Abstract
This report describes formation of a full-thickness macular hole subsequent to an injection of bevacizumab for the treatment of neovascular AMD. This complication may be caused by focal tractional forces on the retinal surface due to either vitreous incarceration at the injection site or contraction of the choroidal neovascularization membrane. Alternatively, it may be due to a toxic effect of bevacizumab on a previously compromised retina.
Key Words: Age-related macular degeneration, Bevacizumab, Choroidal neovascularization, Macular hole, Intravitreal injection
Introduction
Age-related macular degeneration (AMD) is the leading cause of irreversible severe central visual acuity loss in people over 50 years of age in the developed world [1]. Anti-angiogenic drugs have become increasingly popular for the treatment of choroidal neovascularization (CNV) that occurs in a variety of conditions, such as AMD, diabetic retinopathy, retinal venous occlusive disease, high myopia, trauma, and others [2]. Current research has focused on anti-vascular endothelial growth factor (VEGF) drugs, most notably ranibizumab (LucentisTM), which has been approved by the FDA for use in ocular disease. Bevacizumab (AvastinTM) is a recombinant humanized monoclonal antibody that binds VEGF and inhibits its interaction with endothelial receptors. Due to its relatively lower cost, it has been widely used ‘off-label’ in recent years for the aforementioned indications [3].
There are no large-scale, long-term randomized controlled studies on the safety and efficacy of bevacizumab [3], although several studies have focused on its potential complications and adverse effects. Wong et al. [2] reported a very low rate of ocular adverse effects after intravitreal bevacizumab injection, such as retinal pigment epithelium (RPE) tears, retinal ischemia, ocular irritation and cataract progression. Other potential adverse effects are very rare, and include retinal tears, retinal detachment, endophthalmitis and intraocular inflammation. Macular hole formation after intravitreal injections are very rare and have been described only in a small number of case reports [4,5,6,7].
There is a general consensus that adverse effects after intravitreal injections are more likely caused by the injection rather than by the administered medication. A number of studies have shown that the most common adverse effect related to a bevacizumab injection is the formation of RPE tears which occur in 0.8-2.9% of cases and mostly in eyes with neovascular AMD and pigment epithelium detachment (PED) [2, 3, 8]. It should be noted, however, that such tears have also been reported to occur following photodynamic therapy as well as spontaneously [9].
We present a patient with neovascular AMD and PED who developed a full-thickness macular hole after an intravitreal injection of bevacizumab in his right eye.
Case Report
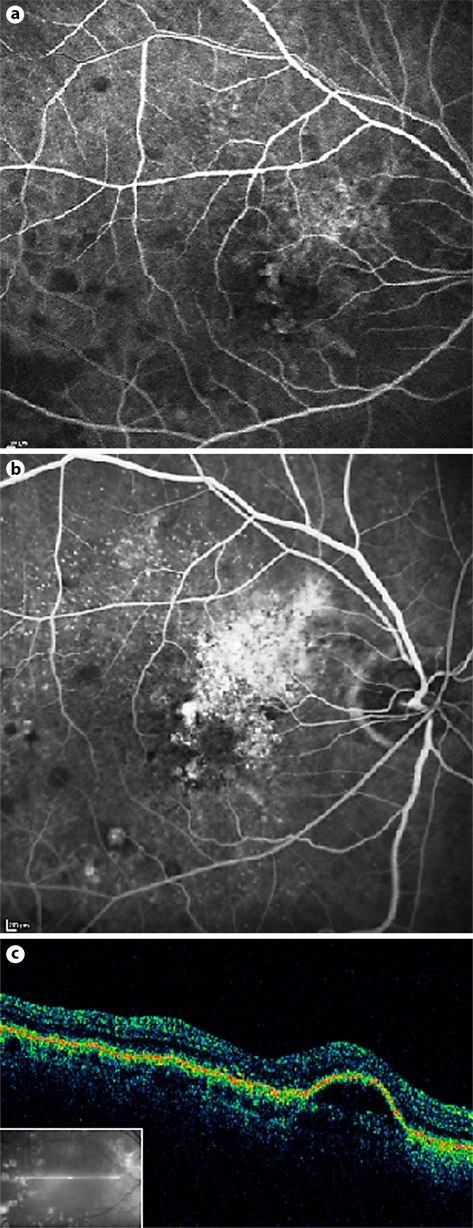
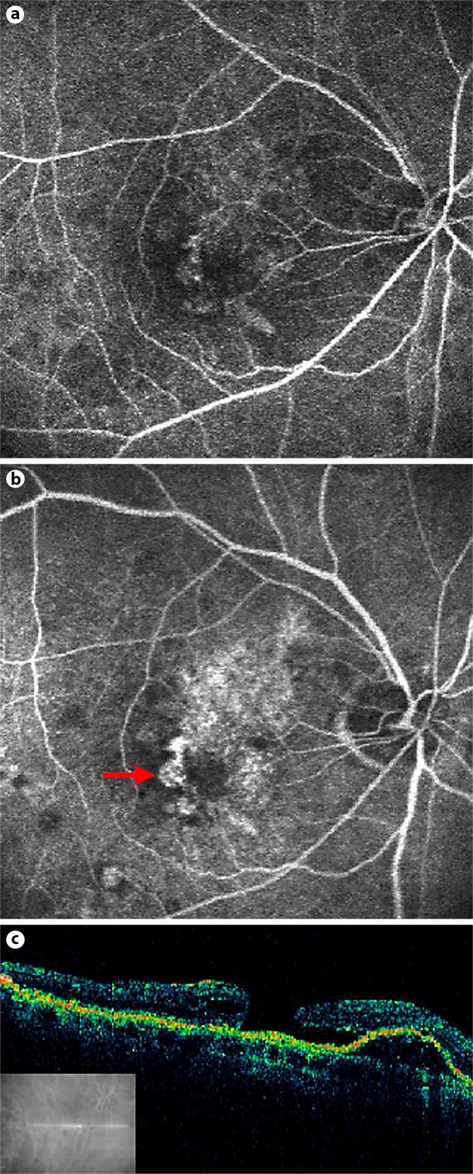
An 86-year-old man was referred to our clinic for treatment of subfoveal CNV and PED in his right eye due to neovascular AMD. His left eye had previously suffered from neovascular AMD, and its visual acuity was reduced to bare light perception due to large subretinal and choroidal hemorrhages following photodynamic therapy treatment for CNV several years earlier. His right eye had been previously treated 6 times with intravitreal bevacizumab after which there were no untoward sequelae. Upon presentation, the visual acuity of the affected eye was 20/40. The initial fluorescein angiographic and optical coherence tomographic (OCT) images (fig. 1) demonstrate early subfoveal hyperfluorescence with staining in the later phases, confirming a still active CNV, retinal pigment elevation, the presence of subretinal fluid and an area of hyper-reflectivity. It is important to note that OCT demonstrated the presence of posterior vitreous detachment. After discussing the available treatment options and their potential benefits and side effects, the patient gave his written informed consent to undergo the recommended intravitreal bevacizumab injection (1.25 mg/0.05 ml). One month after the injection, his visual acuity had worsened to 20/60. Fluorescein angiography demonstrated leakage from the partially fibrotic subfoveal CNV, as well as a round area of hyperfluorescence compatible with a full-thickness macular hole (fig. 2a, b). OCT demonstrated persistence of the PED, a small amount of subfoveal subretinal fluid, and a new grade 4 macular hole (fig. 2). The possibility of surgical repair of the macular hole was discussed, and the patient chose not to undergo any operation in his only functional eye.
Fig. 1.
Initial fluorescein angiograph demonstrating early subfoveal hyperfluorescence (a) with staining in the later phases (b), confirming a still active CNV and retinal pigment elevation, the presence of subretinal fluid and an area of hyperreflectivity. Initial optical coherence tomography demonstrating PED and no macular hole (c).
Fig. 2.
Fluorescein angiograph at one month after an intravitreal bevacizumab injection into the eye described in figure 1, demonstrating early subfoveal hyperfluorescence (a) and in later phases dye leakage consistent with a partially fibrotic subfoveal CNV, and an area of hyperfluorescence compatible with a full-thickness macular hole (arrow) (b). c OCT at one month after intravitreal bevacizumab injection demonstrating persistence of the PED and a small amount of subfoveal subretinal fluid, as well as a new grade 4 macular hole.
Discussion
We report a patient with neovascular AMD and PED who developed a full-thickness macular hole following the 7th intravitreal bevacizumab injection to his right eye. Our review of the literature revealed only 3 publications describing a similar occurrence. The first described a patient with neovascular AMD with subfoveal CNV and vitreomacular traction who developed a stage 2 macular hole [5]. The second described a patient with myopic CNV who developed a retinal detachment with a macular hole [6]. The third described a patient with severe diabetic proliferative retinopathy who developed a retinal detachment with a macular hole [7]. To the best of our knowledge, this is the first description of a macular hole forming subsequent to bevacizumab injection for the treatment of neovascular AMD.
Several mechanisms may have caused the macular hole in our patient. Contraction of the prefoveal vitreous cortex results in focal traction which may cause macular hole formation, as hypothesized by Gass [10]. Vitreous incarceration at the site of injection may have caused such traction. However, as posterior vitreous detachment was demonstrated in the affected eye, tractional forces causing the formation of the macular hole are less likely in this case. The macular hole may be attributed to the pharmacological effect of bevacizumab on the CNV, since it can cause contraction resulting in traction on the retinal surface. Alternatively, it is possible that the macular hole was caused by toxicity of bevacizumab on a previously compromised retina. This explanation is least likely as several studies found no toxic effect of bevacizumab on the retina and RPE, even after repeated injections [11, 12]. Another possible explanation is that the macular hole is the result of progressive retinal and RPE atrophy in a patient with AMD.
In conclusion, macular holes may be a rare adverse effect of bevacizumab injection, whether mechanical or toxic. Patients need to be warned about this potential complication, and it should be included in the differential diagnosis when visual acuity does not improve or if it deteriorates after treatment with bevacizumab.
References
- 1.Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004;291:1900–1901. doi: 10.1001/jama.291.15.1900. [DOI] [PubMed] [Google Scholar]
- 2.Wong LJ, Desai RU, Jain A, et al. Surveillance for potential adverse events associated with the use of intravitreal bevacizumab for retinal and choroidal vascular disease. Retina. 2008;28:1151–1158. doi: 10.1097/IAE.0b013e31817e100f. [DOI] [PubMed] [Google Scholar]
- 3.Garg S, Brod R, Kim D, Lane RG, Maguire J, Fischer D. Retinal pigment epithelial tears after intravitreal bevacizumab injection for exudative age-related macular degeneration. Clin Exp Ophthalmol. 2008;36:252–256. doi: 10.1111/j.1442-9071.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- 4.Georgopoulos M, Polak K, Prager F, Prünte C, Schmidt-Erfurth U. Characteristics of severe intraocular inflammation following intravitreal injection of bevacizumab (Avastin) Br J Ophthalmol. 2009;93:457–462. doi: 10.1136/bjo.2008.138479. [DOI] [PubMed] [Google Scholar]
- 5.Querques G, Souied EH, Soubrane G. Macular hole following intravitreal ranibizumab injection for choroidal neovascular membrane caused by age-related macular degeneration. Acta Ophthalmol. 2009;87:235–237. doi: 10.1111/j.1755-3768.2008.01226.x. [DOI] [PubMed] [Google Scholar]
- 6.Chung Ej, Koh HJ. Retinal detachment with macular hole following combined photodynamic therapy and intravitreal bevacizumab injection. Korean J Ophthalmol. 2007;21:185–187. doi: 10.3341/kjo.2007.21.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitamura Y, Ogata K, Oshitari T, Asaumi N, Yamamoto S. Retinal detachment with macular hole following intravitreal bevacizumab in patient with severe proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92:717–718. doi: 10.1136/bjo.2008.139378. [DOI] [PubMed] [Google Scholar]
- 8.Chan CK, Meyer CH, Gross JG, et al. Retinal pigment epithelial tears after intravitreal bevacizumab injection for neovascular age-related macular degeneration. Retina. 2007;27:541–551. doi: 10.1097/IAE.0b013e3180cc2612. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein M, Heilweil G, Barak A, Loewenstein A. Retinal pigment epithelial tear following photodynamic therapy for choroidal neovascularization secondary to AMD. Eye. 2005;19:1315–1324. doi: 10.1038/sj.eye.6701765. [DOI] [PubMed] [Google Scholar]
- 10.Gass JD. Idiopathic senile macular holes: its early stages and pathogenesis. Arch Ophthalmol. 1988;117:744–751. doi: 10.1001/archopht.1988.01060130683026. [DOI] [PubMed] [Google Scholar]
- 11.Brar VS, Sharma RK, Murthy RK, Chalam KV. Evaluation of differential toxicity of varying doses of bevacizumab on retinal ganglion cells, retinal pigment epithelial cells, and vascular endothelial growth factor-enriched choroidal endothelial cells. J Ocul Pharmacol Ther. 2009;25:507–511. doi: 10.1089/jop.2009.0028. [DOI] [PubMed] [Google Scholar]
- 12.Zayit-Soudry S, Zemel E, Loewenstein A, Perlman I. Safety evaluation of repeated intravitreal injections of bevacizumab and ranibizumab in rabbit eyes. Retina. 2010;30:671–681. doi: 10.1097/IAE.0b013e3181c0858c. [DOI] [PubMed] [Google Scholar]