Abstract
Aim
To determine differences in metal and metalloid exposure between residents of areas in eastern Croatia exposed to heavy fighting during the war in Croatia and residents of areas exposed to moderate fighting.
Methods
Concentrations of aluminum (Al), arsenic (As), barium (Ba), cadmium (Cd), chromium (Cr), copper (Cu), iron (Fe), nickel (Ni), lead (Pb), uranium (U), vanadium (V), and zinc (Zn), reported to be associated with military operations, were determined in hair, serum, and urine samples using inductively-coupled plasma mass spectroscopy. A total of 127 and 46 participants from areas of heavy and moderate fighting, respectively, were included.
Results
Compared with participants from areas exposed to moderate fighting, participants from areas exposed to heavy fighting had significantly higher serum concentrations of Al (87.61 vs 42.75 μg/L, P = 0.007), As (5.05 ± 1.79 vs 4.16 ± 1.55 μg/L, P = 0.003), Ba (7.12 vs 6.01 μg/L, P = 0.044), and V (17.98 vs 16.84 μg/L, P = 0.008); significantly higher urine concentrations of As (43.90 vs 11.51 μg/L, P < 0.001) and Cd (0.67 vs 0.50 μg/L, P = 0.031); and significantly higher hair concentrations of Al (12.61 vs 7.33 μg/L, P < 0.001), As (0.32 vs 0.05 μg/L, P < 0.001), Cd (0.03 vs 0.02 μg/L, P = 0.002), Fe (22.58 vs 12.68 μg/L, P = 0.001), Pb (1.04 vs 0.69 μg/L, P = 0.006), and V (0.07 vs 0.03 μg/L, P < 0.001).
Conclusion
Differences between populations from eastern Croatian areas exposed to heavy and populations exposed to moderate fighting point to the need for extensive monitoring of metal and metalloid exposure, emphasizing the role of biomonitoring through ecologic and preventive activities.
Until recently, in Croatia only food and water have been analyzed for the presence of heavy metals and other toxic elements (1-5). These studies revealed higher than allowable concentrations of toxic metals/metalloid like arsenic (As), cadmium (Cd), mercury (Hg), and lead (Pb) in food products originating from different Croatian counties, in freshwater fish and brassicas from the Zagreb area, and As, Cd, copper (Cu), chromium (Cr), iron (Fe), radium (Ra), uranium (U), and vanadium (V) in water wells in Osijek urban and rural area, which pointed to the need for metal exposure biomonitoring. Such biomonitoring offers an opportunity to define and evaluate the population groups at high exposure and to develop strategies to reduce future exposure (6).
In addition, heavy fighting that took place in Croatia from 1991 to 1995 possibly exposed populations in these areas to excess levels of metals. This points to the need of conducting comprehensive research in order to understand possible long-term consequences of war on population health. Evidence suggests that military action can contaminate the environment with metals (7). Also, lead exposure in professional firearm instructors has been linked to their work with firearms (8,9). Nevertheless, few studies have directly examined long-term effects of metal contamination in the environment following military activity.
Combat activities during the 1991-1995 war in Croatia were most intensive in the eastern part of the country, causing heavy casualties and destruction. The environmental consequences of these military operations have not been well studied (10). The establishment of a military defense headquarters in the eastern Croatian city of Osijek was associated with continuous direct exposure of soldiers and civilians to explosive devices and various contaminants, metals in particular (11,12). Indeed, the predominant use of mines and explosive devices during the 1991-1992 war period points to the need of identifying the long-term health effects in soldiers and civilians wounded by these devices (13). The possible association of carcinogenesis with metals and metal alloys used in modern weaponry has been examined in a few studies (14-16). Aluminum (Al), Cd, Cu, and Pb are associated with bombs, Cu with automatic small weapons, and Al, As, Ba, Cd, Cr, Cu, Fe, Ni, Pb, U, V, and Zn with tank shells (7). Epidemiological studies in occupationally exposed humans, mostly retrospective, have demonstrated that As, Cd, Cr, Ni, and Pb are carcinogens. Exposure to compounds of these metals has been shown to correlate with increased incidences of cancer, such as lung cancer (As, Cr, Ni, Pb), skin cancer, bladder cancer, and cancer of lymphatic and hematopoietic systems (As), prostate cancer (Cd), cancer of the nasal sinuses (Ni), and stomach cancer (Pb). Other weapons-related metals (V, Fe) have been classified as possible carcinogens. In addition, excessive exposure to some metals has been clearly linked to specific conditions like renal intoxication, hypertension, cardiac malfunction (Ba), and liver and kidney damage (Cu). In the case of Zn and U, little or no evidence exists of adverse effect on humans (17,18).
Studies performed in Croatia have suggested a connection between metal exposure and 1991-1995 war actions. Metal monitoring in the Drava river, which has been regularly conducted since 1976, showed that concentrations of Pb, Cd, and Hg increased in the river water during the war period (19). A study performed in 2002 in Baranja, found the concentrations of these three metals in deer kidney samples to be far above the allowed levels, especially in older animals (20).
The aim of our study, designed to be scaled up in the future, was to determine levels of metals and metalloid As derived from explosive devices in adult populations in former combat areas of eastern Croatia, and to compare the levels measured in areas of heavy and moderate fighting.
Methods
Participants
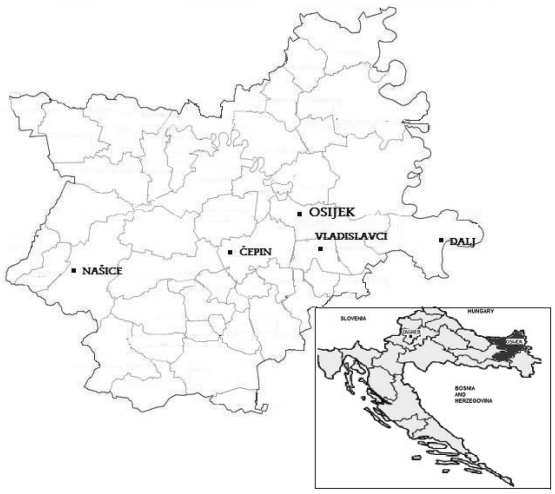
In this cross-sectional study, sample collection was carried out in three, 3-day phases in June 2007, November 2007, and June 2008, in former combat zones throughout the eastern part of Croatia (Osijek-Baranja county, Eastern Slavonia). The position of Osijek-Baranja county and the study sites selected for this investigation are shown in Figure 1. Study sites were selected from the areas exposed to moderate and from the areas exposed to heavy combat, as well as from the areas situated in the Drava depression and from the areas situated on the central plateau, ie, at higher or lower altitude. All sites lay along a horizontal line running through Osijek-Baranja county and belonged to one hydraulic system (21). The study sites of Dalj, Vladislavci, and Našice were chosen, respectively, as the most eastern, central, and most western locations on the elevated central plateau; Osijek was chosen as part of the Drava depression; and Čepin, as a central point on the intersection of the Osijek-Vladislavci longitudinal and Našice-Dalj latitudinal lines, as well as a border area between the Drava depression and central plateau.
Figure 1.
Study sites of moderate fighting (Našice) and heavy fighting (Čepin, Dalj, Osijek, Vladislavci) in Osijek-Baranja county in eastern Croatia. The inset shows the position of Osijek-Baranja county on the map of Croatia.
Čepin, Osijek, and Vladislavci were considered areas of heavy fighting. Located on or close to the front line during the war, they were exposed to continuous heavy artillery fire for more than 3 months (22,23). Dalj was not as heavily bombed, but there was intensive close combat with small arms, it remained under enemy occupation for a long time, and it held many arms storage sites. Našice, as the town away from the front line, was categorized as a place of moderate fighting, because it was only occasionally shelled. These categorizations are supported by data on the number of wounded and killed children and adult civilians, as well as the ratio of killed-to-wounded in hospitals in the respective areas (24-26).
Questionnaire data and samples of hair, blood, and urine were collected from 391 participants randomly selected from patient databases of family medicine practices in the Croatian national health system. Inclusion criteria were age 18 or older; residence in the study location before, during, and after the war period, excluding a possible refugee period of up to 6 months; and absence of clinical conditions connected to excessive metal exposure, like the kidney, liver, pulmonary, neurologic diseases, or cancer.
For financial reasons, a subset of 173 participants (44% of the original sample) was randomly selected using SPSS, version 12.0.0 (SPSS Inc., Chicago, IL, USA). Of these 173 participants, 28 (16.2%) were from Vladislavci, 26 (15.0%) from Dalj, 33 (19.1%) from Čepin, 40 (23%) from Osijek, and 46 (26.7%) from Našice.
The study was approved by the ethics committee of the School of Medicine, Josip Juraj Strossmayer University of Osijek. The purpose of the study was explained to each participant, after which they voluntarily provided written informed consent.
Questionnaire data collection
Using a questionnaire specifically designed for this study, trained staff conducted structured interviews with participants. The questionnaire was used to collect data on age, sex, smoking, alcohol consumption, current residence, possible occupational metal exposure (metal industry, smelter, gas station workers, farmers), and household metal exposure (residence near industrial plants, gas stations, dump sites, heavy traffic roads), participation in war, wound history, and residence during and after the war.
Biological sample collection, sample treatment, and analysis
After the interview, a spot urine sample was collected from each subject by a nurse or medical technician into a urine collection container (100 mL, Greiner Bio-One, Frickenhausen, Germany). A blood sample of at least 3 mL was also obtained from each subject (Vacuette Blod Collection Needle, 38 × 0.9 mm, and Vacuette Serum Gel Tube 3.5 mL, Greiner Bio-One). Serum was separated from the blood on the same day (Cryotube, 3.8 mL, TPP, Trasadingen, Switzerland). A hair sample approximately 1 cm wide and 3 cm long was obtained from each participant from the occipital area of the head, adjacent to the scalp, using stainless steel scissors and polyethylene bags. If the hair was short, several hair cuts from the same head area were combined.
Biological samples were transported in a portable refrigerator to the Public Health Institute in Osijek, where they were stored in a refrigerator. At the end of each sampling stage, the collected samples were transported to the Laboratory of Inorganic Chemistry, Faculty of Chemical Engineering and Technology, University of Zagreb. Complete analysis of the biological samples was performed by inductively-coupled plasma mass spectrometry.
Serum and urine samples were defrosted, and an aliquot of 0.5 mL was removed and mixed with nitric acid (1% v/v, 10 mL). The sample was dried in a microwave oven, transferred to a flask, and analyzed by inductively coupled plasma mass spectroscopy (ICP-MS, ELAN DRC-e, Perkin Elmer, Waltham, MA, USA). Hair was washed in distilled water, soaked for one hour in acetone, rinsed in deionized water, and left to dry at room temperature for 24 hours. Afterwards, the samples were cut into pieces by stainless steel scissors. To hair samples of 0.1 g, nitric acid (65% v/v, 1 mL) was added. Samples were dried in a microwave oven, transferred into a flask and, after addition of deionized water, analyzed by ICP-MS.
All samples were analyzed according to the element determination procedure by ICP-MS (27). Operating conditions of the ICP-MS were: RF power, 1300 W; plasma gas flow, 15 L/min; auxiliary gas flow, 1 L/min; nebulizer gas flow, 0.93-0.98 L/min; peristaltic pump speed, 1 mL/min; and nickel sampler/skimmer cones. Polyatomic interferences with such elements as Fe, As, and Cr were eliminated with an instrumental dynamic reaction cell using reactive gas methane (CH4). The instrument was calibrated after every 12th sample, using the external standard 71-Element Group Multi-Element Standard Solution (Inorganic Ventures, Christiansburg, VA, USA) and internal standards containing the elements yttrium, indium, terbium, and bismuth (Inorganic Ventures). Analytical methods were validated using the following standard reference materials: ICP Multi Element Standard Solution X CertiPUR for Surface Water Testing (Merck, Darmstadt, Germany), Trace Elements Urine Blank, and Trace Elements Urine (SERO AS, Billingstad, Norway).
Statistics
Normality of data distribution was tested with the Smirnov-Kolmogorov test. The χ2 test was used to determine differences in the distribution of qualitative variables, and differences in quantitative variables were tested with the non-parametric Mann-Whitney test or the parametric t test, depending on whether the data were normally distributed or not. The non-parametric Spearman rank correlation test was used to investigate the relationship between concentrations of As, Cd, and Pb in serum, urine, and hair of smokers and non-smokers and between participants with and without occupational exposure to metals. Correlations with a Spearman correlation coefficient (ρ) higher than 0.600 were considered strong and those with a Spearman correlation coefficient ranging from 0.300 to 0.599 were considered medium (28). Statistical analyses were performed with SPSS, version 12.0.0. Comparison of the correlation coefficients was conducted by z-statistics using MedCalc 11.3 (MedCalc Software, Mariakerke, Belgium; www.medcalc.be). In all statistical analyses, two-sided P values of 0.05 were considered significant.
Results
Samples from 173 participants were analyzed, 127 (73%) from areas of heavy fighting and 46 (27%) from areas of moderate fighting. The two groups’ responses on the questionnaire are shown in Table 1. Two groups did not differ significantly in smoking habits or rates of household metal exposure, but did differ significantly in age, sex distribution, and rates of occupational exposure.
Table 1.
Characteristics of study participants from areas of heavy and moderate fighting in eastern Croatia
| Group from areas of |
|||
|---|---|---|---|
| Variable | moderate fighting (n = 46) | heavy fighting (n = 127) | P* |
| Mean age (interquartile range), years* |
41 (23.0-56.0) |
46 (35.0-56.0) |
0.027 |
| Male sex, No. (%) |
18 (39.1) |
88 (69.3) |
<0.001 |
| Smoking, No. (%) |
13 (28.3) |
56 (44.1) |
0.061 |
| Household exposure, No. (%) |
13 (28.3) |
52 (40.9) |
0.128 |
| Professional exposure, No. (%) |
13 (28.3) |
62 (48.8) |
0.016 |
| Wounded in the 1991-1995 war, No. (%) | 0 (0) | 43 (33.9) | <0.001 |
*The values for age were compared using the Mann-Whitney test. All other values were compared using the χ2 test.
To evaluate possible confounding factors, levels of metals were compared between various subgroups from areas of heavy fighting (total n = 127); the subgroups were defined according to age, sex, wound infliction, household exposure, and occupational exposure. Concentrations determined in urine are indicated with “_u,” in hair with “_h,” and in serum with “_s.” Significantly higher concentrations of Cd_u (P = 0.006) were found in participants older than 45 years (n = 69) than in younger participants (n = 58). Significantly higher concentrations of Fe_s (P = 0.045), Cd_u (P = 0.016), Pb_u (P = 0.026), Zn_u (P = 0.001), As_h (P = 0.000), Cd_h (P = 0.034), Pb_h (P = 0.001), and V_h (P = 0.000) were found in men (n = 88) than in women (n = 39). Wounded participants (n = 43) had significantly higher Al_s concentrations (P = 0.035) than non-wounded ones (n = 84); participants exposed due to the location of their residence (n = 52) had higher As_s (P = 0.019) and Cu_s (P = 0.022) concentrations than participants who were not (n = 75); occupationally exposed participants (n = 62) had higher As_s (P = 0.002), Cd_s (P = 0.007), Cu_s (P = 0.036), and As_h (P = 0.040) concentrations than unexposed participants (n = 65). Element concentrations were compared between farmers (n = 5) and non-farmers (n = 122). Differences were not significant for any of the elements regardless of sample (urine, hair, serum), with the exception of Fe_s, which was significantly higher in non-farmers.
Correlations between smoking and occupational exposure and element concentrations in samples were also analyzed. In smokers, positive strong correlations (n = 69) were found between As_s-As_u, As_s-As_h, As_u-As_h, and Cd_h-Pb_h and medium correlations between As_h-Cd_h. In non-smokers (n = 104), positive strong correlations were found between As_u-As_h and Cd_h-Pb_h and medium correlations between As_s-Cd_s, As_s-As_u, As_u-Cd_u, and As_h-Cd_h (Table 2).
Table 2.
Spearman rank correlation coefficients (ρ) for the correlation between concentrations of arsenic (As), cadmium (Cd), and lead (Pb) in serum, urine, and hair from smokers and non-smokers
| Element | Smokers (N = 69) |
Non-smokers (N = 104) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As_s | Cd_s | Pb_s | As_u | Cd_u | Pb_u | As_h | Cd_h | As_s | Cd_s | Pb_s | As_u | Cd_u | Pb_u | As_h | Cd_h | |
| Cd_s* |
0.231 |
0.330‡ |
||||||||||||||
| Pb_s |
0.004 |
-0.140 |
0.027 |
0.128 |
||||||||||||
| As_u† |
0.656‡ |
-0.071 |
0.154 |
0.323‡ |
-0.141 |
-0.042 |
||||||||||
| Cd_u |
0.121 |
0.054 |
0.062 |
0.241† |
0.084 |
0.047 |
-0.258‡ |
0.346‡ |
||||||||
| Pb_u |
-0.509 |
-0.040 |
-0.017 |
-0.007 |
0.112 |
-0.009 |
-0.095 |
-0.058 |
0.066 |
0.283‡ |
||||||
| As_h‡ |
0.643‡ |
0.015 |
0.208 |
0.795‡ |
0.165 |
-0.037 |
0.236† |
-0.044 |
-0.150 |
0.616‡ |
0.075 |
0.100 |
||||
| Cd_h |
0.125 |
0.020 |
-0.280† |
0.168 |
0.013 |
0.020 |
0.327‡ |
-0.450 |
0.051 |
-0.133 |
0.113 |
0.211† |
0.201† |
0.335‡ |
||
| Pb_h | 0.094 | 0.050 | -0.013 | 0.168 | 0.005 | 0.029 | 0.295† | 0.772‡ | -0.100 | -0.072 | -0.131 | -0.022 | 0.160 | 0.306‡ | 0.226† | 0.645‡ |
*Abbreviations: _s – in serum; _u – in urine; _h – in hair.
†P < 0.05.
‡P < 0.01.
Table 3 shows the correlations between metal concentrations in participants with occupational exposure (n = 75) and participants without it (n = 98). In participants with occupational exposure, a strong correlation was found between Cd_h-Pb_h and medium correlations were found between As_s-As_u, As_s-As_h, As_u-Cd_u, As_u-Cd_h, Cd_u-Pb_u, As_h-Cd_h, and As_h-Pb_h. Among participants without occupational exposure, strong correlations were found between As_u-As_h and Cd_h-Pb_h, and medium correlations between As_s-Cd_s, As_s-As_u, and As_u-Cd_u.
Table 3.
Spearman rank correlation coefficients (ρ) for the correlation between concentrations of arsenic (As), cadmium (Cd), and lead (Pb) in serum, urine, and hair from participants with and without occupational exposure
| Participants |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Element | with occupational exposure
(N = 75) |
without occupational exposure
(N = 98) |
||||||||||||||
| As_s | Cd_s | Pb_s | As_u | Cd_u | Pb_u | As_h | Cd_h | As_s | Cd_s | Pb_s | As_u | Cd_u | Pb_u | As_h | Cd_h | |
| Cd_s* |
0.143 |
0.336‡ |
||||||||||||||
| Pb_s |
0.130 |
0.077 |
-0.060 |
-0.002 |
||||||||||||
| As_u† |
0.452‡ |
-0.132 |
0.084 |
0.414‡ |
-0.193 |
0.012 |
||||||||||
| Cd_u |
0.030 |
-0.029 |
-0.060 |
0.323‡ |
0.117 |
0.021 |
-0.113 |
0.366‡ |
||||||||
| Pb_u |
0.069 |
-0.237† |
-0.100 |
0.084 |
0.382‡ |
-0.112 |
0.040 |
0.028 |
0.047 |
0.101 |
||||||
| As_h‡ |
0.504‡ |
-0.026 |
0.073 |
0.714‡ |
0.073 |
0.048 |
0.267† |
-0.127 |
-0.026 |
0.649‡ |
0.197 |
0.077 |
||||
| Cd_h |
0.068 |
-0.054 |
-0.112 |
0.301‡ |
0.245† |
0.193 |
0.420‡ |
0.078 |
0.074 |
-0.231† |
-0.028 |
0.063 |
0.106 |
0.251† |
||
| Pb_h | 0.062 | -0.078 | -0.100 | 0.180 | 0.212 | 0.265† | 0.331‡ | 0.721‡ | -0.180 | -0.039 | -0.068 | -0.056 | 0.045 | 0.149 | 0.186 | 0.632‡ |
*Abbreviations: _s – in serum; _u – in urine; _h – in hair.
†P < 0.05.
‡P < 0.01.
Comparison of all estimated correlation coefficients between smokers and non-smokers and between participants with and without occupational exposure revealed significantly higher correlation coefficients between As_s-As_u (P = 0.004), As_s-As_h (P = 0.001), and As_u-As_h (P = 0.02) in smokers than in non-smokers. The correlation coefficient between As_u-Cd_h was significantly higher (P = 0.03) in participants with occupational exposure than in participants without it.
Participants from areas of heavy fighting had significantly higher serum levels of Al, As, Ba, and V than participants from areas of moderate fighting (Table 4). However, participants from areas of moderate fighting had significantly higher serum levels of Cr and Ni than participants from areas of heavy fighting.
Table 4.
Serum concentrations of metals/metalloid (μg/L) in participants from areas of moderate and heavy fighting in eastern Croatia
| Element |
Participants from areas of moderate fighting (n = 46) |
Participants from areas of heavy fighting (n = 127) |
P† |
PRR
(μg/L) § |
PRR
(μg/L)II |
||
|---|---|---|---|---|---|---|---|
| median (IQR)* |
mean ± SD |
median (IQR) |
mean ± SD |
||||
| Aluminum |
42.75 (8.15-108.00) |
87.61 (17.79-185.63) |
0.007 |
1-10 |
0.5-8.0 |
||
| Arsenic |
4.16 ± 1.55 |
5.05 ± 1.79 |
0.003‡ |
NA |
4.4-14.2 |
||
| Barium |
6.01 (2.89-11.33) |
7.12 (4.75-12.58) |
0.044 |
NA |
0.4-1.7 |
||
| Cadmium |
0.08 (0.04-0.12) |
0.06 (0.04-0.11) |
0.323 |
0.0-4.0 |
0.04-0.36 |
||
| Chromium |
9.4 ± 3.05 |
7.43 ± 3.86 |
0.002‡ |
0.0-0.1 |
0.04-0.48 |
||
| Copper |
989.289 (841.23-1109.22) |
953.15 (842.52-1090.24) |
0.425 |
800-1500 |
601-1803 |
||
| Iron |
1142.74 (858.57-1455.39) |
1147.65 (793.07-1389.97) |
0.747 |
NA |
825-2090 |
||
| Nickel |
7.42 (5.93-9.66) |
4.73 (3.52-6.89) |
<0.001 |
2.6-3.1 |
0.13-2.80 |
||
| Lead |
3.81 (1.54-15.19) |
4.26 (1.40-10.19) |
0.847 |
0.0-150 |
0.1-0.5 |
||
| Uranium |
0.10 (0.02-0.23) |
0.11 (0.04-0.28) |
0.515 |
NA |
0.004-0.11 |
||
| Vanadium |
16.84 (15.34-18.09) |
17.98 (16.32-21.67) |
0.008 |
0.016-0.139 |
0.02-0.11 |
||
| Zinc | 798.95 (736.89-921.22) | 832.16 (748.99-952.77) | 0.204 | 800-1400 | 587-1215 | ||
*Abbreviations: IQR – interquartile range between the 25th and 75th quartiles; SD – standard deviation; NA – not available; PRR – proposed reference range.
†Mann-Whitney test, unless otherwise noted.
‡t test.
§Source: Bogadi-Šare et al (17).
IISource: Chojnacka et al (29) and Heitland and Koster (30).
Participants from areas of heavy fighting had significantly higher urine concentrations of As and Cd than participants from areas of moderate fighting (Table 5). On the other hand, none of the studied elements was present at higher urine concentrations in areas of moderate fighting than in areas of heavy fighting.
Table 5.
Urine concentrations of metals/metalloid (μg/L) in study participants from areas of moderate and heavy fighting in eastern Croatia
| Participants from areas of moderate fighting (n = 46) |
Participants from areas of heavy fighting (n = 127) |
P† |
PRR (μg/L)§ |
PRR (μg/L)II |
|||
|---|---|---|---|---|---|---|---|
| median (IQR)* |
mean ± SD |
median (IQR)* |
mean ±SD |
||||
| Aluminum |
15.69 (0.00-100.20) |
46.36 (6.30-110.17) |
0.255 |
3.0-30.0 |
0.16-11.2 |
||
| Arsenic |
11.51 (8.32-21.93) |
43.9 (25.28-87.00) |
<0.001 |
14-70 |
2.3-161 |
||
| Barium |
7.07 ± 4.44 |
6.19 ± 5.00 |
0.292‡ |
NA |
0.17-3.85 |
||
| Cadmium |
0.5 (0.30-0.78) |
0.67 (0.38-1.41) |
0.031 |
0.0-0.2 |
0.06-0.79 |
||
| Chromium |
4.96 (3.14-8.84) |
5.36 (3.82-8.21) |
0.403 |
0.1-0.5 |
NA |
||
| Copper |
18.85 ± 16.66 |
21.67 ± 15.49 |
0.300‡ |
NA |
4.3-12.1 |
||
| Iron |
153.56 (90.17-274.88) |
128.08 (73.64-193.43) |
0.120 |
NA |
NA |
||
| Nickel |
6.18 (3.65-13.04) |
6.69 (4.54-9.88) |
0.813 |
2.0-2.7 |
0.59-4.06 |
||
| Lead |
3.18 (2.00-7.95) |
4.32 (2.51-8.05) |
0.202 |
NA |
0.01-2.14 |
||
| Uranium |
0.03 (0.00-0.18) |
0.02 (0.00-0.11) |
0.431 |
NA |
0.0002-0.008 |
||
| Vanadium |
22.87 ± 11.86 |
23.42 ± 10.13 |
0.763‡ |
0.0-0.3 |
1.4-10.2 |
||
| Zinc | 348.14 (195.77-503.82) | 439.44 (260.67-708.21) | 0.064 | NA | 44-499 | ||
Hair concentrations of Al, As, Cd, Fe, Pb, and V were significantly higher in participants from areas of heavy fighting than in participants from areas of moderate fighting (Table 6). An unexpected finding was a significantly higher concentration of U in the hair of participants from areas of moderate fighting than in participants from areas of heavy fighting.
Table 6.
Hair concentrations of metals/metalloid (mg/kg) in study participants from areas of moderate and heavy fighting in eastern Croatia
| Participants from areas of moderate fighting (n = 46) |
Participants from areas of heavy fighting (n = 127) |
P† |
PRR (mg/kg)‡ |
PRR (mg/kg)§ |
|
|---|---|---|---|---|---|
| median (IQR)* |
median (IQR)* |
||||
| Aluminum |
7.33 (5.02-12.09) |
12.61 (7.65-21.84) |
<0.001 |
NA |
0.26-5.30 |
| Arsenic |
0.05 (0.03-0.05) |
0.32 (0.16-0.72) |
<0.001 |
NA |
0.03-0.08 |
| Barium |
1.10 (0.59-2.69) |
1.31 (0.79-2.59) |
0.229 |
NA |
0.05-1.58 |
| Cadmium |
0.02 (0.01-0.04) |
0.03 (0.02-0.07) |
0.002 |
NA |
0.004-0.17 |
| Chromium |
0.51 (0.22-0.83) |
0.36 (0.21-1.30) |
0.997 |
0.2-2.0 |
0.11-0.52 |
| Copper |
10.77 (9.41-14.17) |
10.73 (9.04-14.00) |
0.726 |
NA |
9.0-61.3 |
| Iron |
12.68 (8.41-26.26) |
22.58 (13.16-39.71) |
0.001 |
NA |
NA |
| Nickel |
0.28 (0.19-0.50) |
0.36 (0.23-0.63) |
0.085 |
0.2-1.0 |
0.08-0.90 |
| Lead |
0.69 (0.34-1.43) |
1.04 (0.56-2.82) |
0.006 |
NA |
0.13-4.57 |
| Uranium |
0.00 (0.00-0.01) |
0.00 (0.00-0.00) |
<0.001 |
NA |
0.002-0.03 |
| Vanadium |
0.03 (0.01-0.07) |
0.07 (0.04-0.13) |
<0.001 |
0.004-0.140 |
0.001-0.051 |
| Zinc | 117.87 (100.47-141.70) | 122.58 (98.92-147.10) | 0.883 | NA | 129-209 |
Discussion
We found significant differences in metals/metalloid concentrations in biological samples between adult populations in eastern Croatia that had experienced heavy and those that had experienced moderate fighting during the 1991-1995 war. Currently, there are no internationally recommended reference values for exposure to the metals analyzed in this work. Nevertheless, the concentrations of most elements investigated in the present study exceeded the reference ranges recommended by particular authors (17,31,32). To the best of our knowledge, this is the first study relating elevated concentrations of metals and metalloids in human samples to previous armed activities and war actions.
The strengths of this study lie in the fact that three different biological samples were analyzed from each participant, and the analysis was performed using ICP-MS, a remarkably powerful method for (ultra)trace element determinations (29,30,33). Superior to other methods, ICP-MS has extremely low limits of detection for various elements, wide multielement capability, and high sample throughput. The method allows simultaneous detection of a large number of elements in small samples and differentiates between different isotopes of the same element (18,34-37).
Serum concentrations of Al, As, Ba, and V were higher in participants from areas of heavy fighting than in participants from areas of moderate fighting. These results are in accordance with the previously established association between these elements and heavy artillery projectiles (7), and they indicate extensive and indiscriminate use of heavy artillery during the war in Croatia, especially by the Yugoslav Army. The fact that the elevated serum concentrations of these elements in our participants have persisted to the present, almost two decades after the outbreak of war, suggests that the populations living in areas of heavy fighting may still be exposed to residual metal contamination from heavy artillery ammunition.
As and Cd were the only two elements that showed higher urine concentrations in participants from areas of heavy fighting than in participants from areas of moderate fighting. As urine concentrations are indicators of acute exposure, these findings suggest that the level of contamination with most of the metals and the metalloid under study has decreased with time.
Hair concentrations of Al, As, Cd, Fe, Pb, and V, all of which are associated with bombs and heavy artillery ammunition, were higher in participants from areas of heavy fighting than in participants from areas of moderate fighting. These findings confirm hair as a useful indicator of long-term metal exposure, making it a valuable matrix for use in biomonitoring studies, as reported by other authors (29,32). Low U concentration in the hair of participants from areas of heavy fighting confirmed the assumptions that depleted-uranium weapons were rarely used or not used at all in the 1991-1995 war in Croatia (23,38,39).
Unexpectedly, participants from areas of moderate fighting showed higher serum concentrations of Cr and Ni and higher U concentrations in hair. Cr and U exposure may be related to cement industry, which is present in the studied areas (17,40). Non-warfare sources of Ni exposure are unclear, since there are no mining, steel, or electroplating production industries in this area (17,18).
Since some authors have demonstrated an effect of Co, Ni, and U from embedded shrapnel on genotoxic and tumorigenic pathways (14-16), we considered wounding as a possible confounding factor in our study. When we compared levels of metals in wounded participants from areas of heavy fighting with non-wounded participants from the same areas, only one element (Al) in one matrix (serum) was found to be significantly higher in wounded participants. Therefore, we believe that different proportions of wounded participants from areas of heavy and moderate fighting can be excluded as a confounding factor.
A larger proportion of participants from areas of heavy fighting than of participants from areas of moderate fighting was at risk for occupational exposure. Although we did not find higher concentrations of any studied element among farmers compared with non-farmers, we cannot exclude pesticide as a confounding factor due to the small number of farmers in our subgroup of participants with occupational exposure. The differences in serum As, Cd, and Cu concentrations and hair As concentrations between participants with and without occupational exposure may be caused by confounding factors.
We also investigated correlations between the elements usually connected with occupational exposure and smoking. It is unlikely that As, Cd, and Pb had a significant confounding influence on our results: the coefficients for these metals were similar between participants with and without occupational exposure, as well as between smokers and non-smokers. One exception is As, for which significantly stronger correlations between sample matrices were found in smokers than in non-smokers, as expected. Nevertheless, since the percentage of smokers is similar in the groups from areas of moderate and heavy fighting, it seems less likely that smoking affected the overall results.
A limitation of the study is the small sample size. This made it impossible to estimate age, sex, and occupational exposure differences between the groups. Participants from areas of heavy fighting were older and more often male than participants from areas of moderate fighting. This makes sense given the predominance of men among war veterans and the war wounded. Another limitation is that this study did not consider all possible contributions from non-combat factors, such as variations in the concentrations of metals and metalloids in drinking water, local hydrogeologic characteristics, and exposure from local industry (17,18,40,41).
Future scale up of this small-scale study, including a larger number of participants, may help us to verify the results and possibly detect additional important differences, to establish national and international reference values, comprehensively assess the risk of metal and metalloid exposure in populations living in former conflict zones in Croatia, identify other mechanisms of metal exposure in these and other populations of Croatia, and lead to the development and implementation of preventive and corrective measures.
Acknowledgments
The study was supported by the Ministry of Science, Education, and Sports of the Republic of Croatia and was conducted as part of the project “Investigation of Long-Term Consequences of War on Population Health.” This project is part of the scientific program “Croatian Biobank – Resource for Studying Determinants of Health and Disease in the Population,” implemented jointly by Josip Juraj Strossmayer University of Osijek, School of Medicine Osijek; Osijek-Baranja County Health Center; Institute of Public Health of Osijek-Baranja County; Zagreb University Faculty of Chemical Engineering and Technology; and Dr Andrija Štampar Institute of Public Health, Zagreb. The authors thank to all those living in eastern Croatia, especially Osijek-Baranja County, who directly or indirectly contributed to this study.
References
- 1.Bituh T, Marovic G, Petrinec B, Sencar J, Franulovic I. Natural radioactivity of 226Ra and 228Ra in thermal and mineral waters in Croatia. Radiat Prot Dosimetry. 2009;133:119–23. doi: 10.1093/rpd/ncp033. [DOI] [PubMed] [Google Scholar]
- 2.Bosnir J, Puntaric D. Lead concentration in brassicas from Zagreb home gardens. Croat Med J. 1997;38:143–6. [Google Scholar]
- 3.Bosnir J, Puntaric D, Skes I, Klaric M, Simic S, Zoric I, et al. Toxic metals in freshwater fish from the Zagreb area as indicators of environmental pollution. Coll Antropol. 2003;27(Suppl 1):31–9. [PubMed] [Google Scholar]
- 4.Habuda-Stanic M, Kules M, Kalajdzic B, Romic Z. Quality of groundwater in eastern Croatia. The problem of arsenic pollution. Desalination. 2007;210:157–62. doi: 10.1016/j.desal.2006.05.040. [DOI] [Google Scholar]
- 5.Santo V, Grgić J, Laslavić B, Dario M, Valek M. Concentration of arsenic, manganese and iron in drinking water of Osijek-Baranya County. In: 6th Symposium. Water and Public Water Supply; 2002 Oct 16-19; Mlini, Župa Dubrovačka, Croatia. Zagreb: Croatian National Institute of Public Health; 2002. [Google Scholar]
- 6.Angerer J, Ewers U, Wilhelm M. Human biomonitoring: state of the art. Int J Hyg Environ Health. 2007;210:201–28. doi: 10.1016/j.ijheh.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Bordeleau G, Martel R, Ampleman G, Thiboutot S. Environmental impacts of training activities at an air weapons range. J Environ Qual. 2008;37:308–17. doi: 10.2134/jeq2007.0197. [DOI] [PubMed] [Google Scholar]
- 8.Gulson BL, Palmer JM, Bryce A. Changes in blood lead of a recreational shooter. Sci Total Environ. 2002;293:143–50. doi: 10.1016/S0048-9697(02)00003-7. [DOI] [PubMed] [Google Scholar]
- 9.Tripathi RK, Sherertz PC, Llewellyn GC, Armstrong CW. Lead exposure in outdoor firearm instructors. Am J Public Health. 1991;81:753–5. doi: 10.2105/AJPH.81.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croatian Cleaner Production Center. Polychlorinated Biphenyls (PCBs) inventory in Croatia [in Croatian]. Available from: http://www.cro-cpc.hr/projekti/pops/PCBIzvjestaj.pdf Accessed: September 7, 2010.
- 11.Lowe J, Miller JM. Non-Governmental Organization (NGO) Committee on Disarmament, Peace and Security. The ABCs of disarmament, military, and the environment. 2008. Available from: http://disarm.igc.org/2009backup/paperenvtabc.php Accessed: September 7, 2010.
- 12.Plavsic F, Petrovecki M, Fuchs R, Sostaric B, Wolf-Coporda A, Romic Z, et al. The chemical war in Croatia. Lijec Vjesn. 1992;114:1–5. [in Croatian] [PubMed] [Google Scholar]
- 13.Soldo S, Puntaric D. Injuries in Croatian Army brigade soldiers inflicted in an offensive action during the 1991/1992 war in Croatia. Mil Med. 1998;163:420–2. [PubMed] [Google Scholar]
- 14.Kalinich JF, Emond CA, Dalton TK, Mog SR, Coleman GD, Kordell JE, et al. Embedded weapons-grade tungsten alloy shrapnel rapidly induces metastatic high-grade rhabdomyosarcomas in F344 rats. Environ Health Perspect. 2005;113:729–34. doi: 10.1289/ehp.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller AC, Xu J, Stewart M, Prasanna PG, Page N. Potential late health effects of depleted uranium and tungsten used in armor-piercing munitions: comparison of neoplastic transformation and genotoxicity with the known carcinogen nickel. Mil Med. 2002;167:120–2. [PubMed] [Google Scholar]
- 16.Miller AC, Brooks K, Smith J, Page N. Effect of the militarily-relevant heavy metals, depleted uranium and heavy metal tungsten-alloy on gene expression in human liver carcinoma cells (HepG2). Mol Cell Biochem. 2004;255:247–56. doi: 10.1023/B:MCBI.0000007280.72510.96. [DOI] [PubMed] [Google Scholar]
- 17.Bogadi-Šare A, Macan J, Pleština R, Turk R, Zavalić M. Chemical noxiousness [in Croatian]. In: Šarić M, Žuškin E, editors. Medicina rada i okoliša. Zagreb: Medicinska naklada; 2002. p. 129-275. [Google Scholar]
- 18.Nordberg GF, Fowler BA, Nordberg M, Friberg L, editors. Handbook on the toxicology of metals. 3rd ed. Burlington (MA): Academic Press Inc; 2007. [Google Scholar]
- 19.Vitale K, Marijanovic Rajcic M, Senta A. Waters in Croatia between practice and needs: public health challenge. Croat Med J. 2002;43:485–92. [PubMed] [Google Scholar]
- 20.Lazarus M, Vickovic I, Sostaric B, Blanusai M. Heavy metal levels in tissues of red deer (Cervus elaphus) from Eastern Croatia. Arh Hig Rada Toksikol. 2005;56:233–40. [PubMed] [Google Scholar]
- 21.Bacani A, Sparica M, Velic J. Quaternary deposits as the hydrogeological system of Eastern Slavonia. Geol Croat. 1999;52:141–52. [Google Scholar]
- 22.Ropac D, Milas J. Military casualties in Baranja and east Slavonia during the first 9 months of the war in Croatia. Mil Med. 1999;164:643–7. [PubMed] [Google Scholar]
- 23.Labar B, Rudan I, Ivankovic D, Biloglav Z, Mrsic M, Strnad M, et al. Haematological malignancies in childhood in Croatia: investigating the theories of depleted uranium, chemical plant damage and 'population mixing'. Eur J Epidemiol. 2004;19:55–60. doi: 10.1023/B:EJEP.0000013400.65418.60. [DOI] [PubMed] [Google Scholar]
- 24.Hirsl-Hecej V, Fattorini I. Children casualties in the war against Croatia. Croat Med J. 1992;33:26–33. [Google Scholar]
- 25.Judas M, Chudy D, Priscan A, Radonic N, Papic J, Marusic A, et al. Chronology of civilian suffering in the war against Croatia. Croat Med J. 1992;33:15–23. [PubMed] [Google Scholar]
- 26.Kujundzic M, Kern J, Ivankovic D, Budak A, Dragun D, Vuletic S. Person displacement pattern in Croatia. Croat Med J. 1992;33:55–60. [Google Scholar]
- 27.Wolf RE, Denoyer E, Grosser ZUS. EPA method 2008 for the analysis of drinking waters and wastewaters. In: Application Note ENVA-300B: Norwalk (CT): Perkin Elmer Inc; 2001. p. 1-12. [Google Scholar]
- 28.Artusi R, Verderio P, Marubini E. Bravais-Pearson and Spearman correlation coefficients: meaning, test of hypothesis and confidence interval. Int J Biol Markers. 2002;17:148–51. doi: 10.1177/172460080201700213. [DOI] [PubMed] [Google Scholar]
- 29.Chojnacka K, Zielinska A, Gorecka H, Dobrzanski Z, Gorecki H. Reference values for hair minerals of Polish students. Environ Toxicol Pharmacol. 2010;29:314–9. doi: 10.1016/j.etap.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Heitland P, Koster HD. Biomonitoring of 30 trace elements in urine of children and adults by ICP-MS. Clin Chim Acta. 2006;365:310–8. doi: 10.1016/j.cca.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Alimonti A, Bocca B, Mannella E, Petrucci F, Zennaro F, Cotichini R, et al. Assessment of reference values for selected elements in a healthy urban population. Ann Ist Super Sanita. 2005;41:181–7. [PubMed] [Google Scholar]
- 32.Goulle JP, Mahieu L, Castermant J, Neveu N, Bonneau L, Laine G, et al. Metal and metalloid multi-elementary ICP-MS validation in whole blood, plasma, urine and hair. Reference values. Forensic Sci Int. 2005;153:39–44. doi: 10.1016/j.forsciint.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 33.Barany E, Bergdahl IA, Bratteby LE, Lundh T, Samuelson G, Schutz A, et al. Trace element levels in whole blood and serum from Swedish adolescents. Sci Total Environ. 2002;286:129–41. doi: 10.1016/S0048-9697(01)00970-6. [DOI] [PubMed] [Google Scholar]
- 34.Fiket Z, Roje V, Mikac N, Kniewald G. Determination of arsenic and other trace elements in bottled waters by high resolution inductively coupled plasma mass spectrometry. Croat Chem Acta. 2007;80:91–100. [Google Scholar]
- 35.Holmes L. Determination of thorium by ICP-MS and ICP-OES. Radiat Prot Dosimetry. 2001;97:117–22. doi: 10.1093/oxfordjournals.rpd.a006647. [DOI] [PubMed] [Google Scholar]
- 36.McCarty KM, Chen YC, Quamruzzaman Q, Rahman M, Mahiuddin G, Hsueh YM, et al. Arsenic methylation, GSTT1, GSTM1, GSTP1 polymorphisms, and skin lesions. Environ Health Perspect. 2007;115:341–5. doi: 10.1289/ehp.9152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reimann C, Bjorvatn K, Frengstad B, Melaku Z, Tekle-Haimanot R, Siewers U. Drinking water quality in the Ethiopian section of the East African Rift Valley I – data and health aspects. Sci Total Environ. 2003;311:65–80. doi: 10.1016/S0048-9697(03)00137-2. [DOI] [PubMed] [Google Scholar]
- 38.Lang S. From Gulf War Syndrome to Balkan War Syndrome. Croat Med J. 2001;42:205–9. [PubMed] [Google Scholar]
- 39.Dmitrović B, Kurbel S, Margaretić D, Blažičević V, Vuletić T. The war and its influence on the malignant tumors incidence. Med Glas. 2006;3:26–9. [in Croatian] [Google Scholar]
- 40.Sutton M, Warwick P, Hall A, Jones C. Carbonate induced dissolution of uranium containing precipitates under cement leachate conditions. J Environ Monit. 1999;1:177–82. doi: 10.1039/a809262a. [DOI] [PubMed] [Google Scholar]
- 41.Cavar S, Klapec T, Grubesic RJ, Valek M. High exposure to arsenic from drinking water at several localities in eastern Croatia. Sci Total Environ. 2005;339:277–82. doi: 10.1016/j.scitotenv.2004.12.013. [DOI] [PubMed] [Google Scholar]