Abstract
This unit describes techniques and approaches that can be used to study the functions of the ADP-ribosylation factor (Arf) GTP-binding proteins in cells. There are 6 mammalian Arfs and many more Arf-like proteins (Arls) and these proteins are conserved in eukaryotes from yeast to man. Like all GTPases, Arfs cycle between GDP-bound, inactive and GTP-bound active conformations, facilitated by guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs) that catalyze GTP binding and hydrolysis respectively. Here we describe approaches that can be taken to examine the localization and function of Arf and Arl proteins in cells (Protocol 1). We also provide a simple protocol for measuring activation (GTP-binding) of specific Arf proteins in cells using a pull-down assay (Protocol 2). We then discuss approaches that can be taken to assess function of GEFs and GAPs in cells (Protocol 3).
Key terms: Arf, GTP-binding proteins, guanine nucleotide exchange factors, GTPase activating proteins
INTRODUCTION
The ADP-ribosylation factor (Arf) family of guanine nucleotide binding proteins were originally identified and named for their ability to act as cofactors during cholera toxin-catalyzed ADP-ribosylation of Gas. Subsequently, 6 mammalian and 3 yeast Arfs proteins were identified and Arf1 was found to specifically associate with the Golgi complex and regulate vesicle trafficking between the endoplasmic reticulum (ER) and Golgi (D’Souza-Schorey and Chavrier, 2006; Donaldson and Jackson, 2000). Arfs are conserved 175–183 amino acid G proteins that have defined and nearly identical Switch I and Switch II effector domains. All Arfs are co-translationally myristoylated at the amino terminus and this modification is required for Arfs to interact with membrane surfaces, regulators and effectors. Thus, addition of epitope tags onto Arf proteins must be made at the carboxyl terminus to retain biological activity. Arfs 1–5 reversibly associate with the Golgi complex and cycle into the cytosol during GTP-binding and GTP-hydrolysis, respectively. Arf6, on the other hand, appears to spend more time associated with membranes while GDP-bound.
In their active, GTP-bound form Arfs modify membrane surfaces by recruitment of coat proteins that sort membrane proteins into forming vesicles, and by stimulating the activity of lipid modifying enzymes (phosphatidylinositol 4-phosphate 5-kinase and phospholipase D) that lead to production of phosphatidylinositol 4,5-bisphosphate and phosphatidic acid, respectively (D’Souza-Schorey and Chavrier, 2006; Donaldson and Jackson, 2000). Through these activities Arfs mediate membrane trafficking in cells. Most of the studies on Arfs have concerned the function of Arf1 at the Golgi complex and Arf6 at the plasma membrane in standard tissue culture cell lines. Specific localization and functions for the other Arf proteins and assessment of Arf function in specialized cells and tissues have yet to be determined. The first protocol describes ways to examine localization and function of Arf or Arl proteins in cells in culture. Cells expressing wild type or mutant Arfs can be examined for effects on a cellular function of interest. If an Arf is involved in a particular physiological process or membrane trafficking step, it is often desirable to be able to determine what fraction of a particular Arf is in the active, GTP-bound state. The second protocol describes a simple assay to determine the amount of active Arf in cells.
Arf proteins cycle between GTP-bound, active and GDP-bound, inactive forms. This cycle is regulated by GEFs that catalyze GDP release and GTP binding, and GAPs that catalyze GTP hydrolysis and return to the GDP-bound form. There are over 15 GEFs and 24 GAPs expressed in mammalian cells, and although we know the Arf specificities and functions for some of these regulators, for many their specificities, localization and function have not been evaluated. There are several families of Arf GEFs whose members all share the catalytic Sec7 domain and then differ in other domains. The BIG and GBF GEFs are large proteins that are localized to the Golgi and sensitive to inhibition by the drug brefeldin A (BFA). The ARNO/Cytohesin, EFA6, and BRAG families of Arf GEFs localize to the cytosol and PM, and are not inhibited by BFA (Casanova, 2007). There are also many Arf GAP families of proteins that contain the catalytic Arf GAP domain. Arf GAPs also contain many other domains that function to regulate and recruit the GAP to membranes as well as domains that serve as scaffolding platforms to recruit other Arf effectors. In this sense, Arf GAPs may be effectors as well as signal terminators (Inoue and Randazzo, 2007). Since there are many more GEFs and GAPs than there are Arfs, there is much to learn about the function of these proteins and we suggest approaches to assessing their cellular function in Protocol 3.
Strategic Planning
Although the Arf proteins are ubiquitously expressed, high affinity, specific antibodies to examine the localization and function of endogenous proteins are not available. Hence, the transient expression of Arf proteins is a useful tool for examining Arf function in cells. Expression of the wild type Arf protein is a reasonable way to determine how an Arf localizes and behaves in a cell since expression of the wild type protein, especially at low levels, does not usually cause a strong phenotype by itself, presumably because the Arf can cycle between GDP-bound inactive and GTP-bound active forms. As mentioned earlier, due to the importance of the amino terminal myristoyl group for biological function, epitope tags, if used, should be placed at the carboxyl terminus of an Arf protein. A number of tags have been appended to the carboxyl terminus of Arf proteins including peptide tags like HA, myc, FLAG®, and also fluorescent proteins like green fluorescent protein (GFP) and red fluorescent proteins (RFP). Small epitope tags are not benign, however, and in many cases are highly charged. Thus, it is important to consider the consequence of each tag when examining Arf function in cells. Although antibodies are not available that can detect endogenous Arfs, which might be expressed at low levels, commercial antibodies are available that can detect over-expressed Arf proteins (in particular for Arf1 and Arf6); thus the behavior of an epitope-tagged over-expressed Arf protein can be compared to that of an untagged over-expressed Arf protein.
When over-expressed, Arfs1–5 typically have a prominent localization at the Golgi complex that can be verified by co-staining cells with antibodies to Golgi resident proteins. Arfs1–5 also have a substantial cytosolic pool, which represents an inactive pool of the molecules. Because Arfs1–5 are released into the cytosol upon GTP hydrolysis, it is important to realize that they can be recruited to extra-Golgi sites as part of their activation cycle. Indeed there are reports of Arf1 at the PM and on peripheral endosomal structures. Arf6 is not localized to the Golgi, and instead localizes to the plasma membrane, and on endosomal structures. In some cells, like HeLa cells, Arf6 is mostly localized on membranes, whereas in other cells types Arf6 constructs show a significant cytosolic pool. The cytosolic pool can be considered inactive, but Arf6 can localize to membrane regardless of its GTP/GDP status, so it cannot be assumed that all Arf6 localized to membranes is GTP bound.
A useful pharmacological tool for examining Arf function in cells is the fungal metabolite BFA. BFA is an uncompetitive inhibitor of certain Arf GEFs, stabilizing a complex of the GEF with GDP-bound Arf. In this way it inhibits activation of Arfs by that GEF. BFA inhibits Arf activation catalyzed by GBF and BIG family GEFs that contain the residues necessary to form this complex whereas other Arf GEFs, such as the Cytohesin/ARNO and EFA6 families, lack these residues. An Arf-dependent process that is BFA sensitive would point to activation of the Arf through GBF1 or BIG family exchange factors. An Arf-dependent process that is not BFA-sensitive would suggest activation of Arf through other GEFs. Treating cells with BFA results in a rapid release of Arf1 and Golgi-associated coat proteins from the Golgi, reflecting a block in activation of Arf at the Golgi. At later times of BFA treatment, Golgi membranes and content fuse with the ER and in some cells an alteration of endosomal and lysosomal membrane morphology is also observed.
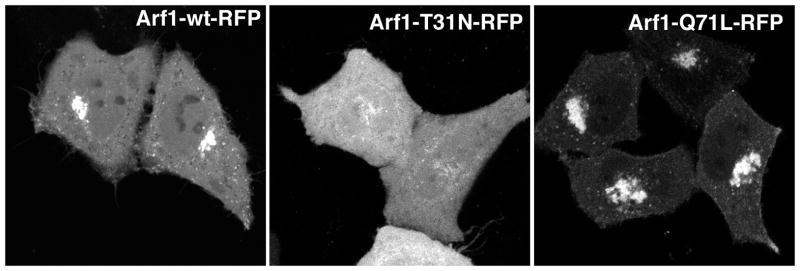
Another useful approach to examining Arf function in cells is expression of mutant Arf proteins. Threonine to asparagine mutations in the nucleotide binding pocket (T31N in Arfs1–5 and T27N in Arf6) act as dominant negative constructs, used to inhibit function of an endogenous Arf. These mutants are inactive and may sequester endogenous Arf GEFs by mimicking the inactive, GDP-bound or nucleotide free from of the Arf, preventing activation of the endogenous Arf. Indeed, the first Arf exchange factor was identified as a supressor of an Arf1T31N mutant in a genetic screen in yeast. We have observed that both Arf1T31N-HA and Arf1T31N-GFP function effectively as dominant negatives when expressed in cells. Arf1T31N itself is more cytosolic, as it is in a GDP-bound state and its expression results in cytosolic distribution of Golgi coat proteins and loss of Golgi structure, similar to what is observed with acute BFA treatment (see Fig. 1).
Figure 1.
Expression of Arf1-RFP and its mutants in HeLa cells. Wild type Arf1-RFP has both a prominent localization at the Golgi complex and a cytosolic pool. In cells expressing Arf1-T31N-RFP, the Arf is largely cytosolic and staining for a Golgi marker would reveal that the Golgi is largely absent (redistributed to the ER). Arf1-Q71L-RFP is almost entirely membrane bound, and causes vesiculation and swelling of the Golgi complex.
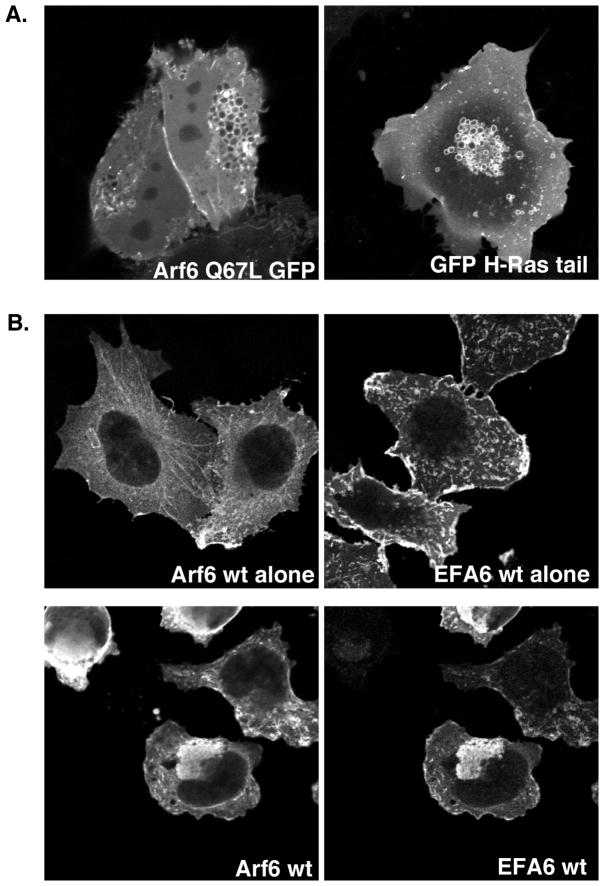
Mutation of a glutamine residue to leucine in the guanine nucleotide binding pocket (Q71L in Arfs1–5 or Q67L in Arf6) creates GTP-hydrolysis-defective mutants of Arf proteins, which are constitutively GTP bound, and can be used to mimic a persistently activated state. Although activated Arfs can presumably function, Arfs must cycle between the GTP-bound, active and GDP-bound, inactive state for biological activity. Thus, expression of these mutants often inhibits Arf-specific processes. For instance, expression of Arf1Q71L leads to the stable association of coat proteins with the Golgi complex causing vesiculation and swelling of Golgi cisternae (see Fig. 1), which inhibits transport through the secretory pathway. Expression of the Arf6Q67L mutant promotes cellular protrusions and also stimulates endocytosis and leads to the accumulation of endosomal membranes (see Fig. 2). This is often accompanied by a reduction of cell footprint, especially with high levels of expression and at longer times after expression. The association of Arf6 Q67L with these membranes is difficult to discern in fixed preparations, and often it is necessary to label the endosomal structures with a marker that retains strong membrane association (see protocol). The vacuole phenotype is strongest in the non-tagged version of Arf6Q67L, followed by Arf6Q67L-HA, and Arf6Q67L-GFP. Length and level of expression is also an important variable in experiments using Arf6 Q67L. Cells tend to round up after long or high level expression of this mutant.
Figure 2.
A) Expression of Arf6-Q67L in cells induces the formation of distinctive vacuoles. The left image shows a HeLa cell expressing Arf6-Q67L-GFP, which causes accumulation of large vacuolar structures. The right image shows a COS-7 cell co-expressing Arf6 Q67L (not shown) and the carboxyl terminal sequence of H-Ras fused to GFP. The H-Ras-tail-GFP highlights the Q67L vacuole clearly. B) Expression of Arf6 and EFA6 in cells promotes a phenotype similar to Arf6 Q67L.
Arf6 wild type localizes on tubular endosomes and at the PM in HeLa cells. Its GEF, EFA6, is localized mostly at the PM and causes cell ruffling. Co-expression of Arf6 and EFA6 induces the formation of vacuoles similar to expression of Arf6 Q67L.
Basic Protocol 1: Localization and Function of Specific Arf Proteins in cells
This protocol describes how to evaluate Arf function in HeLa cells by exogenous expression of wild type and mutant Arf proteins in cells. It can easily be adapted to other systems.
Materials
1M HCl
70% Ethanol
12-mm No 1 round, glass coverslips
100-mm tissue culture dishes
Culture media (DMEM containing 10% FBS and 1% penicillin/streptomycin solution)
6-well tissue culture dish
Mammalian expression plasmid containing Arf construct of interest
PBS
2% Formaldhyde solution
12-well plate
PBS/FBS (10% FBS in PBS + .02% sodium azide)
10% saponin
parafilm
15-cm tissue culture dish
watchmaker forceps
Primary antibody
Secondary antibody (Alexa Fluor 488 or 594 conjugated)
Fluoromont G
1.2 mm thick glass slide
clear nail polish
small kimwipes
Prepare cells and coverslips
-
Acid wash coverslips by incubating them in 1M HCL at 65–75°C for 6–8 hours. Rinse coverslips 4x in distilled water, and 3x in 70% ethanol. Store coverslips in 70% ethanol.
Appropriate care should be taken when heating 1M HCL as it is corrosive. Coverslips should be acid washed in a glass beaker under a chemical fume hood. Acid washing coverslips helps to provide a good surface for cells to adhere to, and helps ensure reproducibility between different batches of coverslips. Coverslips can be treated in large batches in advance and stored in 70% ethanol until needed. Specific cell lines may require different coverslip treatment. In a tissue culture hood, place 10 ml of media into a 100-mm tissue culture plate. Remove several coverslips from 70% ethanol with watchmaker forceps, and carefully flame to sterilize and remove ethanol. Place coverslips in tissue culture dish, being sure to carefully separate them from one another. Gently push each coverslip down onto the bottom of the culture dish to help prevent them from moving around. Continue until you have placed the desired number of coverslips in the dish.
Remove media from coverslips and replace with a fresh 10 ml of media to remove any residual ethanol.
-
Plate 0.5 –0.75×106 cells into the dish with coverslips.
We try to plate the cells to be 40–60% confluent the day after plating; If they are less crowded the cells often do not transfect well. If they are more crowded it is often hard to clearly distinguish subcellular structures clearly by immunofluorescence. This condition could vary with different cell lines. -
The following day when cells are 40–60% confluent place 2 ml of media into the well of a 6-well plate. Place 1–4 coverslips in each well. Transfect cells with 1 μg DNA for all Arf constructs except Arf6 Q67L. Transfect 0.25 μg Arf6 Q67L constructs to avoid toxicity.
We routinely use Fugene from Roche to transfect HeLa cells, and obtain 70% transfection efficiency for most constructs. -
Incubate the cells for 18 hours to allow expression of proteins from plasmids.
Expression of Arf6Q67L causes cells to round up. This is exacerbated by high levels of expression so we often use a smaller amount of DNA and transfect for shorter times (12 h instead of 18) to induce a milder phenotype. Other Arf mutants may also have toxicity issues under different conditions and both level and length of expression may have to be varied similarly.
Immunoflourescent staining of cells to detect expressed Arf proteins
(see also Protocol on Immunofluorescence Staining - Unit 4.3)
After transfection period, remove media and gently rinse cells with 2ml of PBS, and then replace with 2 ml of media. Cells can then be treated with drugs, growth factors, etc as desired.
Fix cells by placing coverslip into 1 ml of 2% formaldehyde in a 12 well plate. Incubate for 10 minutes.
-
Remove formaldehyde and rinse with 1 ml of PBS and then with 1 ml of PBS/FBS. Incubate for 1 hr.
The PBS/FBS solution is used to inhibit non-specific binding of antibodies to the cells; other blocking solutions can be used containing other proteins such as BSA or gelatin, for example. If the entire immunofluorescence protocol cannot be completed in one day, this is an appropriate place to stop. Cells can be stored in PBS/FBS overnight at 4° C. -
Dilute primary antibody to the epitope tag of the Arf used in PBS/FBS with 0.2% saponin. Place a piece of parafilm in a 15-cm tissue culture dish. Place a 30μl drop of the primary antibody on the parafilm. Invert coverslip (cells facing down) over the drop. Incubate for 1 hr at room temperature.
If using GFP-tagged proteins the fluorescence can be observed directly or the fluorescent signal can be enhanced with an antibody against GFP, followed by 488-conjugated secondary antibodies. Because Arf1 is discretely localized to the Golgi it is often easily detected by fluorescence alone. To help evaluate how expression of Arf proteins affects the system under study, it is often useful to co-stain the cells with antibodies to specific cellular components. Golgi structure can be monitored by staining with antibodies to Golgi resident proteins (like GM130). Arf6 associated membrane structures can be stained with antibodies directed against endosomal protein markers such as clathrin-independent endosomal cargo proteins (Eyster et al., 2009), transferrin receptor or co-expression of a GFP-tagged membrane marker like carboxyl terminal sequence of H-Ras (see Fig. 2). Place coverslips (cells facing up) back in 1 ml PBS/FBS in a 12-well plate. Wash coverslips 3 times for 5 minutes each.
-
Dilute appropriate fluorescently-conjugated secondary antibodies in PBS/FBS with 0.2% saponin. Again place a 30 μl drop on a piece of parafilm in a 15 cm culture dish. Invert coverslip onto drop. Incubate for 30 minutes at room temperature.
We typically use Alexa dye conjugated secondary antibodies such as 488, 594 and 633. Place coverslip back in 12 well plate, cells facing up. Wash twice in PBS/FBS and once in PBS, incubating 5 minutes for each wash.
Place a drop of glycerol-based mounting fluid such as FluoromountG in the middle of a 1.2 mm thick glass slide. Invert coverslip on top of drop (cells facing down). Gently press down on the coverslip using 3–4 kimwipes to remove excess mounting solution.
Allow coverslips to air-dry for 5 minutes and then seal coverslips around edges with clear nail polish.
-
Allow nail polish to dry (about 10 min) prior to examination of slides. Slides can be stored in covered boxes at 4°C for longer periods.
If fluorescently tagged Arfs are used, in particular Arf1-GFP (or -RFP), the localization and dynamics of Arf disssociation and association with Golgi membranes can be examined in living cells (see Unit 21 on imaging of GFP-tagged proteins).
Basic Protocol 2: Assessment of GTP-bound Arfs
In this protocol we describe a pull-down assay using the GGA3 VHS-GAT to assess the amount of GTP-bound Arf in a population of cells. This is a variation of the protocol first described by Santy et al (Santy and Casanova, 2001). GGAs are a family of Golgi-associated coat proteins that bind to GTP-bound Arf. The domain that binds to Arf-GTP is restricted to the GAT domain of GGA (Boman et al., 2000; Dell’Angelica et al., 2000). In vitro this region binds to Arf-GTP with high affinity, with little binding to Arf-GDP. In addition, like many Arf effectors, GGA can bind all Arf isoforms. This protocol uses the purified recombinant VHS-GAT domains of GGA3 fused to GST to probe cellular lysates for GTP-bound Arf. The VHS-GAT domain binds to active Arf, and thus can provide an estimate of the percentage of Arf in the cell that is active. The first protocol describes using this pull-down to look at activation of endogenous Arfs. The second protocol describes an adaptation of this protocol for assessing the activation of transfected Arf proteins and the effect of co-expression of regulators.
Materials
Frozen bacterial stock for GST-VHS-GAT expression
LB/Amp (LB + 100μg/ml Ampicillin)
1 M IPTG
PBS
Protease inhibitors (such as 1 mg/ml pepstatin, 1 mM leupeptin, 5 mg/ml aproptinin, and 1 mM PMSF)
Lysozyme (lyophilized powder)
10% Triton X-100 stock
DNase I (10 Units/μl)
RNase (1mg/ml stock)
1M DTT
glutathione sepharose beads
100mM tissue culture dish
lysis buffer (see recipe)
5x SDS page sample buffer
1.7 ml microfuge tubes
microcentrifuge spin columns (for example, PierceR Spin Cups with cellulose Acetate filters)
12% polyacrylamide gel or 4–20% gradient gel
Antibodies for Western blot
Expressions of GST-VHS GAT from GGA3
From a frozen bacterial stock, streak out GST-VHS GAT on an LB/Amp plate to obtain single colonies after growth overnight at 37°C.
Inoculate one colony into a 2ml culture of LB/Amp and grow overnight.
In the morning, inoculate a 100 ml culture of LB/Amp 1: 1000.
When the OD of the culture reaches 0.8–1.0 (about mid – day), add 500μM IPTG from a 1M stock. Grow for 3 hours.
-
Spin down pellet, remove culture media, and freeze at −80°C.
Pellet can be kept at −80°C for a couple of days if necessary.
Purification of GST-VHS-GAT
This is the protocol we use to purify GST-VHS-GAT. Other protocols for purifying GST fusion proteins will also work.
Thaw pellet on ice.
Resuspend in 20 ml ice cold PBS containing 2mM EDTA, 1 mg/ml lysozyme plus protease inhibitors.
Incubate on ice for 30 minutes.
Add 0.4 ml of 10% Triton-X 100 stock (for final concentration of 0.2%).
Add 30 μl DNAse I (10 U/μl stock), and 70 μl of a 1mg/ml solution of RNAse.
Incubate in a cold room with tube rotation for 10 min.
Add DTT to a final concentration of 1mM (20μl of 1M stock).
-
Spin down at 10,000 rpm in a Sorvall SS-34 for 30 min at 4°C.
For convenience the resultant supernatant may be aliquoted into single use size and frozen in liquid N2 and stored at −80°C. It is best to avoid repeated rounds of freeze/thaw at this point. Wash 250 μl of glutathione sepharose beads 1 x with 1ml 0.2% Triton x-100 in PBS.
Incubate glutathione sepharose with 5 ml of bacterial lysate for 30 min at 4 C.
-
Wash beads 3x in 1 ml 0.2% Triton x-100 in PBS. Wash beads 1x in lysis buffer. Resuspend beads at a 1:1 ratio of beads to lysis buffer.
It can be useful to verify that 30 ml of the 1:1 suspension contains 50–100 mg of GST-VHS-GAT. This can be done by running a 30 ml sample on an SDS-PAGE gel with appropriate BSA standards and Coomassie staining the gel. The GST-VHS-GAT fusion is approximately 40 kDa, and should be the major band. Adjust the amount of beads used in the assay if necessary.
Preparing cell lysates
The amount of cells used in this experiment will vary depending on what you are trying to examine, the cell type used, the amount of Arf expressed, and your ability to detect an Arf by western blot. This protocol is based on looking for endogenous Arf1 or Arf6 in one 10-cm tissue culture dish that is 70–80% confluent. If you have trouble detecting the endogenous Arf by western blot, you may need to use more cells or make adjustments to this protocol. An alternative approach is to transiently express an epitope tagged Arf in cells to do this assay. Specific considerations for this approach are outlined in the variation of this protocol that follows.
Plate 0.75–1.0 × 106 cells in 10ml media in a 10-cm tissue culture dish, and return to the incubator overnight.
Treat cells according to experimental design.
Wash cells once in ice cold PBS. Carefully remove all excess liquid.
-
Add 250μl lysis buffer containing protease inhibitors, and use a cell scraper to collect cells.
The amount of lysis buffer used will vary depending on the level of expression of endogenous Arf in your cell line, but generally we have found that you want to keep your lysates as concentrated as possible so that you can easily detect the protein in your loading control. To have a reliable result it is very important that you can clearly detect the endogenous Arf in the whole cell lysate fraction. Place lysate into a 1ml microcentrifuge tube, and spin down in a microcentrifuge at 13,000 x g for 5 minutes at 4°C.
Place 20 μl of lysate in 5 μl of 4x sample buffer with reducing agent and boil (for whole cell lysate sample).
Incubate 200 μl of lysate with 30 μl glutathione beads bound with GST-VHS-GAT for 30 min on a rotating rack at 4°C.
Spin down beads at 6,000xg for 30 sec in a microcentrifuge at 4°C, and remove supernate.
Resuspend beads in 0.75 ml of lysis buffer and transfer to a microcentrifuge spin column like those from Pierce.
Wash beads 2x in 0.75 ml of lysis buffer at 4°C. Spin columns at 6,000 x g for 30 sec, removing wash from collection tubes with each spin.
Place columns in fresh collection tubes and add 25 μl 2x sample buffer without reducing agent, vortex briefly to mix buffer and beads, and incubate at room temperature for 10 min.
Spin column at 13,000x g in a microcentrifuge to collect sample.
Add 5 μl of 1M DTT to sample, and boil sample for 5 min (this is the pull-down sample).
-
Run whole cell lysate sample (from step 22) and pull-down sample on SDS-PAGE gel (either 13% or a 4–20% Tris-HCL gradient mini gel) and transfer to nitrocellulose paper.
It is useful to run a pre-stained molecular weight marker along with your samples. To get good resolution of Arfs which are about 20kDa, it is often necessary to run the dye front off the gel, and run the gel until a marker around 5kDa is close to the bottom of the mini gel. -
Conduct western blot using Arf specific antibodies.
Of commercially available antibodies we have experience using the Arf1/3 antibody produced in sheep from Sigma (A4594), which can be used at 1:1000 for western blot, and the Arf6 (3A-1) mouse IgG2b antibody from Santa Cruz biotechnology that can be used at 1:100. It is important to verify the specificity of the antibody that you are using. One convenient way to do this is to transient express different epitope-tagged Arf isoforms and make individual cell lysates. However, be aware that the tag at the carboxyl terminus sometimes may interfere with recognition of an Arf, particularly if the epitope from the Arf is at the extreme carboxyl terminus. Quantify the intensity of your bands, to determine the fraction of total Arf that is GTP bound. In this scenario that would be the (intensity of the band from the pull down)/(10 x the intensity of the whole cell lysate band).
Assessment of Arf GTP levels in transfected cells
Reliable and reproducible detection of endogenous Arf proteins can be difficult, making interpretation of the pull-down assay an issue. Transient transfection of low levels of epitope-tagged wild type Arf proteins is an alternative approach. This approach allows you to directly compare activation of different Arf isoforms using the same antibody. It can also be used to look at the effect of expression of regulatory proteins (such as GEFs and GAPs) on Arf isoforms. This protocol is a modification of the above protocol.
Preparation of GST-VHS GAT fusion protein
This is the same as outlined in protocol 2.
Preparation of cell lysates
-
Plate 1.0 × 105 HeLa cells in the wells of a 6-well plate. On the following day when the cells are about 50–60% confluent, transfect the cells with relevant plasmids.
It is important to have a control lane in which you express the wild type Arf alone, and one in which you express the Arf with the regulator. This allows you to see a fold change in GTP-bound Arf upon expression of the regulator.Generally this has worked well when transfecting 0.25 μg of the wild type Arf, and 1.0 μg of the regulator. It is important that most of the cells transfected with the Arf also have the regulator in them. To monitor whether this is happening, include 1 coverslip in the plate and assess the efficiency of co-transfection by immunofluorescence.If you wish to compare the specificity of a regulator for one or another Arf you should express Arfs with the same eptitope tag and use the same antibody to conduct your western blot. On the following day, wash cells in 1x ice cold PBS.
Carefully remove excess liquid.
Lyse cells in 100 μl of lysis buffer.
Place lysate into a 1 ml microcentrifuge tube, and spin down in a microcentrifuge at 13,000 x g for 5 minutes at 4°C.
Place 25 μl of lysate in 5 μl of 4x sample buffer with reducing agent and boil (for whole cell lysate sample).
Dilute 50 μl of lysate into 0.4 ml lysis buffer, and incubate with 30 μl glutathione beads bound with GST-VHS-GAT for 30 min on a rotating rack at 4°C.
Spin down beads at 6,000 x g for 30 sec in a microcentrifuge at 4°C, and remove supernate.
Resuspend beads in 0.75 ml of lysis buffer and transfer to a microcentrifuge spin column like those from Pierce.
Wash beads 2x in 0.75 ml of lysis buffer at 4°C. Spin columns at 6,000 x g for 30 sec, removing wash from collection tubes with each spin.
Place columns in fresh collection tubes and add 25 μl 2x sample buffer without reducing agent, vortex briefly to mix buffer and beads, and incubate at room temperature for 10 min.
Spin column at 13,000 x g in a microcentrifuge to collect sample.
Add 5 μl of 1M DTT to sample, and boil sample for 5 min (this is the pull-down sample).
Run whole cell lysate sample (from step 6) and pull-down sample on SDS-PAGE gel (either 13% or a 4–20% Tris-HCL gradient mini gel) and transfer to nitrocellulose paper.
Quantify the intensity of the Arf bands and calculate the fraction of Arf that is GTP bound. In this scenario that would be the (intensity of the band from the pull down)/(2 x the intensity of the whole cell lysate band).
Reagents and Solutions
-
Lysis Buffer
50 mM Tris pH 7.5
100 mM NaCl
2 mM MgCl2
1% Triton X-100
10% glycerol
Basic Protocol 3: Assessment of specificity and function of Arf GEFs and GAPs
In this section we discuss strategies for evaluating the function and specificity of Arf GEFs and GAPs in cells. Identifying which isoform of Arf is the target of a particular GEF or GAP in cells is a challenge. While cell-free, biochemical assays often point to a preferred substrate, these assays cannot recreate the cellular environment. Furthermore, since GEFs and GAPs have catalytic activity, the expression of these regulators can reveal distinct cellular phenotypes due to activation or inactivation of particular Arf proteins.
Strategies for GEFs
The localization of endogenous GEFs is generally difficult due to low abundancew of these proteins and the lack of specific antibodies. Thus, it is advantageous to transiently over-express epitope-tagged GEFs in cells, and look at them by immunofluorescence modifying Protocol 1. The localization of the GEF can give you some information about the likely specificity of the GEF. If it is localized to the Golgi it probably is not using Arf6 as a substrate. However, if it is localized in the cell periphery it cannot be assumed that it is acting on Arf6, as other Arfs may be recruited to extra Golgi sites.
A second important consideration is whether the GEF affects the localization of an Arf protein. This can be addressed by co-expressing the GEF with wild type Arf proteins and conducting indirect immunofluorescene similar to what is outlined in protocol 1. A GEF that uses a particular Arf might co-localize with that Arf, and sometimes might change the normal distribution of the Arf. For example co-expression of Arf1 with the Arf exchange factor ARNO, can recruit Arf1 to the plasma membrane where it co-localizes with ARNO. Further a GEF would be expected to bind to and co-localize with the dominant negative mutant of the Arf it activates.
Unlike over-expression of the wild type Arf, over-expression of the wild type GEF sometimes creates an obvious phenotype. For instance over-expression of the Arf6 exchange factor, EFA6A, induces plasma membrane ruffling and macropinocytosis (Brown et al., 2001), not observed in cells expressing Arf6 alone (see Fig. 2). All Arf GEFs contain a conserved glutamic acid residue within their sec7 domain that is critical to their activity as exchange factors. When this residue is mutated to lysine (E156K in ARNO or E794K in GBF1) the exchange factor is unable to activate an Arf. In some cases, these mutants have also been reported to act like dominant negatives, and inhibit a process an Arf is involved in. Over-expression of a BFA sensitive Arf GEF can sometimes provide protection against the effects of BFA treatment by shifting the dose-response curve to the drug, and prevent or slow loss of its substrate Arf from Golgi membranes.
Co-expression of an Arf GEF with its substrate Arf often mimics the phenotype observed for the constitutively active mutants of the Arf. For example, co-expression of Arf6 with its GEF, EFA6A, can promote a vacuolar phenotype that is similar to expression of Arf6Q67L (see Fig. 2). Finally, looking at the effect the GEF has on activation of specific Arf proteins can be assessed by using the VHS-GAT pull-down assay described in Protocol 2. Comparing how different Arf proteins respond to a particular GEF when co-expressed in cells may provide some insight into specificity issues.
Strategies for GAPs
Since GAPs inactivate Arf proteins, their expression often antagonizes Arf function. The localization of an Arf GAP can give you information about its specificity. If it is localizied to the Golgi it is probably using a Golgi Arf (Arf 1–5) as a substrate. However, Arf GAPs that localize outside of the Golgi may still work on Arf 1–5 as a substrate since other Arfs can be recruited to extra Golgi sites. In some cases there may be antibodies available to look at the localization of endogenous ArfGAPs, however exogenous expression of epitope-tagged GAPs can be readily performed using Protocol 1.
Just like Arf GEFs, expression of Arf GAPs sometimes exhibits a distinct cellular phenotype. In this case however, an Arf GAP would be expected to mimic the Arf dominant negative and antagonize Arf function. For example over-expression of ArfGAP1 leads to loss of the COP I coat from the Golgi and dissolution of the Golgi similar to that induced by expression of Arf1 T31N (Aoe et al., 1997). In some cases, co-expression of a GAP with the Arf substrate can ameliorate the phenotype of expression of the GAP by itself. The Arf GAP would also be expected to co-localize with the constitutively active mutant of its Arf substrate. In the VHS-GAT pull-down, co-expression of the GAP with its substrate should lead to a reduction in the amount of Arf which is GTP bound.
Inactive mutants for Arf GAPs have also been described. If expression of the wild type GAP causes a phenotype, then expression of the inactive GAP should not cause that phenotype, if Arf activity is important in producing the phenotype. GAPs are often large proteins with many other protein domains that are important to their biological activity. Some GAPs have been reported to have phenotypes that are independent of their ability to activate Arf (Inoue and Randazzo, 2007). For instance over-expression of ASAP1, a peripheral Arf1 GAP, can induce podosome formation, but the ASAP1 mutant that lacks GAP activity (ASAP1-R495K) also can induce podosme formation suggesting that the GAP activity is not critical to this aspect of ASAP1’s function (Bharti et al., 2007). For this reason it is very important to determine whether the GEF or GAP activity of the regulator is important to the biological function being examined.
Commentary
Background
Cell biologists have gained a great deal of information about the functions of Arf proteins in cells using transient expression of wild type and mutant forms of the GTPase. The ability to examine phenotypes in cells expressing constitutively active and dominant negative forms of Arfs has been an effective tool for figuring out the function of Arf1 at the Golgi complex and Arf6 at the PM (D’Souza-Schorey and Chavrier, 2006; Donaldson and Jackson, 2000). This experimental tool has also been used for discovering functions of the numerous Rab proteins, which also affect membrane traffic in cells. Although there are some concerns about indirect effects of expression of some of these mutants in cells (see below), a careful evaluation of observed localization and phenotypes for expression of wild type and mutant forms of Arfs should ensure that a reasonable understanding of Arf function can be revealed.
Studies of Arf proteins have benefited greatly from the identification of the many Arf GEFs and GAPs in the genome (Casanova, 2007; Inoue and Randazzo, 2007). By contrast, few GEFs and GAPs have been identified for the Rab GTPases. Although analysis of the function of these regulatory molecules can in themselves be complex, their anticipated actions in terms of turning on and off a particular Arf can complement the analysis of the effect of expressing the corresponding Arf-GDP vs Arf-GTP mutant. Results from studies on transfected cells expressing specific Arfs, GEFs and GAPs should complement cell-free biochemical assays using recombinant proteins where GTP binding and hydrolysis can be measured directly.
The use of the GGA-VHS-GAT pull-down assay to determine the fraction of Arf in the GTP-bound state has been used widely to demonstrate Arf activation and inactivation in response to a physiological signal. For Arf6, EGF treatment results in an increase in Arf6-GTP (Morishige et al., 2008) whereas platelet activation leads to an immediate drop in Arf6-GTP (Choi et al., 2006). These changes in Arf6 GTP/GDP state can be due to either stimulation or inhibition of actions of the GEFs and GAPs. The Arf investigator thus has many tools with which to investigate these effects.
In addition to the expression of mutant and wild type Arfs, GEFs, and GAPs, another approach is to deplete cells of these proteins using siRNA protocols. These studies have many challenges and require good immunological reagents to determine extent of depletion of the protein, however they offer the possibility of rescuing a given phenotype by expression of an siRNA-resistant, wild type or mutants of the protein. In addition to the classical dominant negative and constitutively active mutants of Arf proteins, other mutations have been described including effector domain mutants in Arf1 (N52R) and Arf6 (N48R) that are defective in stimulating PLD, but are normal with respect to other activities (Skippen et al., 2002).
Critical Parameters
In attempting to investigate potential Arf function in a physiological process it is important to gather reliable reagents to undertake these studies. For expression of Arfs, GEFs and GAPs, vectors encoding these proteins for expression in mammalian cells can usually be obtained from active investigators in the field. Depending on whether antibodies are available to detect these proteins, epitope tagging of these constructs is often desirable so that commercial, anti-epitope antibodies can be used.
The choice of cell type for these studies is usually dictated by the particular biological process under study. If the process is apparent in all cells, the standard use of HeLa or Cos cells will ensure high efficiency of transfection and ease of analysis. More specialized cell types may, for example, require alternative transfection strategies. The amount of plasmid DNA to use in the transfection and also the time of transfection may also vary with the cell type and type of protein being expressed. Pilot experiments to maximize expression of protein are advisable. It is generally helpful to examine first the localization and cellular phenotype of expression of the Arfs, GEFs and GAPs in cells by immunofluorescence prior to looking specifically at whether a particular GEF or GAP or physiological stimulus affects Arf-GTP levels in the pull-down assay. Looking at the whole cell can also alert the investigator to toxic or secondary, indirect effects. For example, when examining effects of Arf1T31N on an endosomal compartment, one should examine whether in these cells the Golgi complex is still functional. Since Arf1T31N often disassembles the Golgi complex and blocks the secretory pathway, the effect observed on the endosomal compartment could reflect a requirement for normal secretory pathway traffic. Another consideration when examining specific Arf function in cells is possible redundancy among Arf proteins in the biological processes under study. From siRNA depletion studies, there is evidence that Arf proteins may act in pairs at particular locations (Volpicelli-Daley et al., 2005).
Troubleshooting
Described here are common problems encountered when conducting these types of experiments.
No immunoflourescence signal for epitope-tagged Arf
Check to see that a protein with the same epitope tag, that you have experience expressing under your experimental conditions, is detectable. If no fluorescence signal is detected it might point to a more general problem in the transfection protocol, or a general problem detecting the epitope tag by immunofluorescence. If you can detect fluorescence of your protein, it suggests a specific problem expressing or detecting the Arf protein of interest. Check the quality of your plasmid DNA source as poor quality plasimid DNA might interfere with expression. Consider using an anti-GFP antibody to boost the fluorescence signal if you are visualizing a GFP fusion protein.
Vary the amount of DNA used, and the time of expression, making it either shorter or longer. Arf mutants can have toxic effects on cells, particularly when expressed for a longer period of time, and some cells may be more sensitive to these effects than others. Observing a lot of floating cells after the transfection might indicate a toxicity problem. Shortening the time of expression and decreasing the amount of DNA used may help with toxicity issues.
Low co-transfection efficiency
Sometimes Arfs or their mutants are refractory to being co-expressed with other proteins. Try varying the ratio of the Arf to the other protein of interest, and shorten the length of time of expression. Consider your experimental design when varying the ratio. For instance, when using the GAT assay to look the effect of a protein on Arf’s nucleotide status, it is critical that most of the Arf is expressed in cells with the protein of interest. Therefore the ratio should be biased to favor expression of the protein of interest. Occasionally this is hard to overcome, but be aware that often the problem is co-transfecting the protein with a specific mutant (constitutively active or dominant negative), so you may be able to gain information by using the wildtype or a different mutant.
Little or no Arf GTP signal in VHS-GAT pulldown assay
Verify that the GST fusion protein is present by staining nitrocellulose membrane with Ponceau S. There should be a prominent band around 40kDa. There might also be some degradation products of the GST fusion protein present, but as long the 40kDa band in abundant it should not interfere with the assay. If there is no 40kDa band present there is a problem with the purification or expression of the GST fusion protein.
Verify that the GST fusion protein can precipitate constitutively active Arf. If it cannot it suggests there is a problem with the fusion protein or the assay. Check to see that the fusion protein is intact. For this assay to work well, it is also important once the cells containing the Arf are lysed that the assay is completed in a timely fashion (2–3 hrs), and kept cold. If the GST-VHS GAT beads can precipitate constitutively active Arf, it suggests the fusion protein and assay are working correctly. Consider increasing the amount of lysate used in the pulldown.
Reproducibility of the VHS-GAT pull-down Assay
Results for the GAT assay need to be verified by repetition of the experiment. If results are variable from one experiment to another, it suggests a problem in the assay. Consider monitoring the ability of the VHS-GAT-GST fusion to distinguish between the consitutively active and dominant negative mutants. If the constitutively active and dominant negative mutants are pulled down in the assay in similar amounts, try loading less lysate for the pulldown, or increasing the number of washing steps.
It is critical to be able to detect the Arf of interest in the whole cell lysate. A very weak signal could make it difficult to quantify band intensity reliably, and this could add a large amount of error into your final calculation. Optimize the conditions for you western blot. Consider increasing the amount of total protein used in your whole cell lysate sample. One way to do this is to reduce the volume of total lysis buffer used to make the initial sample more concentrated, or to use more cells and the same amount of lysis buffer. Although the ultimate measure in this assay is the percentage of Arf that is GTP, and slight differences for the level of Arf expression in the whole cell lysate will be accounted for in this calculation, large difference may artificially bias the results. If protein loading between samples in the whole cell lysate is uneven consider quantifying total protein concentration using a Bradford or BCA assay, and correcting for differences. Sometimes when co-expressing an Arf and a regulator, the amount of Arf expressed decreases. Consider using less of the regulator.
Anticipated Results
This unit is meant to help investigators evaluate whether an Arf is involved in a biological process under study by providing basic tools and strategies to investigate Arf protein function. Investigators should be able to examine whether an Arf localizes on cellular structures of interest, or co-localizes with proteins of interest. They can also evaluate whether a particular biological process is affected by expression of dominant negative or constitutively active mutants of Arfs. They can evaluate whether an Arf is activated or inactivated during a particular treatment or process. Finally, they can address how an Arf might be regulated in their system by addressing GEF and GAP function using wild type and mutant proteins.
Time Considerations
For localization and function of Arf proteins in cells (Basic Protocol 1), cells are plated on day 1, transfected on day 2, and an experiment performed 12–24 hours after the start of transfection. After fixing the cells in formaldehyde and removing the formaldehyde and replacing with a blocking solution the cells may be placed at 4°C for up to 24 hours. After fixation the immunofluorescence staining should take about 4 hours to complete.
For assessment of GTP-bound Arfs (Basic Protocol 2), a bacterial lysate of the GST-VHS-GAT protein can be made in advance, and stored at −80°C. Allow 2 days for growth of the bacteria, and 2 hours for making the bacterial lysate. Purifcation of the GST-VHS-GAT fusion will take about 1 hour. The purification of the GST-VHS-GAT fusion protein should be done on the same day as the preparation of cell lysates. For preparation of cell lysates, cells are plated on day 1. The experiment is either performed on day 2, or the cells are transfected on day 2, and the experiment is performed 12–24 hours after transfection. It will take about 3 hours to perform preparation of cell lyates and pull-down portion of this experiment. Once boiled, samples can be stored at −20°C. Allow 2 more days to finish experiment; to run and transfer SDS-PAGE gels, and conduct the western blot.
Literature Cited
- Aoe T, Cukierman E, Lee A, Cassel D, Peters PJ, Hsu VW. The KDEL receptor, ERD2, regulates intracellular traffic by recruiting a GTPase-activating protein for ARF1. Embo J. 1997;16:7305–16. doi: 10.1093/emboj/16.24.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti S, Inoue H, Bharti K, Hirsch DS, Nie Z, Yoon HY, Artym V, Yamada KM, Mueller SC, Barr VA, Randazzo PA. Src-dependent phosphorylation of ASAP1 regulates podosomes. Mol Cell Biol. 2007;27:8271–83. doi: 10.1128/MCB.01781-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman AL, Zhang C, Zhu X, Kahn RA. A family of ADP-ribosylation factor effectors that can alter membrane transport through the trans-Golgi. Mol Biol Cell. 2000;11:1241–55. doi: 10.1091/mbc.11.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG. Phosphatidylinositol 4,5- bisphosphate and Arf6-regulated membrane traffic. J Cell Biol. 2001;154:1007–17. doi: 10.1083/jcb.200103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova JE. Regulation of Arf activation: the Sec7 family of guanine nucleotide exchange factors. Traffic. 2007;8:1476–85. doi: 10.1111/j.1600-0854.2007.00634.x. [DOI] [PubMed] [Google Scholar]
- Choi W, Karim ZA, Whiteheart SW. Arf6 plays an early role in platelet activation by collagen and convulxin. Blood. 2006;107:3145–52. doi: 10.1182/blood-2005-09-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–58. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- Dell’Angelica EC, Puertollano R, Mullins C, Aguilar RC, Vargas JD, Hartnell LM, Bonifacino JS. GGAs: a family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J Cell Biol. 2000;149:81–94. doi: 10.1083/jcb.149.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG, Jackson CL. Regulators and effectors of the ARF GTPases. Current Opinion in Cell Biology. 2000;12:475–482. doi: 10.1016/s0955-0674(00)00119-8. [DOI] [PubMed] [Google Scholar]
- Eyster CA, Higginson JD, Huebner R, Porat-Shliom N, Weigert R, Wu WW, Shen RF, Donaldson JG. Discovery of New Cargo Proteins that Enter Cells through Clathrin-Independent Endocytosis. Traffic. 2009;10:590–599. doi: 10.1111/j.1600-0854.2009.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Randazzo PA. Arf GAPs and their interacting proteins. Traffic. 2007;8:1465–75. doi: 10.1111/j.1600-0854.2007.00624.x. [DOI] [PubMed] [Google Scholar]
- Morishige M, Hashimoto S, Ogawa E, Toda Y, Kotani H, Hirose M, Wei S, Hashimoto A, Yamada A, Yano H, Mazaki Y, Kodama H, Nio Y, Manabe T, Wada H, Kobayashi H, Sabe H. GEP100 links epidermal growth factor receptor signalling to Arf6 activation to induce breast cancer invasion. Nat Cell Biol. 2008;10:85–92. doi: 10.1038/ncb1672. [DOI] [PubMed] [Google Scholar]
- Santy LC, Casanova JE. Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D. J Cell Biol. 2001;154:599–610. doi: 10.1083/jcb.200104019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skippen A, Jones DH, Morgan CP, Li M, Cockcroft S. Mechanism of ADP ribosylation factor-stimulated phosphatidylinositol 4,5-bisphosphate synthesis in HL60 cells. J Biol Chem. 2002;277:5823–31. doi: 10.1074/jbc.M110274200. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Li Y, Zhang CJ, Kahn RA. Isoform-selective effects of the depletion of ADP-ribosylation factors 1–5 on membrane traffic. Mol Biol Cell. 2005;16:4495–508. doi: 10.1091/mbc.E04-12-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]