Abstract
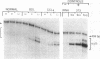
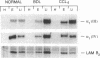
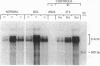
Whether parenchymal or nonparenchymal liver cells play a predominant role in the pathophysiology of hepatic fibrosis has not been firmly established in vivo. We have addressed this question by quantitating the relative abundance of specific mRNAs for collagen types I, III, and IV, and laminin in purified populations of hepatocytes, sinusoidal endothelial cells, and lipocytes from normal and fibrotic rat liver. In normal liver, type I collagen gene expression was minimal in all cell types; mRNA for types III and IV collagen were apparent in endothelial cells and lipocytes, but not in hepatocytes. Laminin mRNA was present in all cell types. Induction of fibrogenesis by either bile duct ligation or carbon tetrachloride administration was associated with a substantial increase in mRNA for types I and III collagen in nonparenchymal cells. Lipocytes from fibrotic animals exhibited a greater than 30-fold increase in type I collagen mRNA relative to normal lipocytes, and greater than 40-fold relative to hepatocytes. Type III collagen mRNA reached 5 times that in normal lipocytes and greater than 120 times that in hepatocytes. Endothelial cells exhibited an isolated increase in type I collagen mRNA, reaching five times that in normal liver. Type IV collagen and laminin gene expression were not significantly increased in nonparenchymal cells during fibrogenesis; in fact, mRNA for type IV collagen and laminin decreased by up to 50% in endothelial cells. Despite the pronounced changes that occurred in matrix gene expression in nonparenchymal cells during fibrogenesis, no change was noted in hepatocytes. We conclude that nonparenchymal liver cells, particularly lipocytes, are important effectors of hepatic fibrosis in vivo.
Full text
PDF

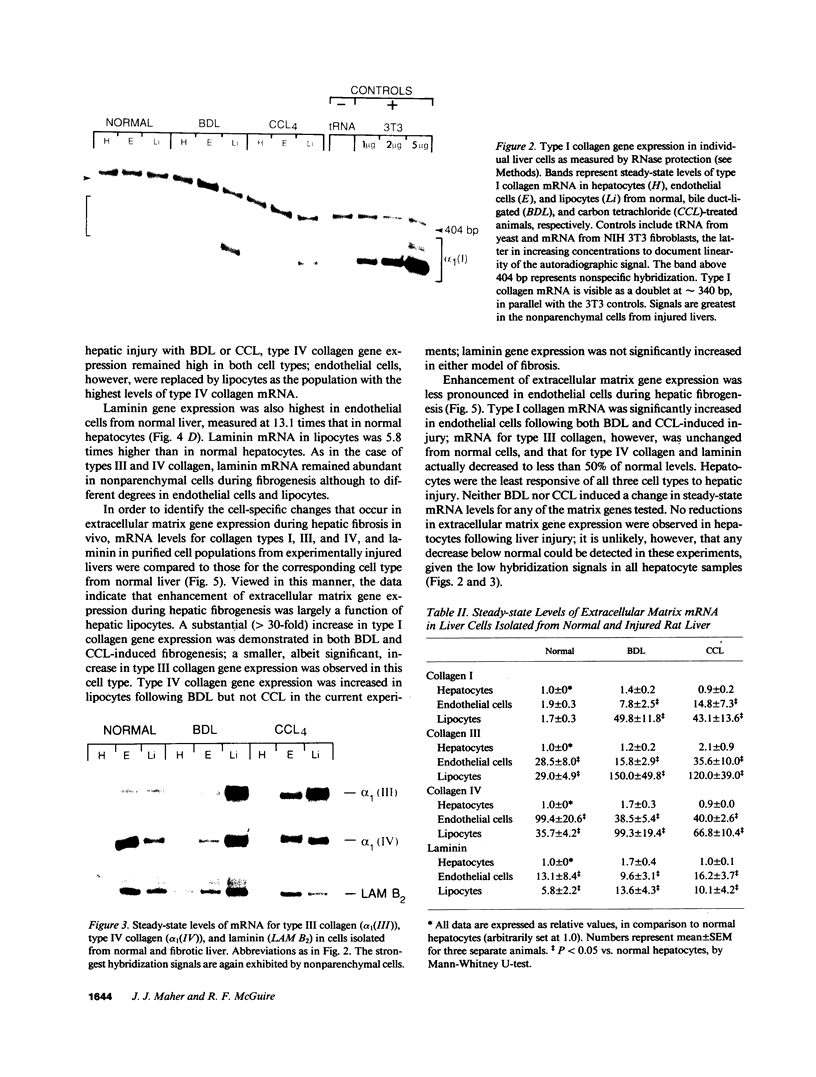
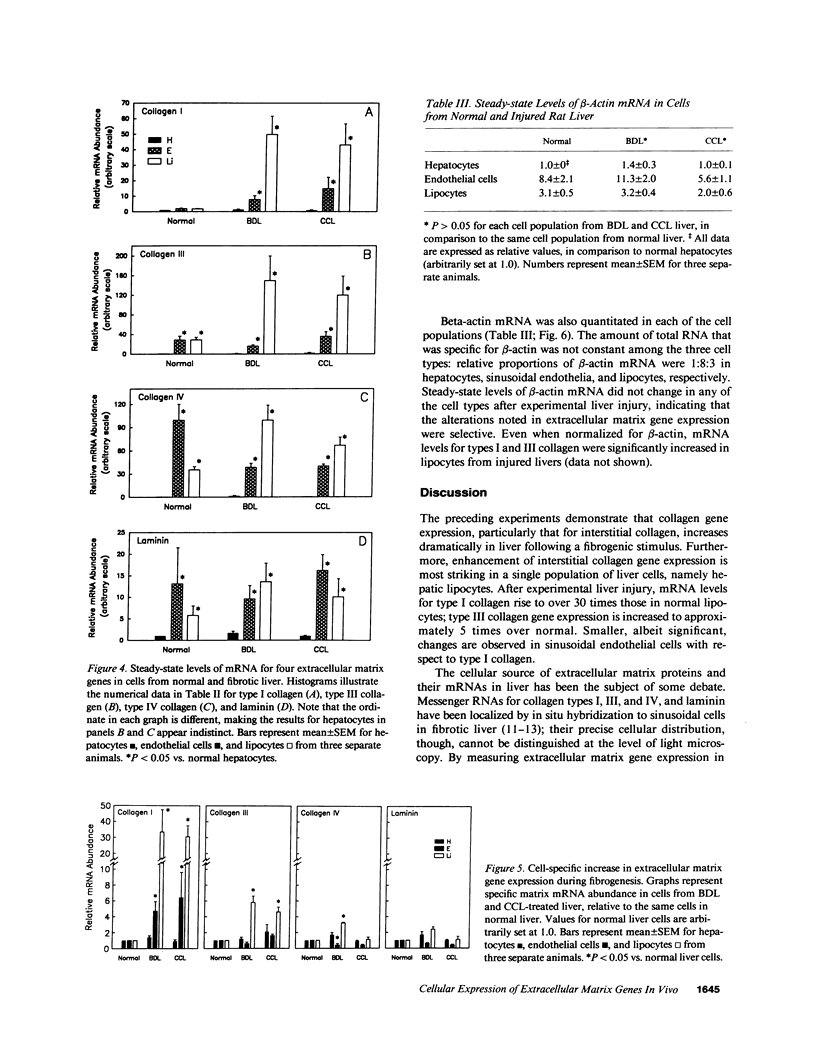



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur M. J., Friedman S. L., Roll F. J., Bissell D. M. Lipocytes from normal rat liver release a neutral metalloproteinase that degrades basement membrane (type IV) collagen. J Clin Invest. 1989 Oct;84(4):1076–1085. doi: 10.1172/JCI114270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow D. P., Green N. M., Kurkinen M., Hogan B. L. Sequencing of laminin B chain cDNAs reveals C-terminal regions of coiled-coil alpha-helix. EMBO J. 1984 Oct;3(10):2355–2362. doi: 10.1002/j.1460-2075.1984.tb02140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. L. Preparation and characterization of RNA: overview. Methods Enzymol. 1987;152:215–219. doi: 10.1016/0076-6879(87)52022-5. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., Alcorn J. M., Feitelberg S. P., Leffert H. L., Chojkier M. Expression of collagen genes in the liver. Mol Biol Med. 1990 Apr;7(2):105–115. [PubMed] [Google Scholar]
- Chojkier M. Hepatocyte collagen production in vivo in normal rats. J Clin Invest. 1986 Aug;78(2):333–339. doi: 10.1172/JCI112581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojkier M., Lyche K. D., Filip M. Increased production of collagen in vivo by hepatocytes and nonparenchymal cells in rats with carbon tetrachloride-induced hepatic fibrosis. Hepatology. 1988 Jul-Aug;8(4):808–814. doi: 10.1002/hep.1840080419. [DOI] [PubMed] [Google Scholar]
- Clayton D. F., Darnell J. E., Jr Changes in liver-specific compared to common gene transcription during primary culture of mouse hepatocytes. Mol Cell Biol. 1983 Sep;3(9):1552–1561. doi: 10.1128/mcb.3.9.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement B., Rissel M., Peyrol S., Mazurier Y., Grimaud J. A., Guillouzo A. A procedure for light and electron microscopic intracellular immunolocalization of collagen and fibronectin in rat liver. J Histochem Cytochem. 1985 May;33(5):407–414. doi: 10.1177/33.5.3886779. [DOI] [PubMed] [Google Scholar]
- Clément B., Emonard H., Rissel M., Druguet M., Grimaud J. A., Herbage D., Bourel M., Guillouzo A. Cellular origin of collagen and fibronectin in the liver. Cell Mol Biol. 1984;30(5):489–496. [PubMed] [Google Scholar]
- Czaja M. J., Weiner F. R., Flanders K. C., Giambrone M. A., Wind R., Biempica L., Zern M. A. In vitro and in vivo association of transforming growth factor-beta 1 with hepatic fibrosis. J Cell Biol. 1989 Jun;108(6):2477–2482. doi: 10.1083/jcb.108.6.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. H., Pratt B. M., Madri J. A. Retinol and extracellular collagen matrices modulate hepatic Ito cell collagen phenotype and cellular retinol binding protein levels. J Biol Chem. 1987 Jul 25;262(21):10280–10286. [PubMed] [Google Scholar]
- Flaim K. E., Hutson S. M., Lloyd C. E., Taylor J. M., Shiman R., Jefferson L. S. Direct effect of insulin on albumin gene expression in primary cultures of rat hepatocytes. Am J Physiol. 1985 Nov;249(5 Pt 1):E447–E453. doi: 10.1152/ajpendo.1985.249.5.E447. [DOI] [PubMed] [Google Scholar]
- Friedman S. L., Arthur M. J. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989 Dec;84(6):1780–1785. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. L., Roll F. J., Boyles J., Arenson D. M., Bissell D. M. Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J Biol Chem. 1989 Jun 25;264(18):10756–10762. [PubMed] [Google Scholar]
- Friedman S. L., Roll F. J., Boyles J., Bissell D. M. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. L., Roll F. J. Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Anal Biochem. 1987 Feb 15;161(1):207–218. doi: 10.1016/0003-2697(87)90673-7. [DOI] [PubMed] [Google Scholar]
- Genovese C., Rowe D., Kream B. Construction of DNA sequences complementary to rat alpha 1 and alpha 2 collagen mRNA and their use in studying the regulation of type I collagen synthesis by 1,25-dihydroxyvitamin D. Biochemistry. 1984 Dec 4;23(25):6210–6216. doi: 10.1021/bi00320a049. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn E., Wick G., Pencev D., Timpl R. Distribution of basement membrane proteins in normal and fibrotic human liver: collagen type IV, laminin, and fibronectin. Gut. 1980 Jan;21(1):63–71. doi: 10.1136/gut.21.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving M. G., Roll F. J., Huang S., Bissell D. M. Characterization and culture of sinusoidal endothelium from normal rat liver: lipoprotein uptake and collagen phenotype. Gastroenterology. 1984 Dec;87(6):1233–1247. [PubMed] [Google Scholar]
- Kountouras J., Billing B. H., Scheuer P. J. Prolonged bile duct obstruction: a new experimental model for cirrhosis in the rat. Br J Exp Pathol. 1984 Jun;65(3):305–311. [PMC free article] [PubMed] [Google Scholar]
- Kurkinen M., Condon M. R., Blumberg B., Barlow D. P., Quinones S., Saus J., Pihlajaniemi T. Extensive homology between the carboxyl-terminal peptides of mouse alpha 1(IV) and alpha 2(IV) collagen. J Biol Chem. 1987 Jun 25;262(18):8496–8499. [PubMed] [Google Scholar]
- Madri J. A., Williams S. K. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983 Jul;97(1):153–165. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher J. J., Bissell D. M., Friedman S. L., Roll F. J. Collagen measured in primary cultures of normal rat hepatocytes derives from lipocytes within the monolayer. J Clin Invest. 1988 Aug;82(2):450–459. doi: 10.1172/JCI113618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher J. J., Friedman S. L., Roll F. J., Bissell D. M. Immunolocalization of laminin in normal rat liver and biosynthesis of laminin by hepatic lipocytes in primary culture. Gastroenterology. 1988 Apr;94(4):1053–1062. doi: 10.1016/0016-5085(88)90566-5. [DOI] [PubMed] [Google Scholar]
- Mak K. M., Lieber C. S. Lipocytes and transitional cells in alcoholic liver disease: a morphometric study. Hepatology. 1988 Sep-Oct;8(5):1027–1033. doi: 10.1002/hep.1840080508. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A. The hepatic extracellular matrix. I. Electron immunohistochemical studies in normal rat liver. Lab Invest. 1984 Jul;51(1):57–74. [PubMed] [Google Scholar]
- Martinez-Hernandez A. The hepatic extracellular matrix. II. Electron immunohistochemical studies in rats with CCl4-induced cirrhosis. Lab Invest. 1985 Aug;53(2):166–186. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Hahn E. G., Stein H. In situ hybridization for procollagen types I, III and IV mRNA in normal and fibrotic rat liver: evidence for predominant expression in nonparenchymal liver cells. Hepatology. 1989 Jul;10(1):84–92. doi: 10.1002/hep.1840100117. [DOI] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Kim K. Y., Riecken E. O., Stein H. Procollagen expression by nonparenchymal rat liver cells in experimental biliary fibrosis. Gastroenterology. 1990 Jan;98(1):175–184. doi: 10.1016/0016-5085(90)91307-r. [DOI] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Riecken E. O., Stein H. Cellular localization of laminin gene transcripts in normal and fibrotic human liver. Am J Pathol. 1989 Jun;134(6):1175–1182. [PMC free article] [PubMed] [Google Scholar]
- Murata K., Kudo M., Onuma F., Motoyama T. Changes of collagen types at various stages of human liver cirrhosis. Hepatogastroenterology. 1984 Aug;31(4):158–161. [PubMed] [Google Scholar]
- Pierce R. A., Glaug M. R., Greco R. S., Mackenzie J. W., Boyd C. D., Deak S. B. Increased procollagen mRNA levels in carbon tetrachloride-induced liver fibrosis in rats. J Biol Chem. 1987 Feb 5;262(4):1652–1658. [PubMed] [Google Scholar]
- Pérez-Tamayo R. Cirrhosis of the liver: a reversible disease? Pathol Annu. 1979;14(Pt 2):183–213. [PubMed] [Google Scholar]
- Rojkind M., Giambrone M. A., Biempica L. Collagen types in normal and cirrhotic liver. Gastroenterology. 1979 Apr;76(4):710–719. [PubMed] [Google Scholar]
- Sakakibara K., Igarashi S., Hatahara T. Localization of type III procollagen aminopeptide antigenicity in hepatocytes from cirrhotic human liver. Virchows Arch A Pathol Anat Histopathol. 1985;408(2-3):219–228. doi: 10.1007/BF00707984. [DOI] [PubMed] [Google Scholar]
- Seyer J. M., Hutcheson E. T., Kang A. H. Collagen polymorphism in normal and cirrhotic human liver. J Clin Invest. 1977 Feb;59(2):241–248. doi: 10.1172/JCI108634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstoshev P., Haber R., Trapnell B. C., Crystal R. G. Procollagen messenger RNA levels and activity and collagen synthesis during the fetal development of sheep lung, tendon, and skin. J Biol Chem. 1981 Sep 25;256(18):9672–9679. [PubMed] [Google Scholar]
- Tseng S. C., Savion N., Gospodarowicz D., Stern R. Modulation of collagen synthesis by a growth factor and by the extracellular matrix: comparison of cellular response to two different stimuli. J Cell Biol. 1983 Sep;97(3):803–809. doi: 10.1083/jcb.97.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner F. R., Giambrone M. A., Czaja M. J., Shah A., Annoni G., Takahashi S., Eghbali M., Zern M. A. Ito-cell gene expression and collagen regulation. Hepatology. 1990 Jan;11(1):111–117. doi: 10.1002/hep.1840110119. [DOI] [PubMed] [Google Scholar]