Abstract
Summary
Objective
In a significant proportion of patients with hematologic malignancies (5-30%) poor mobilization of hematopoietic stem cells (HSC) is observed. This compromises the application of effective and potentially curative high-dose chemotherapy (HDC) treatment.
Case Report
Here we report the case of a 38-year-old female patient who was treated for recurrent follicular B-cell non-Hodgkin's lymphoma grade III. In this patient, we failed twice to mobilize stem cells using chemotherapy followed by granulocyte-colony stimulating factor (G-CSF). Recently a new chemokine receptor CXCR4 antagonist, AMD3100 (plerixafor), was introduced which can be combined with G-CSF mobilization and has been reported to increase the number of harvested stem cells significantly. Using this protocol, we were able to harvest a HSC product. This product was transplanted 3 weeks after the harvest (after HDC), and the patient had an uncomplicated recovery of granulopoiesis (day 11 after transplantation of autologous HSC).
Conclusion
Plerixafor has the potency to become an important tool in mobilizing HSC, especially in those patients in whom HSC cannot be mobilized by the combination of G-CSF and chemotherapy alone.
Key Words: Hematopoietic stem cells, Peripheral blood progenitor cells, Stem cell collection, CD34, Plerixafor
Abstract
Zusammenfassung
Hintergrund
Bei einer ganzen Reihe von Patienten mit hämatologischen Erkrankungen (5–30%) wird eine schlechte Mobilisation von hämatopoetischen Stammzellen (HSC) beobachtet. Das beeinträchtigt den Einsatz von effektiven und möglicherweise kurativen HochdosisChemotherapie (HDC).
Fallbericht
Hier beschreiben wir den Fall einer 38 Jahre alten Frau, die wegen eines rekurrenten follikularen B-Zell-Non-Hodgkin-Lymphoms Grad III behandelt wurde und bei der es zweimal nicht gelang, Stammzellen nach Chemotherapie und Verabreichung von Granulozyten-Kolonie stimulierenden Faktor (G-CSF) zu mobilisieren. Vor kurzem wurden ein neuer Chemokinrezeptor-CXCR4-Antagonist, AMD3100 (Plerixafor) vorgestellt, der mit einer G-CSF-Mobilisation kombiniert werden kann und für den ein signifikanter Anstieg der gesammelten Stammzellen berichtet wurde. Durch Anwendung dieses Protokolls waren wir in der Lage, auch bei unserer Patientin Stammzellen zu sammeln. Diese Stammzellen wurden 3 Wochen nach der Apherese (nach HDC) transplantiert. Die Patientin zeigte eine unkomplizierten Erholung der Granulopoese (Tag 11 nach Transplantation der autologen HSC).
Schlussfolgerung
Plerixafor hat das Potential, ein wichtiges Element bei der Mobilisierung von HSC zu werden, besonders bei solchen Patienten, bei denen die HSC nicht durch die Kombination von Chemotherapie und G-CSF allein mobilisiert werden können.
Introduction
There is a significant proportion of patients with hematologic malignancies (5–30%) [1,2,3,4,5,6] in which poor mobilization of hematopoietic stem cells (HSC) is observed. This compromises the application of effective and potentially curative high-dose chemotherapy (HDC) treatment. Here we report a case of a 38-year-old female patient who was treated for recurrent follicular B-cell non-Hodgkin's lymphoma (B-NHL) grade III and in whom we attempted twice to mobilize stem cells unsuccessfully using chemotherapy followed by granulocyte-colony stimulating factor (G-CSF).
Case Report
A 38-year-old female patient was admitted to our hospital for treatment of a recurrent follicular B-NHL grade III. After diagnosis in October 2007 she received 6 chemotherapy cycles (6x R-CHOP21) and was in complete remission. In September 2008 she relapsed with axillar, mediastinal, ret-roperitoneal and inguinal lymphoma manifestations and 80% bone marrow infiltration (NHL grade III). During 2008 we attempted twice to harvest autologous HSC during the recovery phase after chemotherapy using G-CSF (Neupogen). Both attempts remained unsuccessful. She never reached levels of more than 2 CD34+ cells/μl (peripheral blood) during these attempts, and therefore she falls within the definition of a poor mo-bilizer. Because no other curative option besides HDC plus autologous HSC transplantation (HSCT) was within reach for this patient, we discussed the possibility of steady-state G-CSF mobilization in combination with plerixafor, and she agreed.
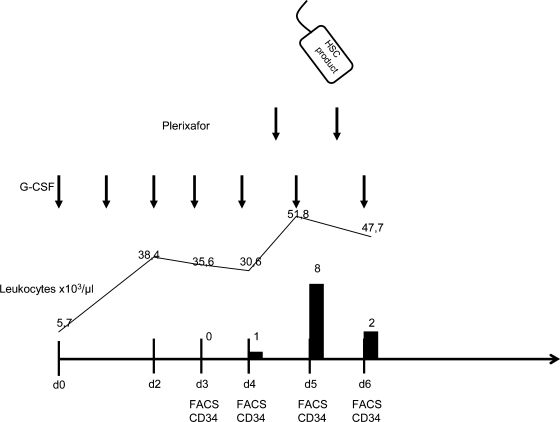
After a steady-state mobilization with G-CSF (10 μg/kg body weight (BW)), again no CD34+ HSC could be detected in her peripheral blood (day 4). On day 4, plerixafor (Genzyme, Boston, MA, USA) (240 μg/kg BW per day) was applied s.c., 10 h before the planned apheresis. Peripheral blood monitoring showed 8 CD34+ cells/μl (pre-apheresis, day 5). Large-volume apheresis (4 times patient's blood volume) using a Cobe Spectra,(Caridian, Lakewood, CO, USA) resulted in a HSC product of 1 × 106 CD34+ cells/kg BW. We planned to repeat the mobilization procedure including plerixafor the next day. However pre-apheresis monitoring showed only 2 CD34+ cells/μl in the peripheral blood and we decided to refrain from further apheresis. Plerixafor was well tolerated by this patient, we observed no adverse events or systemic allergic reactions. Figure 1 presents a schematic overview of the mobilization procedure, leukocyte counts, and CD34+ counts in the peripheral blood.
Fig. 1.
Schematic overview of the steady-state mobilization procedure with G-CSF and plerixafor (arrows) and the CD34+ counts in the peripheral blood (black bloxes). On day 5 a stem cell product was harvested indicated by the bag.
Functional assays (colony assays of thawed cryoconserved samples) showed 0.6 × 105 CFU/kg BW (goal >1 × 105 CFU/kg BW). Three weeks after the mobilization the patient was transplanted with the cryoconserved autologous HSC product after HD-BEAM chemotherapy. The patient showed uncomplicated recovery of granulopoiesis within the normal time frame (day 11 after HSCT). Probably as a consequence of the low numbers of transplanted CD34+ cells/kg BW, recovery of megakaryopoi-esis (>20,000 platelets/μl) took more time (day 18 after HSCT). 28 days after HSCT, no further blood products were required.
Discussion
Why some patients do not mobilize adequate numbers of CD34+ cells is still not completely understood. High-risk factors for poor mobilization of CD34+ cells certainly include extent of prior therapy, disease status, and extensive irradiation.
CD34+ HSC are normally located in the bone marrow. Through interactions of integrins such as LFA-1 and adhesion molecules such as ICAM-1 they are bound to endoosteal osteoblasts and to stromal cells [7, 8].
Recent studies point out the crucial pathway in which the chemokine stromal-cell-derived factor-1 (SDF-1) in combination with its ligand, the CXCR4 receptor on CD34+ cells, has a high impact on the release of CD34+ HSC from the bone marrow to the peripheral blood [9, 10]. Plerixafor disrupts the physiological binding of SDF-1 to CXCR4 by acting as a competitive antagonist. In combination with G-CSF, a synergistic effect has been observed [11, 12]. One explanation could be that G-SCF decreases the expression and secretion of SDF-1 in marrow stromal cells [13].
Recent clinical trials in multiple myeloma and non-Hodgkin's lymphoma patients compare the mobilization of HSC using G-CSF with or without plerixafor [14, 15]. In both trials plerixafor is enhancing the mobilization of HSC quantitatively without serious adverse effects. The investigators included good and poor HSC mobilizers in these trials. We feel that the separation of good and poor or non-mobilizers is important in this context. In patients defined as good HSC mobilizers we are quite comfortable in continuing the classical mobilization approach with G-CSF (plus chemotherapy) because we see no significant advantage compared to mobilization using plerixafor additionally. In poor non-mobilizers however plerixafor has the potential to enable autologous HSCT, and therefore must be regarded as an important novel therapeutic tool in this patient group. An important point in this context is that in case of non-mobilizers, these patients never reach the apheresis units because they are ‘of the list’ already because of low CD34+ counts in their CD34+ monitoring. This could be an explanation of the wide variability of reported poor mobilizers in different institutions.
Although plerixafor is only available as a compassionate-use drug in Germany at present, we are convinced about its effectiveness in the case described here.
To date we performed G-CSF plus plerixafor mobilization in 2 additional non-mobilizers. One was successful, and we were able to harvest a therapeutic unit (2 × 106 CD34+ cells/kg BW), the other not.
The patient in whom mobilization was not successful had a history of extensive irradiation treatments (34 procedures) and chemotherapy. However, we observed a discrete increase in CD34+ HSC in the peripheral blood (maximally 3 CD34+ cells/μl) in this patient after additional application of plerixafor. In our opinion these cases demonstrate that a critical minimal stem cell pool must be present in the bone marrow. If this critical mass is not available, CD34 mobilization with either G-CSF alone or in combination with plerixafor will be not successful.
Conclusion
Plerixafor has the potency to become an important tool in mobilizing HSC, especially in those patients in whom HSC cannot be mobilized by giving G-CSF and chemotherapy alone. HDC treatment in combination with autologous HSC transplantation is extremely important for this group of patients because of limited alternative curative treatment options. Performing HSC apheresis using the combination of plerixafor and G-CSF in non-mobilizers means an ‘all hands on deck’ approach. Because the increase in HSC is very discrete and short-lasting; intensive (daily) CD34 monitoring and large-volume apheresis are a prerogative for successful harvesting in these patients.
References
- 1.Chabannon C, Le Corroller AG, Viret F, Eillen C, Faucher C, Moatti JP, Viens P, Vey N, Braud AC, Novakovitch G, et al. Cost-effectiveness of repeated aphereses in poor mobilizers undergoing highdose chemotherapy and autologous hematopoietic cell transplantation. Leukemia. 2003;17:811–813. doi: 10.1038/sj.leu.2402867. [DOI] [PubMed] [Google Scholar]
- 2.Corso A, Mangiacavalli S, Nosari A, Castagnola C, Zappasodi P, Cafro AM, Astori C, Bonfichi M, Varettoni M, Rusconi C, et al. Efficacy, toxic-ity and feasibility of a shorter schedule of DCEP regimen for stem cell mobilization in multiple myeloma. Bone Marrow Transplant. 2005;36:951–954. doi: 10.1038/sj.bmt.1705166. [DOI] [PubMed] [Google Scholar]
- 3.Stiff PJ. Management strategies for the hard-to-mobilize patient. Bone Marrow Transplant. 1999;23(suppl 2):S29–S33. doi: 10.1038/sj.bmt.1701671. [DOI] [PubMed] [Google Scholar]
- 4.Lefrere F, Zohar S, Beaudier S, Audat F, Ribeil JA, Ghez D, Varet B, Cavazzana-Calvo M, Dal CL, Letestu R, et al. Evaluation of an algorithm based on peripheral blood hematopoietic progenitor cell and CD34+ cell concentrations to optimize peripheral blood progenitor cell collection by apheresis. Transfusion. 2007;47:1851–1857. doi: 10.1111/j.1537-2995.2007.01407.x. [DOI] [PubMed] [Google Scholar]
- 5.Perry AR, Watts MJ, Peniket AJ, Goldstone AH, Linch DC. Progenitor cell yields are frequently poor in patients with histologically indolent lymphomas especially when mobilized within 6 months of previous chemotherapy. Bone Marrow Transplant. 1998;21:1201–1205. doi: 10.1038/sj.bmt.1701267. [DOI] [PubMed] [Google Scholar]
- 6.Majado MJ, Minguela A, Gonzalez-Garcia C, Salido E, Blanquer M, Funes C, Insausti CL, Garcia-Hernandez AM, Moraleda JM, Morales A. Large-volume-apheresis facilitates autologous transplantation of hematopoietic progenitors in poor mobilizer patients. J Clin Apher. 2009;24:12–17. doi: 10.1002/jca.20191. [DOI] [PubMed] [Google Scholar]
- 7.Dar A, Kollet O, Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol. 2006;34:967–975. doi: 10.1016/j.exphem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631–2639. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- 9.Liles WC, Broxmeyer HE, Rodger E, Wood B, Hubel K, Cooper S, Hangoc G, Bridger GJ, Hen-son GW, Calandra G, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102:2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 10.Devine SM, Flomenberg N, Vesole DH, Liesveld J, Weisdorf D, Badel K, Calandra G, DiPersio JF. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin's lymphoma. J Clin Oncol. 2004;22:1095–1102. doi: 10.1200/JCO.2004.07.131. [DOI] [PubMed] [Google Scholar]
- 11.Flomenberg N, Devine SM, Dipersio JF, Liesveld JL, McCarty JM, Rowley SD, Vesole DH, Badel K, Calandra G. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106:1867–1874. doi: 10.1182/blood-2005-02-0468. [DOI] [PubMed] [Google Scholar]
- 12.Liles WC, Rodger E, Broxmeyer HE, Dehner C, Badel K, Calandra G, Christensen J, Wood B, Price TH, Dale DC. Augmented mobilization and collection of CD34+ hematopoietic cells from normal human volunteers stimulated with granulocyte-colony-stimulating factor by single-dose administration of AMD3100, a CXCR4 antagonist. Transfusion. 2005;45:295–300. doi: 10.1111/j.1537-2995.2005.04222.x. [DOI] [PubMed] [Google Scholar]
- 13.Juarez J, Bendall L. SDF-1 and CXCR4 in normal and malignant hematopoiesis. Histol Histopathol. 2004;19:299–309. doi: 10.14670/HH-19.299. [DOI] [PubMed] [Google Scholar]
- 14.Fruehauf S, Ehninger G, Hubel K, Topaly J, Goldschmidt H, Ho AD, Muller S, Moos M, Badel K, Calandra G. Mobilization of peripheral blood stem cells for autologous transplant in non-Hodgkin's lymphoma and multiple myeloma patients by plerixafor and G-CSF and detection of tumor cell mobilization by PCR in multiple myeloma patients. Bone Marrow Transplant. 2009 doi: 10.1038/bmt.2009.142. doi: 10.1038/ bmt.2009.142. [DOI] [PubMed] [Google Scholar]
- 15.Dipersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, Maziarz RT, Hosing C, Fruehauf S, Horwitz M, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113:5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]