that the replication of different viruses, bacteria species [4], parasites [5], and leucocytes [6] is inhibited after PRT.
Considering the implementation of this technique into routine use, the question arises whether this pathogen reduction method has an influence on the quality and storability of the platelets. We collected single-donor platelets resuspended in autologous plasma and investigated the influence of PRT procedure on the in vitro quality of platelets in comparison to single-donor standard PC. We focused on platelet metabolism and activation over a storage period of 7 days, assessing different initial resting times before PRT treatment.
Material and Methods
In accordance with the German regulations for blood donation, single-donor PC were collected from regular blood donors using the Amicus™ cell separator (Fenwal Europe, Mont Saint Guibert, Belgium). Each donor gave informed consent to participate in the study. A double dose of 6.0 × 1011 (1,180-2,100 × 106 cells/ml) platelets was harvested and resuspended in 450 ml autologous plasma using ACD-A as the anticoagulant.
-
–
Series I: PC double dose units (n = 14) were stored for 2 h without agitation. Afterwards, each unit was split into 2 identical PC subunits of approximately 225 ml, followed immediately by PRT.
-
–
Series II/III: PC double dose units (n = 6 each) split into 2 subunits after the initial storage of 2 h without agitation and stored for 4 h (series II) and for 6 h (series III) under agitation before PRT was carried out.
-
–
Series IV: PC double dose units (n = 6) were split into 2 subunits after the initial storage of 2 h without agitation and stored over night with agitation. PRT was carried out the next day within 24 h of collection.
Test Platelet Units – Pathogen Reduction Technology
The test subunit (T-PC) was transferred to an illumination/storage bag (CaridianBCT, Lakewood, CO, USA). RB is packed in a disposable poly-olefin bag to maintain appropriate solution stability through the sterilisation process and prior to use. It is wrapped in a foil pouch protecting the vitamin from ambient light. RB is connected to the PC using a sterile docking device. The mixture of PC and RB (35 ± 5 ml, 500 μml/l RB in a 0.9% saline chloride, pH 4.0-5.0) leads to a final RB concentration of approximately 65-70 μmol/l. The Mirasol process has been validated for RB concentrations up to 160 μmol/l. For illumination, the PC was placed in a fix position in the illumination device and exposure to light took place (6.2 J/ml of UV light, 265-370 nm). During this process, the device agitated the PC and controlled the temperature of the product and the UV lamps' outputs. Depending on the volume of the PC, the illumination time was about 6-10 min.
As further bag transfer or adsorption of the resulting photoproducts is not required, the PC were stored on a platelet incubator under agitation (22 ± 2 °C) over a period of 7 days.
Control Platelet Units
The control subunit (C-PC) was transferred into the same storage bag as the T-PC (CaridianBCT). Autologous plasma (35 ml) was added to the corresponding T-PC at the same time as RB. Thus, we were able to directly compare both products due to identical storage container characteristics and volume. The C-PC was also stored in a platelet incubator under agitation for 7 days.
Laboratory Parameters
Using a sterile transfer tubing set connected to the storage bag, samples were withdrawn under laminar flow using a syringe. In series I/II/III, investigations for C-PC and T-PC were carried out on day 0 after addition of RB or plasma, and before PRT treatment as well as on days 1, 3, 5, and in the morning of day 8 with respect to a potential storage up to day 7. In series IV, sampling was carried out on day 1 after addition of RB or plasma, and before PRT, and on days 2, 4, 6, and 8.
The samples were assayed for platelet count, red blood cell (RBC) and white blood cell (WBC) counts (only series I), blood gas analysis, glucose consumption and lactate production rate, lactate dehydrogenase (LDH), soluble p-selectin (sCD62p) as well as surface-expressed p-selectin (CD62p) with and without activation using TRAP (thrombin receptor-activating peptide) and swirling.
Blood Gas Analysis and Cell Count
Samples were immediately analysed for pH (22 °C), HCO3-, pO2, and pCO2 with a blood gas analyser (Synthesis 10; Instrumentation Laboratory, Kirchheim, Germany). Platelet count was analysed automatically (CELL-DYN 3200; Abbott, Wiesbaden, Germany). RBC and WBC were analysed using FACS (Coulter Epics XL; MCL Beckmann Coulter, Krefeld, Germany).
Platelet Activation Markers
For estimation of baseline activation, CD62p expression (GMP140, p-selectin) was determined by flow cytometry using 2-color labelling with monoclonal antibodies. PC samples were adjusted to 25,000 platelets/μl with PBS. A 30 μl diluted PC sample was incubated with 10 μl of anti-CD41-PE and 20 μl of anti-CD62p-FITC for 5 min in the dark at room temperature. The reaction was stopped by adding 1 ml of PBS (pH 7.4, 4 °C). For the negative control, 20 μl of anti-IgG1-FITC was used instead of the anti-CD62p-FITC. 20,000 events were counted by the flow cytometre (FACSCalibur; Becton Dickinson, Heidelberg, Germany) and results were reported in percent positive within a range defined by the negative control. In a second PC sample, CD62p expression was measured after activation by 100 μmol/l TRAP. This analysis gives information on the maximal activation capacity of the platelets.
Levels of p-selectin in plasma (sCD62p) were quantified by an ELISA (Human sl-selectin; R & D System GmbH, Wiesbaden-Nordenstadt, Germany).
Evaluating cell dilatation or cell lysis due to platelet activation, LDH was quantified by automated methods (Dimension RxL; Siemens Medical System, Dade Behring, Eschborn, Germany) according to the manufacturer's recommendations.
Biochemical Studies
In the supernatant of the PC, glucose and lactate concentration were measured by automated methods (Dimension RxL) according to the manufacturer's recommendations.
Statistics
All data are expressed as mean ± 1 standard deviation (SD). The Wilcoxon paired t-test was used for comparison of test versus control units. To compare the data within 1 group over the storage period, the Friedman test was used.
Statistically significant differences were based on p-values < 0.05. Statistical analyses were performed with commercially available software for personal computers (SPSS for Windows XP; SPSS Software GmbH, Munich, Germany).
Results
Volume, Platelet Content, Red Blood Cells, White Blood Cells, Blood Gas Analysis
-
–
Series I: On average, PC were resuspended in a collection volume of 455 ± 11 ml. After 2 h of rest without agitation, PC were split into 2 subunits with a volume of 223 ± 4 ml for T-PC and 223 ± 6 ml for C-PC. Due to sampling, the volume dropped during storage in both series.
On average, 5.8 ± 0.7 × 1011 platelets were collected. After splitting, T-PC contained 2.8 ± 0.3 × 1011 and C-PC 2.9 ± 0.2 × 1011 platelets per unit. Due to sampling, the content dropped until the end of the storage period (table 1).
WBC and RBC values were measured immediately afterwards. Results were comparable between T-PC and C-PC, with WBC counts of 0.05 ± 0.001 × 106 per unit and RBC counts of 0.28 ± 0.003 × 109 per unit.
The pH dropped significantly for T-PC from day 1 on. On day 5, T-PC showed values of 6.8 ± 0.2 in comparison to C-PC with values of 7.4 ± 0.1 (p = 0.001) (table 1).
This was paralleled by a drop of the HCO3- values from 17.3 ± 1.5 mmol/l (day 0) to 3.8 ± 1.3 mmol/l (day 5) to 0.7 ± 0.1 mmol/l (day 8) for the T-PC and from 22.4 ± 1.3 mmol/l (day 0) to 11.4 ± 1.5 mmol/l (day 5) to 6.7 ± 2.0 mmol/l (day 8) for the C-PC. Values of T-PC were overall significantly lower. But as the course of HCO3− was paralleled in both groups from day 0 to day 8, the differences resulted due to the addition of the 35 ml of plasma to the C-PC.
-
–
Series IV: On average, PC were resuspended in a collection volume of 428 ± 10 ml. After splitting, C-PC and T-PC volume were about 216 ± 4 ml. On average, 5.8 ± 0.4 × 1011 platelets were collected. After splitting, T-PC contained 2.8 ± 0.1 × 1011 and C-PC 2.7 ± 0.2 × 1011 platelets (table 2). The pH dropped significantly for T-PC from day 2 on. On day 6, T-PC showed values of 6.8 ± 0.2 in comparison to C-PC with values of 7.3 ± 0.1 (p = 0.018) (table 2).
This was paralleled by a drop of the HCO3- values from 16.8 ± 2.0 mmol/l (day 1) to 4.8 ± 1.2 mmol/l (day 6) to 1.1 ± 0.1 mmol/l (day 8) for the T-PC and from 17.1 ± 1.8 mmol/l (day 1) to 11.0 ± 1.3 mmol/l (day 6) to 6.9 ± 1.6 mmol/l (day 8) for the C-PC. But as the course of HCO3- was paralleled in both groups from day 0 to day 8, the differences resulted due to the addition of the 35 ml of plasma to the C-PC.
Table 1.
Series I (n = 14): preparation data
| Original bag | After addition of RB before illumination | Day 1 | Day 3 | Day 5 | Day 8 | |
|---|---|---|---|---|---|---|
| Platelet yield, × 1011 | ||||||
| T-PC | 5.8 ± 0.7 | 2.8 ± 0.3* | 2.4 ± 0.3* | 2.3 ± 0.2* | 2.2 ± 0.3* | 2.1 ± 0.3* |
| C-PC | 5.8 ± 0.7 | 2.9 ± 0.2 | 2.7 ± 0.2 | 2.5 ± 0.2 | 2.4 ± 0.2 | 2.4 ± 0.2 |
| Platelet content, × 103/μl | ||||||
| T-PC | 1,277 ± 159 | 1,099 ± 119* | 1,048 ± 117* | 1,064 ± 108* | 1,101 ± 124 | 1,125 ± 143 |
| C-PC | 1,277 ± 159 | 1,147 ± 70 | 1,148 ± 89 | 1,133 ± 68 | 1,153 ± 93 | 1,201 ± 107 |
| pH, 22 °C | ||||||
| T-PC | 7.4 ± 0.1 | 7.4 ± 0.1* | 7.3 ± 0.1* | 7.1 ± 0.1* | 6.8 ± 0.2* | 6.3 ± 0.0* |
| C-PC | 7.4 ± 0.1 | 7.2 ± 0.3 | 7.4 ± 0.0 | 7.4 ± 0.1 | 7.4 ± 0.1 | 7.2 ± 0.2 |
p < 0.05; T-PC (PRT-treated unit) versus C-PC (untreated control unit).
Table 2.
Series IV (n = 6): preparation data
| Original bag | After addition of RB before illumination day 1 | Day 2 | Day 4 | Day 6 | Day 8 | |
|---|---|---|---|---|---|---|
| Platelet yield, × 1011 | ||||||
| T-PC | 5.8 ± 0.4 | 2.8 ± 0.1 | 2.5 ± 0.1 | 2.4 ± 0.1 | 2.4 ± 0.1 | 2.2 ± 0.1 |
| C-PC | 5.8 ± 0.4 | 2.7 ± 0.2 | 2.6 ± 0.2 | 2.4 ± 0.2 | 2.4 ± 0.3 | 2.3 ± 0.2 |
| Platelet content, × 103/μl | ||||||
| T-PC | 1,364 ± 75 | 1,164 ± 48 | 1,048 ± 50 | 1,038 ± 48 | 1,046 ± 52 | 1,009 ± 67 |
| C-PC | 1,364 ± 75 | 1,094 ± 98 | 1,066 ± 97 | 1,029 ± 88 | 1,031 ± 114 | 1,008 ± 98 |
| pH, 22 °C | ||||||
| T-PC | 7.2 ± 0.1 | 7.5 ± 0.1 | 7.3 ± 0.4* | 7.1 ± 0.1* | 6.8 ± 0.2* | 6.3 ± 0.0* |
| C-PC | 7.3 ± 0.1 | 7.5 ± 0.2 | 7.6 ± 0.1 | 7.5 ± 0.1 | 7.3 ± 0.1 | 7.2 ± 0.1 |
p < 0.05; T-PC (PRT-treated unit) versus C-PC (untreated control unit).
Biochemical Measurements
Glucose and lactate were calculated in mmol/l per 1012 platelets. The rate of glucose consumption and lactate production was calculated as mmol/1012 platelets per hour.
-
–
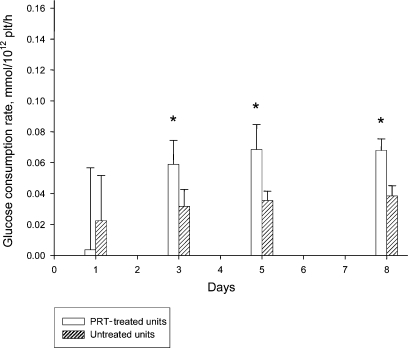
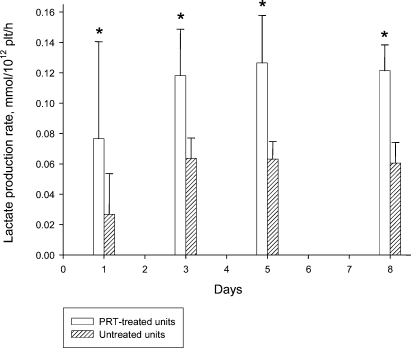
Series I: Glucose concentration per 1012 cells was generally higher in C-PC due to the addition of autologous plasma (table 3). Nevertheless, values indicate a higher consumption in T-PC. Lactate results per 1012 cells were generally lower in C-PC (table 3). The accumulation of lactate over time was higher in T-PC, indicating a higher production. This was confirmed by calculating the glucose consumption rate (fig. 1) and the lactate production rate (fig. 2) which were significantly higher for T-PC throughout the storage period.
-
–
Series IV: Glucose concentration per 1012 cells was generally higher in C-PC (table 4) due to the addition of autologous plasma. Values indicate again a higher consumption of glucose in T-PC. With the exception of day 2, the glucose consumption rate was significantly higher for T-PC compared to C-PC. Values ranged from 0.07 ± 0.031 versus 0.040 ± 0.028 mmol/1012 cells/h (day 2, p = 0.063), 0.064 ± 0.033 versus 0.036 ± 0.027 mmol/1012 cells/h (day 4, p = 0.018), 0.067 ± 0.011 versus 0.033 ± 0.012 mmol/1012 cells/h (day 6, p = 0.018) to 0.069 ± 0.009 versus 0.036 ± 0.010 mmol/1012 cells/h (day 8, p = 0.018). Comparable to series I, lactate results per 1012 cells were generally lower in C-PC (table 4). Lactate production was higher in T-PC, and the lactate production rate was significantly higher for T-PC throughout storage. Values (T-PC vs. C-PC) ranged from 0.142 ± 0.027 versus 0.063 ± 0.020 mmol/1012 cells/h (day 2, p = 0.018), 0.156 ± 0.054 versus 0.090 ± 0.033 mmol/1012 cells/h (day 4, p = 0.018), 0.146 ± 0.012 versus 0.083 ± 0.013 mmol/1012 cells/h (day 6, p = 0.018) to 0.150 ± 0.011 versus 0.080 ± 0.011 mmol/1012 cells/h (day 8, p = 0.018).
Table 3.
Series I (n = 14): laboratory findings
| After addition of RB before illumination | Day 1 | Day 3 | Day 5 | Day 8 | |
|---|---|---|---|---|---|
| CD62p, % | |||||
| T-PC | 24.7 ± 8.2* | 22.8 ± 6.2* | 30.2 ± 6.0* | 35.2 ± 11.5* | 41.7 ± 11.6* |
| C-PC | 15.8 ± 5.2 | 16.2 ± 5.6 | 25.7 ± 8.3 | 28.5 ± 15.4 | 24.6 ± 9.7 |
| CD62p + TRAP, % | |||||
| T-PC | 65.8 ± 8.9 | 53.3 ± 13.3* | 50.3 ± 10.6* | 49.8 ± 10.8* | 41.6 ± 10.8 |
| C-PC | 61.5 ± 8.7 | 63.8 ± 12.7 | 61.8 ± 13.0 | 62.5 ± 13.8 | 47.6 ± 13.3 |
| LDH, IU/l | |||||
| T-PC | 179 ± 80 | 133 ± 46* | 147 ± 48* | 167 ± 51* | 200 ± 47 |
| C-PC | 154 ± 57 | 159 ± 56 | 169 ± 57 | 179 ± 57 | 188 ± 57 |
| Glucose, mmol/1012 cells | |||||
| T-PC | 12.7 ± 0.7* | 12.5 ± 1.3* | 8.8 ± 0.8* | 5.0 ± 1.4* | 0.4 ± 0.6* |
| C-PC | 14.5 ± 0.7 | 14.0 ± 1.1 | 12.3 ± 0.8 | 10.3 ± 1.0 | 7.2 ± 1.3 |
| Lactate, mmol/1012 cells | |||||
| T-PC | 4.1 ± 1.4* | 6.0 ± 1.3* | 12.3 ± 2.5* | 18.9 ± 4.2* | 26.6 ± 3.8* |
| C-PC | 3.5 ± 1.1 | 4.0 ± 1.0 | 7.7 ± 1.0 | 10.6 ± 1.6 | 14.5 ± 3.1 |
p < 0.05; T-PC (PRT-treated unit) versus C-PC (untreated control unit).
Fig. 1.
Series I (n = 14): glucose consumption rate. *p < 0.05 PRT-treated units versus untreated units.
Fig. 2.
Series I (n = 14): lactate production rate. *p < 0.05 PRT-treated units versus untreated units.
Table 4.
Series IV (n = 6): laboratory findings
| After addition of RB before illumination day 1 | Day 2 | Day 4 | Day 6 | Day 8 | |
|---|---|---|---|---|---|
| CD62p, % | |||||
| T-PC | 26.4 ± 16.7 | 27.5 ± 6.2* | 45.9 ± 9.8* | 45.4 ± 10.5 | 61.9 ± 9.6* |
| C-PC | 19.5 ± 8.6 | 21.4 ± 6.7 | 24.2 ± 7.0 | 36.9 ± 16.8 | 26.8 ± 3.9 |
| CD62p + TRAP, % | |||||
| T-PC | 81.9 ± 7.3 | 82.0 ± 6.0* | 72.8 ± 13.4* | 61.1 ± 10.2* | 66.1 ± 7.2 |
| C-PC | 81.1 ± 5.7 | 85.2 ± 4.7 | 80.9 ± 7.6 | 71.1 ± 10.8 | 71.1 ± 3.9 |
| LDH, IU/l | |||||
| T-PC | 136 ± 28 | 132 ± 29* | 144 ± 29 | 153 ± 36* | 164 ± 39 |
| C-PC | 151 ± 26 | 150 ± 26 | 159 ± 25 | 166 ± 28 | 164 ± 33 |
| Glucose, mmol/1012 cells | |||||
| T-PC | 11.9 ± 0.8* | 11.7 ± 1.0* | 7.5 ± 1.7* | 4.4 ± 1.0* | 0.7 ± 1.1* |
| C-PC | 15.2 ± 1.8 | 14.3 ± 1.8 | 12.6 ± 1.1 | 11.2 ± 1.7 | 9.0 ± 1.6 |
| Lactate, mmol/1012 cells | |||||
| T-PC | 4.5 ± 0.8 | 7.9 ± 1.0* | 15.6 ± 3.7* | 21.8 ± 1.7* | 30.0 ± 2.3* |
| C-PC | 4.3 ± 0.6 | 5.8 ± 0.9 | 10.6 ± 2.4 | 13.9 ± 2.0 | 17.5 ± 2.4 |
p < 0.05; T-PC (PRT-treated unit) versus C-PC (untreated control unit).
Platelet Activation
-
–
Series I: We investigated the baseline expression of the platelet surface marker CD62p and the platelet maximal activation capacity using TRAP. The results of the baseline expression were significantly higher for T-PC throughout the entire storage period (table 3). On day 0 before PRT, both T-PC and C-PC showed the same maximal CD62p expression. From day 1 on, the activation capacity was significantly reduced for T-PC. In the morning of day 8, the C-PC as well as the T-PC showed again comparable expression levels (table 3). The difference between baseline and TRAP-induced CD62p expression was significantly higher in C-PC on day 5 than in T-PC.
Soluble sCD62p was significantly different only in units after RB addition and before UV illumination compared to units after plasma addition (127 ± 45 vs. 102 ± 52 ng/ml, p = 0.034). Throughout storage, values increased in T-PC and C-PC and reached levels of 135 ± 42 versus 152 ± 56 ng/ml on day 5 (p = 0.041) and 155 ± 39 versus 162 ± 62 ng/ml (p = 0.638) on day 8.
LDH release from the cytoplasma is related to shear stress. In series I, C-PC showed higher LDH levels until day 5 of storage, but not on day 8 of storage (table 3).
-
–
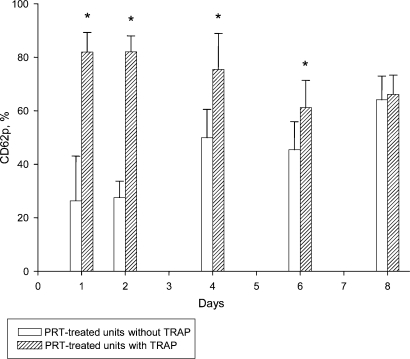
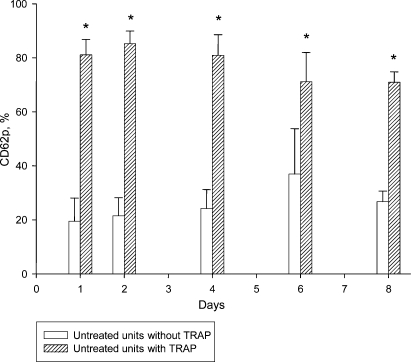
Series IV: CD62p baseline expression levels were significantly higher in the T-PC throughout the entire storage period (table 4). CD62p expression after TRAP activation was identical in T-PC and C-PC on day 1. After PRT treatment, the activation capacity was significantly reduced in T-PC up to day 6. In the morning of day 8, the C-PC and T-PC showed again comparable levels (table 4). Comparable to series I, baseline versus maximal expression demonstrate that T-PC at baseline (61.9 ± 9.6%) and maximal activation (66.1 ± 7.2%) on day 8 is equal (fig. 3) while the C-PC still show a significant difference between baseline (26.8 ± 3.9%) and TRAP-induced CD62p expression (71.1 ± 3.9%) (fig. 4).
No differences in sCD62p levels could be found for T-PC and C-PC throughout storage. Values increased for T-PC from 105 ± 23 (day 1) to 182 ± 28 ng/ml (day 8) and for C-PC from 107 ± 31 (day 1) to 189 ± 44 ng/ml (day 8).
LDH levels were higher in C-PC until day 5 of storage, but not on day 8 of storage (table 4).
Fig. 3.
Series IV (n = 6): PRT-treated units; CD62p expression (%) with and without TRAP. *p < 0.05.
Fig. 4.
Series IV (n = 6): untreated units; CD62p expression (%) with and without TRAP. *p < 0.05.
Discussion
The PRT uses RB and UV light. The aim of the study was to evaluate the practicality of this technique and its impact on in vitro platelet quality.
The handling procedure can be easily integrated into PC manufacturing and takes on average less than half an hour until the PC is ready for transfusion. Platelets collected by the Amicus platform have to be transferred to an illumination/storage bag (CaridianBCT). After RB addition and UV illumination, no further platelet manipulation and transfer is necessary. The platelet unit can be directly transfused.
Several publications described that platelet exposure to mechanical or chemical influences results in changes, which is collectively referred to as platelet storage lesion (PSL) [7,8,9]. The question arises whether PRT treatment might also lead to PSL, and if it does, to what extent.
Our results demonstrate platelet alteration due to this treatment by means of higher glucose consumption and lactate production rates indicating accelerated glycolysis. We could confirm findings from Ruane et al. [4] and AuBuchon et al. [10] who described an increased metabolic activity as a result of PRT, though in both studies the glucose consumption and lactate production rates were lower. Beyond day 5 of storage, we could not detect any remaining glucose. This is in contrast to results from Li et al. [11] who detected glucose levels of 8 ± 2 on day 4 or day 5, and 6 ± 2 mmol/l 1012 cells on day 6 or 7.
The accumulation of the lactic acid resulted in a decline of pH to levels of 6.7 (day 5) after PRT. This value was lower in comparison to 7.4 (day 5) observed in other studies [4, 10, 11], although the same storage bag was used and platelets were stored in plasma in all studies. Due to increase protons, our data showed reduced HCO3− values in PRT-treated PC up to day 5 (3.8 mmol/l), indicating limited buffer capacity.
Moreover, we could demonstrate that PRT leads to increased activation of platelets. In contrast to other studies, data from study I (n = 14) showed that the addition of RB alone activated platelets by increasing baseline CD62p expression. Values obtained from other studies (n = 18) after initial prolonged resting time (study II/II/IV) showed non-significant increases in p-selectin expression in T-PC compared to C-PC. In addition, we found higher baseline expression of CD62p throughout storage, which is comparable to published results on PRT-treated platelets [4, 10-12]. Values observed in these studies range from 35% (day 1) up to 60% (day 5), which is comparable to our values of 23% (day 1) to 35% (day 5) in series I and of 28% (day 1) to 45% (day 6) in series IV.
Furthermore, we found a decline of the activation capacity in response to agonist stimulation using TRAP. It has been described that reduced responsiveness diminishes adhesion, secretion, and aggregation in vivo [13, 14].
The correlation between the extent of platelet activation in vitro and in vivo parameters is discussed controversially [15,16,17,18]. Some authors found a positive correlation between increased activated platelets in vitro and the shortness of survival in vivo [15, 16]. Others described little evidence that the degree of platelet activation impairs the ability of transfused platelets to produce acceptable post-transfusion recovery or decreased bleeding [17, 18]. Michelson et al. [17] demonstrated in baboons that surface-expressed CD62p is rapidly cleaved from the platelet membrane and released into the plasma pool in transfused, activated, degranulated platelets. The activated platelets continue to circulate and function without affecting the life span in comparison to unactivated platelets. This could also be confirmed by Berger et al. [18] who carried out experiments with wild-type and p-selectin knockout mice. In contrast, it has been published that enhanced expression of CD62p leads to platelet recognition by macrophages of the reticuloendothelial system. This resulted in an increased formation of platelet-leucocyte aggregation through the PSGL-1 receptor and subsequent clearance from the circulation [19,20,21]. Some investigators have suggested that soluble sCD62p is a more sensitive marker for platelet activation than CD62p surface expression [22]. We could not detect any difference in sCD62p levels due to PRT, but we found increased baseline levels and decreased activation capacity that was more prominent after PRT treatment.
To evaluate PSL by means of platelet cell integrity loss, LDH, which can be found in the cytoplasma of platelets, was investigated. Values of treated and untreated PC were comparable and in range with published data from Klinger et al. [23]. Therefore, we conclude that no increased lysis of platelets due to PRT occurs.
The collection technique itself might have an impact on the extent and progression of PSL. We therefore extended the resting time of the PC on agitation before PCT was carried out. Nevertheless, findings on platelet metabolism and activation could not be improved. We conclude that the reason for platelet activation seen after PRT lies in the underlying mechanism of action.
In summary, PRT leads to increased platelet metabolism and activation resulting from the underlying mechanism of action and can not be improved by longer rest times of the platelets before treatment. Due to low pH values, shelf-life of PRT-treated platelets stored in plasma is limited to 5 days, which is in line with the manufacturer's recommendations and national guidelines in most European countries [24, 25]. Furthermore, it needs to be investigated whether other platelet preparation methods may improve platelet quality. A first clinical evaluation of PRT-treated platelets in thrombocytopenic patients showed that patients receiving treated platelets had comparable haemostasis and support requirements. A significant difference in corrected count increment (CCI) 1 h was observed, but the CCI 24 h, number of platelet transfusions, total platelet dose, and number of RBC transfusions per subject were not significantly different [26].
Disclosure
The authors declared no conflict of interest.
References
- 1.Kumar V, Lockerbie O, Keil SD, Ruane PH, Platz MS, Marin CB, Ravanat JL, Cadet J, Goodrich RP. Riboflavin and UV-light based pathogen reduction: extent and consequence of DNA damage at the molecular level. Photochem Photobiol. 2004;80:15–21. doi: 10.1562/2003-12-23-RA-036.1. [DOI] [PubMed] [Google Scholar]
- 2.Tsugita A, Okada Y, Uehara K. Photosensitized inactivation of nucleic acids in the presence of riboflavin. Biochem Biophys Acta. 1965;103:360–363. doi: 10.1016/0005-2787(65)90182-6. [DOI] [PubMed] [Google Scholar]
- 3.Cadet J, Decarroz C, Wang SY, Midden WR. Mechanisms and products of photosensitized degradation of nucleic acids and related models compounds. Israel J Chem. 1983;23:420–429. [Google Scholar]
- 4.Ruane PH, Edrich R, Gampp D, Keil SD, Leonard L, Goodrich RP. Photochemical inactivation of selected viruses and bacteria in platelet concentrates using riboflavin and light. Transfusion. 2004;44:877–885. doi: 10.1111/j.1537-2995.2004.03355.x. [DOI] [PubMed] [Google Scholar]
- 5.Cardo LJ, Rentas FJ, Ketchum L, Salata J, Har-man R, Melvin W, Weina PJ, Mendes J, Reddy H, Goodrich RP. Pathogen inactivation of Leishmania donovani infantum in plasma and platelet concentrates using riboflavin and ultraviolet light. Vox Sang. 2006;90:85–91. doi: 10.1111/j.1423-0410.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- 6.Fast LD, DiLeone G, Li J, Goodrich RP. Functional inactivation of white blood cells by Mirasol treatment. Transfusion. 2006;46:642–648. doi: 10.1111/j.1537-2995.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- 7.Maurer-Spurej E, Chiperfield K. Past and future approaches to assess the quality of platelets for transfusion. Transfus Med Rev. 2007;21:295–306. doi: 10.1016/j.tmrv.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Klinger MHF. The storage lesion of platelets: ultrastructural and functional aspects. Ann Hematol. 1996;73:103–112. doi: 10.1007/s002770050210. [DOI] [PubMed] [Google Scholar]
- 9.Seghatchian J, Krailadsiri P. The platelet storage lesion. Transfus Med Rev. 1997;11:130–144. doi: 10.1053/tm.1997.0110130. [DOI] [PubMed] [Google Scholar]
- 10.AuBuchon JP, Herschel L, Roger J, Taylor H, Whitley P, Li J, Edrich R, Goodrich RP. Efficacy of apheresis platelets treated with riboflavin and ultraviolet light for pathogen reduction. Transfusion. 2005;45:1335–1341. doi: 10.1111/j.1537-2995.2005.00202.x. [DOI] [PubMed] [Google Scholar]
- 11.Li J, de Korte D, Woolum MD, Ruane PH, Keil SD, Lockerbie O, McLean R, Goodrich RP. Pathogen reduction of buffy coat platelet concentrates using riboflavin and light: comparisons with pathogen reduction technology-treated apheresis platelet products. Vox Sang. 2004;87:82–90. doi: 10.1111/j.1423-0410.2004.00548.x. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Pujol S, Tonda R, Lozano M, Fuste B, Lopez-Vilchez I, Galan AM, Li J, Goodrich RP, Escolar G. Effects of a new pathogen-reduction technology (Mirasol PRT) on functional aspects of platelet concentrates. Transfusion. 2005;45:911–919. doi: 10.1111/j.1537-2995.2005.04350.x. [DOI] [PubMed] [Google Scholar]
- 13.Curvers J, van Pampus EC, Feijge MA, RomboutSestrienkova E, Giesen PL, Heemskerk JW. Decreased responsiveness and development of activation markers of PLTs stored in plasma. Transfusion. 2004;44:49–58. doi: 10.1111/j.0041-1132.2004.00628.x. [DOI] [PubMed] [Google Scholar]
- 14.Cauwenberghes S, van Pampus E, Curvers J, Akkerman JWN, Heemskerk JWM. Hemostatic and signalling functions of transfused platelets. Transfus Med Rev. 2007;21:287–294. doi: 10.1016/j.tmrv.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Rinder HM, Murphy M, Mitchell JG, Stocks J, Ault KA, Hillman RS. Progressive platelet activation with storage: evidence for shortened survival of activated platelets after transfusion. Transfusion. 1991;31:409–414. doi: 10.1046/j.1537-2995.1991.31591263195.x. [DOI] [PubMed] [Google Scholar]
- 16.Holme S, Sweeney JD, Sawyer S, Elfath MD. The expression of p-selectin during collection, processing and storage of platelet concentrates: relationship to loss of in vivo viability. Transfusion. 1997;37:12–17. doi: 10.1046/j.1537-2995.1997.37197176945.x. [DOI] [PubMed] [Google Scholar]
- 17.Michelson AD, Barnard MR, Hechtman HB, MacGregor H, Connolly RJ, Loscalzo J, Valeri CR. In vivo tracking of platelets: circulating degranulated platelets rapidly lose surfact p-selctin but continue to circulate and function. Proc Natl Acad Sci U S A. 1996;93:11877–11882. doi: 10.1073/pnas.93.21.11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger G, Hartwell DW, Wagner DD. P-selectin and platelet clearance. Blood. 1998;92:4446–4452. [PubMed] [Google Scholar]
- 19.Leytin V, Allen DJ, Gwozdz A, Garvey B, Freedman J. Role of platelet surface glycoprotein Ib alpha and p-selectin in the clearance of transfused platelet concentrates. Transfusion. 2004;44:1487–1495. doi: 10.1111/j.1537-2995.2004.04042.x. [DOI] [PubMed] [Google Scholar]
- 20.Larson E, Celi A, Gilbert GE, Furie BC, Erban JK, Bonfanti R, Wagner DD, Furie B. PADGEM protein: a receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 1989;59:305–312. doi: 10.1016/0092-8674(89)90292-4. [DOI] [PubMed] [Google Scholar]
- 21.Mehta P, Patel KD, Laue TM, Erikson HP, McEver RP. Soluble monomeric p-selectin containing only the lectin and epidermal growth factor domains bind to p-selectin glycoprotein ligand-1 on leukocytes. Blood. 1997;90:2381–2389. [PubMed] [Google Scholar]
- 22.Stohlawetz P, Hergovich N, Stiegler G, Eichler HG, Höcker P, Kapiotis S, Jilma B. Differential induction of p-selectin expression on platelets by two cell separators during plateletpheresis and the effect of gender on the release of soluble p-selectin. Transfusion. 1998;38:24–30. doi: 10.1046/j.1537-2995.1998.38198141494.x. [DOI] [PubMed] [Google Scholar]
- 23.Klinger MHF, Josch M, Klüter H. Platelets stored in a glucose-free additive solution or in autologous plasma – an ultrastructural and morphometric evaluation. Vox Sang. 1996;71:13–20. doi: 10.1046/j.1423-0410.1996.7110013.x. [DOI] [PubMed] [Google Scholar]
- 24.Council of Europe . Guide to the preparation, use and quality assurance of blood components. ed 14. Strasbourg: Council of Europe Publishing; 2008. [Google Scholar]
- 25.Bundesärztekammer . Richtlinien zur Gewinnung von Blut und Blutbestandteilen und zur Anwendung von Blutprodukten (Hämotherapie). Gesamtnovelle 2005 mit Änderungen und Ergänzungen 2007. ln: Deutscher Ärzte-Verlag; 2007. [Google Scholar]
- 26.Goodrich RP, Folléa G, Robert T. Clinical evaluation of Mirasol PRT treated apheresis platelets in thrombocytopenic patients. Transfusion. 2008;48s:20A. [Google Scholar]