Abstract
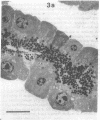
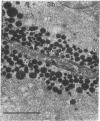
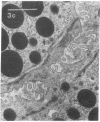
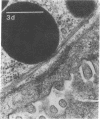
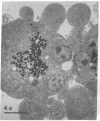
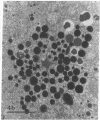
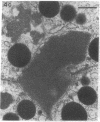
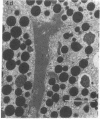
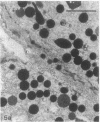
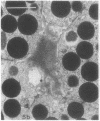
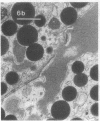
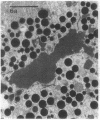
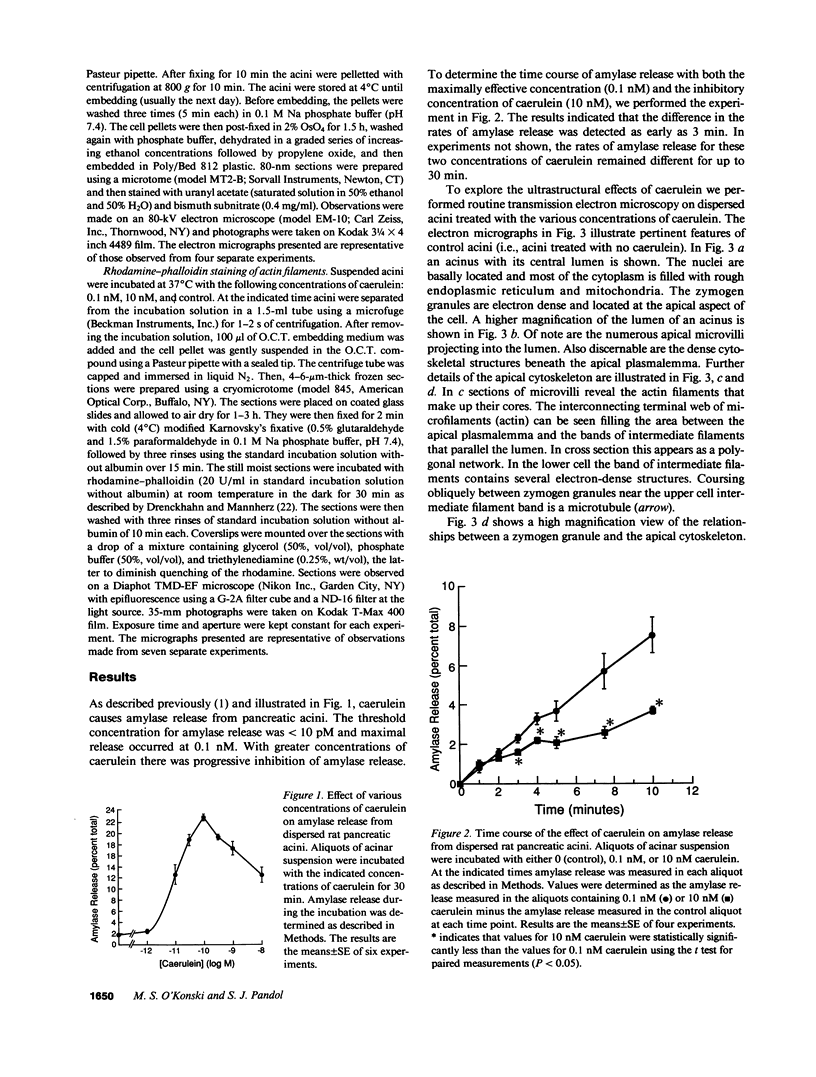
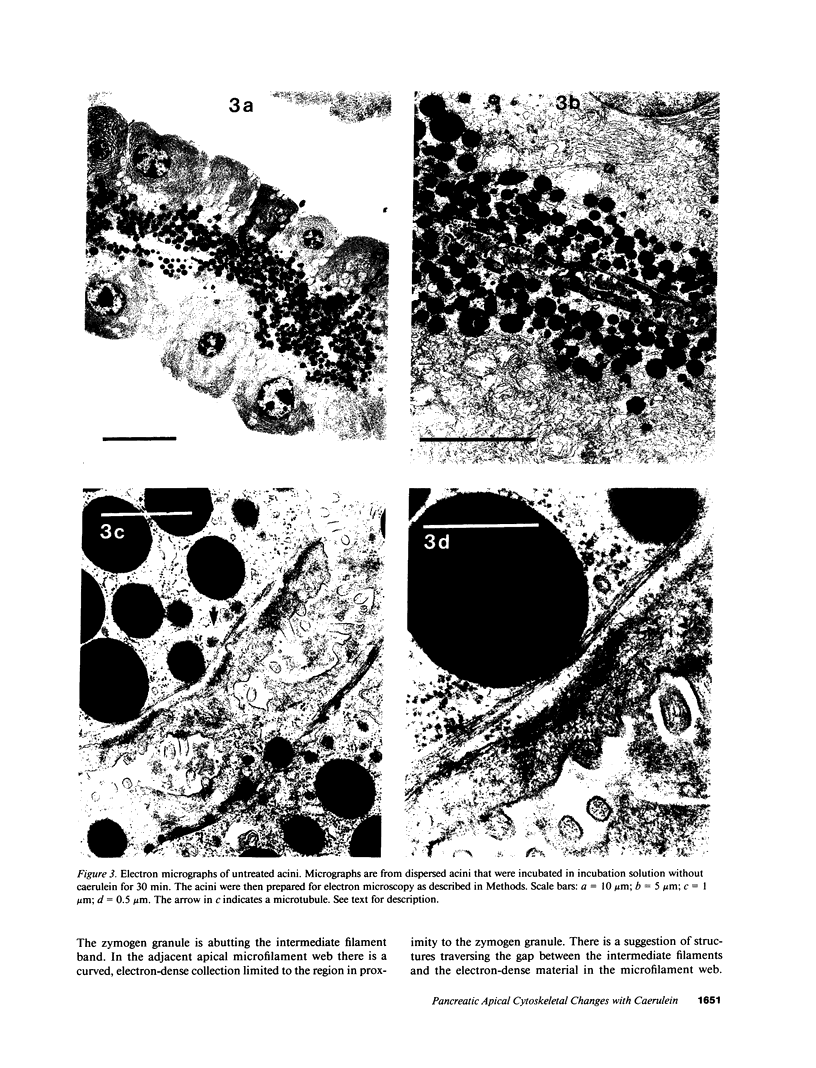
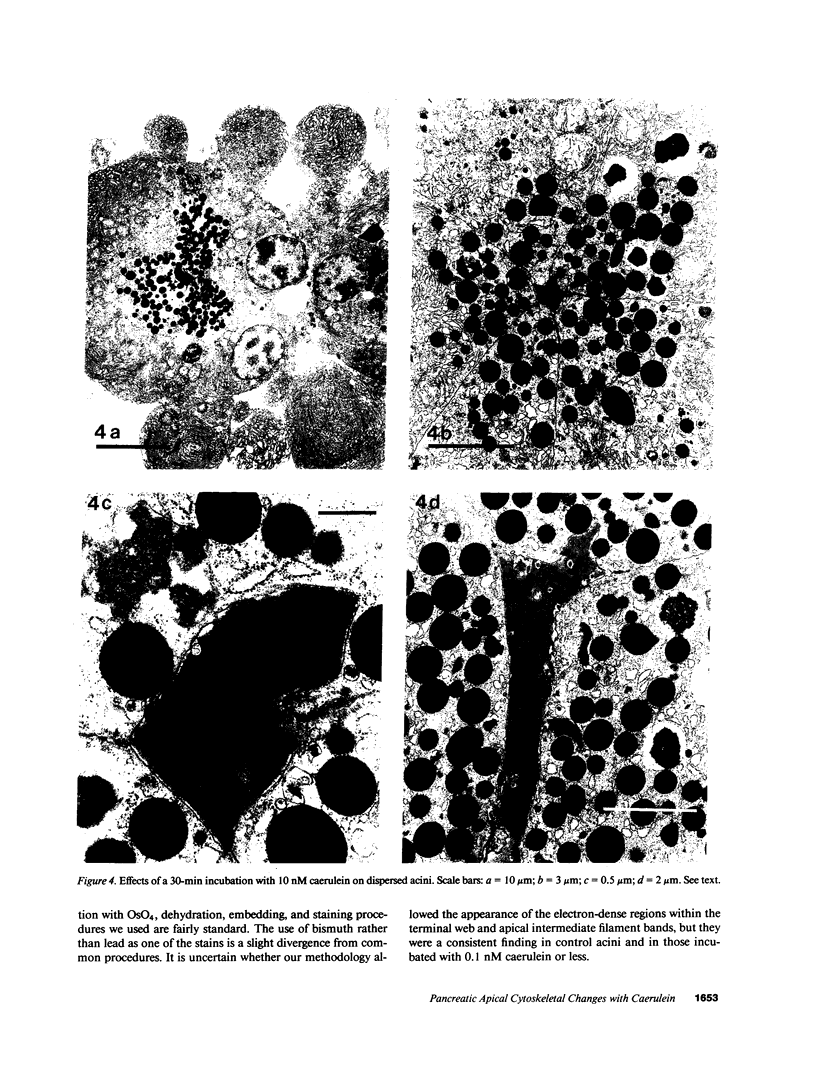
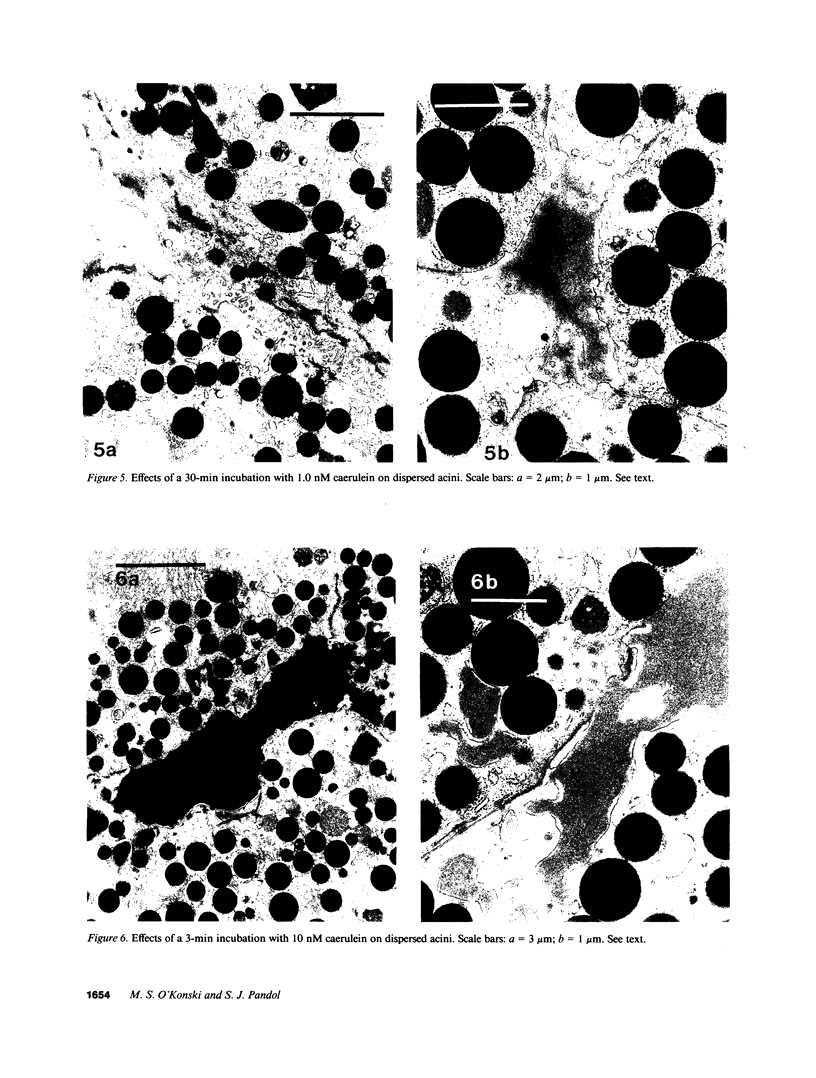
In this study experiments were performed to correlate the rate of digestive enzyme secretion to morphologic observations of the apical cytoskeleton using dispersed rat pancreatic acini with various concentrations of caerulein. Caerulein at concentrations of 10 pM to 0.1 nM stimulated increasing rates of secretion of amylase, a digestive enzyme. Greater concentrations of caerulein caused progressively less amylase secretion. Transmission electron microscopy demonstrated several characteristics of the apical cytoskeleton in untreated acini that were altered with the "inhibitory" concentrations of caerulein. In control acini and acini stimulated with concentrations of caerulein up to 0.1 nM, the micrographs reveal an apical actin network extending into microvilli, an intermediate filament band, and electron-dense structures contained in both the actin filament network and the intermediate filament band. With concentrations of caerulein greater than 0.1 nM, these structures were progressively ablated. The findings with respect to the actin filament network were confirmed with light microscopic observations of dispersed acini stained with rhodamine-phalloidin. These results indicate that caerulein has marked morphologic effects on the pancreatic acinar cell cytoskeleton and that the cytoskeletal changes may modulate the secretory response.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler G., Kern H. F., Pan G. Z., Gardner J. D. Secretagogue-induced membrane alterations in dispersed acini from rat pancreas. Eur J Cell Biol. 1984 Mar;33(2):234–241. [PubMed] [Google Scholar]
- Bruzzone R., Pozzan T., Wollheim C. B. Caerulein and carbamoylcholine stimulate pancreatic amylase release at resting cytosolic free Ca2+. Biochem J. 1986 Apr 1;235(1):139–143. doi: 10.1042/bj2350139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J., Coluccio L. M. Kinetic analysis of F-actin depolymerization in the presence of platelet gelsolin and gelsolin-actin complexes. J Cell Biol. 1985 Oct;101(4):1236–1244. doi: 10.1083/jcb.101.4.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J., Kurth M. C. Actin-gelsolin interactions. Evidence for two actin-binding sites. J Biol Chem. 1984 Jun 25;259(12):7480–7487. [PubMed] [Google Scholar]
- Burnham D. B., Williams J. A. Effects of high concentrations of secretagogues on the morphology and secretory activity of the pancreas: a role for microfilaments. Cell Tissue Res. 1982;222(1):201–212. doi: 10.1007/BF00218300. [DOI] [PubMed] [Google Scholar]
- Dixon J. F., Hokin L. E. Secretogogue-stimulated phosphatidylinositol breakdown in the exocrine pancreas liberates arachidonic acid, stearic acid, and glycerol by sequential actions of phospholipase C and diglyceride lipase. J Biol Chem. 1984 Dec 10;259(23):14418–14425. [PubMed] [Google Scholar]
- Drenckhahn D., Mannherz H. G. Distribution of actin and the actin-associated proteins myosin, tropomyosin, alpha-actinin, vinculin, and villin in rat and bovine exocrine glands. Eur J Cell Biol. 1983 May;30(2):167–176. [PubMed] [Google Scholar]
- Gardner J. D., Jackson M. J. Regulation of amylase release from dispersed pancreatic acinar cells. J Physiol. 1977 Sep;270(2):439–454. doi: 10.1113/jphysiol.1977.sp011961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOKIN L. E., HOKIN M. R. Phosphoinositides and protein secretion in pancreas slices. J Biol Chem. 1958 Oct;233(4):805–810. [PubMed] [Google Scholar]
- HOKIN M. R., HOKIN L. E. Effects of acetylcholine on phospholipides in the pancreas. J Biol Chem. 1954 Aug;209(2):549–558. [PubMed] [Google Scholar]
- HOKIN M. R., HOKIN L. E. Enzyme secretion and the incorporation of P32 into phospholipides of pancreas slices. J Biol Chem. 1953 Aug;203(2):967–977. [PubMed] [Google Scholar]
- Halenda S. P., Rubin R. P. Phospholipid turnover in isolated rat pancreatic acini. Consideration of the relative roles of phospholipase A2 and phospholipase C. Biochem J. 1982 Dec 15;208(3):713–721. doi: 10.1042/bj2080713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokin-Neaverson M. Acetylcholine causes a net decrease in phosphatidylinositol and a net increase in phosphatidic acid in mouse pancreas. Biochem Biophys Res Commun. 1974 Jun 4;58(3):763–768. doi: 10.1016/s0006-291x(74)80483-3. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Chaponnier C., Lind S. E., Zaner K. S., Stossel T. P., Yin H. L. Interactions of gelsolin and gelsolin-actin complexes with actin. Effects of calcium on actin nucleation, filament severing, and end blocking. Biochemistry. 1985 Jul 2;24(14):3714–3723. doi: 10.1021/bi00335a046. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Iida K., Yin H. L., Stossel T. P. Polyphosphoinositide micelles and polyphosphoinositide-containing vesicles dissociate endogenous gelsolin-actin complexes and promote actin assembly from the fast-growing end of actin filaments blocked by gelsolin. J Biol Chem. 1987 Sep 5;262(25):12228–12236. [PubMed] [Google Scholar]
- Janmey P. A., Stossel T. P. Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature. 1987 Jan 22;325(6102):362–364. doi: 10.1038/325362a0. [DOI] [PubMed] [Google Scholar]
- Jensen R. T., Lemp G. F., Gardner J. D. Interaction of cholecystokinin with specific membrane receptors on pancreatic acinar cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2079–2083. doi: 10.1073/pnas.77.4.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampel M., Kern H. F. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977 Mar 11;373(2):97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature. 1985 Apr 4;314(6010):472–474. doi: 10.1038/314472a0. [DOI] [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specificity of the interaction between phosphatidylinositol 4,5-bisphosphate and the profilin:actin complex. J Cell Biochem. 1988 Jul;37(3):255–267. doi: 10.1002/jcb.240370302. [DOI] [PubMed] [Google Scholar]
- Merritt J. E., Rubin R. P. Pancreatic amylase secretion and cytoplasmic free calcium. Effects of ionomycin, phorbol dibutyrate and diacylglycerols alone and in combination. Biochem J. 1985 Aug 15;230(1):151–159. doi: 10.1042/bj2300151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S., Schoeffield M., Pandol S., Sachs G. Inositol trisphosphate modification of ion transport in rough endoplasmic reticulum. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4433–4437. doi: 10.1073/pnas.82.13.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs D. L., Korenbrot J. I., Williams J. A. Relation between free cytosolic calcium and amylase release by pancreatic acini. Am J Physiol. 1985 Sep;249(3 Pt 1):G389–G398. doi: 10.1152/ajpgi.1985.249.3.G389. [DOI] [PubMed] [Google Scholar]
- Orchard J. L., Davis J. S., Larson R. E., Farese R. V. Effects of carbachol and pancreozymin (cholecystokinin-octapeptide) on polyphosphoinositide metabolism in the rat pancreas in vitro. Biochem J. 1984 Jan 1;217(1):281–287. doi: 10.1042/bj2170281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Gabbay K. H., Malaisse W. J. Pancreatic beta-cell web: its possible role in insulin secretion. Science. 1972 Mar 10;175(4026):1128–1130. doi: 10.1126/science.175.4026.1128. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Pandol S. J., Jensen R. T., Gardner J. D. Mechanism of [Tyr4]bombesin-induced desensitization in dispersed acini from guinea pig pancreas. J Biol Chem. 1982 Oct 25;257(20):12024–12029. [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield M. S. 1,2-Diacylglycerol, protein kinase C, and pancreatic enzyme secretion. J Biol Chem. 1986 Apr 5;261(10):4438–4444. [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield M. S., Sachs G., Muallem S. Role of free cytosolic calcium in secretagogue-stimulated amylase release from dispersed acini from guinea pig pancreas. J Biol Chem. 1985 Aug 25;260(18):10081–10086. [PubMed] [Google Scholar]
- Pandol S. J., Thomas M. W., Schoeffield M. S., Sachs G., Muallem S. Role of calcium in cholecystokinin-stimulated phosphoinositide breakdown in exocrine pancreas. Am J Physiol. 1985 May;248(5 Pt 1):G551–G560. doi: 10.1152/ajpgi.1985.248.5.G551. [DOI] [PubMed] [Google Scholar]
- Peikin S. R., Rottman A. J., Batzri S., Gardner J. D. Kinetics of amylase release by dispersed acini prepared from guinea pig pancreas. Am J Physiol. 1978 Dec;235(6):E743–E749. doi: 10.1152/ajpendo.1978.235.6.E743. [DOI] [PubMed] [Google Scholar]
- Powers R. E., Johnson P. C., Houlihan M. J., Saluja A. K., Steer M. L. Intracellular Ca2+ levels and amylase secretion in Quin 2-loaded mouse pancreatic acini. Am J Physiol. 1985 May;248(5 Pt 1):C535–C541. doi: 10.1152/ajpcell.1985.248.5.C535. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr, Burgess G. M., Halenda S. P., McKinney J. S., Rubin R. P. Effects of secretagogues on [32P]phosphatidylinositol 4,5-bisphosphate metabolism in the exocrine pancreas. Biochem J. 1983 May 15;212(2):483–488. doi: 10.1042/bj2120483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. P., Godfrey P. P., Chapman D. A., Putney J. W., Jr Secretagogue-induced formation of inositol phosphates in rat exocrine pancreas. Implications for a messenger role for inositol trisphosphate. Biochem J. 1984 Apr 15;219(2):655–659. doi: 10.1042/bj2190655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savion N., Selinger Z. Morphological changes in rat pancreatic slices associated with inhibition of enzyme secretion by high concentrations of secretagogues. J Cell Biol. 1978 Feb;76(2):467–482. doi: 10.1083/jcb.76.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. V. Organization of actin in the leading edge of cultured cells: influence of osmium tetroxide and dehydration on the ultrastructure of actin meshworks. J Cell Biol. 1981 Dec;91(3 Pt 1):695–705. doi: 10.1083/jcb.91.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock C., Launay J. F., Grenier J. F., Bauduin H. Pancreatic acinar cell changes induced by caerulein, vinblastine, deuterium oxide, and cytochalasin B in vitro. Lab Invest. 1978 Feb;38(2):157–164. [PubMed] [Google Scholar]
- Streb H., Heslop J. P., Irvine R. F., Schulz I., Berridge M. J. Relationship between secretagogue-induced Ca2+ release and inositol polyphosphate production in permeabilized pancreatic acinar cells. J Biol Chem. 1985 Jun 25;260(12):7309–7315. [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Watanabe O., Baccino F. M., Steer M. L., Meldolesi J. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am J Physiol. 1984 Apr;246(4 Pt 1):G457–G467. doi: 10.1152/ajpgi.1984.246.4.G457. [DOI] [PubMed] [Google Scholar]
- Williams J. A. Effects of cytochalasin B on pancreatic acinar cell structure and secretion. Cell Tissue Res. 1977 Apr 29;179(4):453–466. doi: 10.1007/BF00219848. [DOI] [PubMed] [Google Scholar]
- Wulf E., Deboben A., Bautz F. A., Faulstich H., Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4498–4502. doi: 10.1073/pnas.76.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. L., Iida K., Janmey P. A. Identification of a polyphosphoinositide-modulated domain in gelsolin which binds to the sides of actin filaments. J Cell Biol. 1988 Mar;106(3):805–812. doi: 10.1083/jcb.106.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. L., Stossel T. P. Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature. 1979 Oct 18;281(5732):583–586. doi: 10.1038/281583a0. [DOI] [PubMed] [Google Scholar]