Abstract
Progressive multifocal leukoencephalopathy (PML) is a severe neurological disorder due to JC virus (JCV) infection. Pre-diagnostic biological markers and risk factors for PML are not well understood. We conducted a case-control study nested within the Multicenter AIDS Cohort Study to examine the association between JCV viruria and viremia and serum antibody to JCV capsids, in relation to subsequent PML diagnoses, 5 months to 12 years later. Other demographic and immunologic factors were examined also. The study population included 28 incident cases of PML, 26 matched HIV-positive controls, and 50 HIV-negative controls. Prevalence of JCV viruria was 37% in cases, 42% in HIV-positive controls, and 28% in HIV-negative controls (p = 0.43). Among persons with JCV viruria, persistent viruria was more common in cases (89%) than in HIV- positive controls (33%) (p = 0.02). Presence of JCV viruria was not related to the time to PML diagnosis (OR: 1.03, 95% CI: 0.8 – 1.4); however, the urinary concentration of JCV DNA increased with proximity to the date of PML diagnosis in cases. JCV seropositivity did not differ between cases or controls (p = 0.42). Four cases tested JCV seronegative, including one case only 5 months prior to diagnosis with PML. JCV DNA was detected in the serum of one HIV-positive control. Smoking was the only demographic variable analyzed associated with an increased risk for PML (MOR: 9.0, 95% CI: 1.2–394.5). The results suggest that persistent JCV viruria and increasing urinary concentration of JCV DNA may be predictive of PML for some patients.
INTRODUCTION
Progressive multifocal leukoencephalopathy (PML) is a fatal demyelinating disorder of the central nervous system caused by a lytic infection of oligodendrocytes with JC virus (JCV), a human polyomavirus (Astrom et al., 1958) (ZuRhein, 1969). JCV infects during late childhood and then persists indefinitely as a latent infection of the kidneys and B-lymphocytes (Chesters et al., 1983; Dorries and Meulen, 1983; Gallia et al. 1997). JCV may also persist as a latent infection of the brain (Eisner and Dorries, 1992; Ferrante et al., 1997). Between 40 and 75% of people worldwide have antibody to JCV (Carter et al., 2003; Rollison et al., 2003), and in approximately 20 to 30% of infected persons, JCV actively replicates in the kidneys and is shed in the urine (Markowitz et al., 1993; Shah, 1996).
PML, which has a case fatality rate of almost 100% and no specific treatment, occurs on a background of conditions associated with T-cell deficiencies, such as HIV-infection (Richardson, 1988; Berger and Major, 1999; Weber et al., 2001). PML was a rare disease prior to the advent of the AIDS pandemic in the 1980's (Brooks and Walker, 1984), but its incidence since has increased dramatically (Berger, 2003; Holman et al., 1998). Today, PML is recognized as an AIDS-defining illness occurring in three to five percent of all AIDS patients (Selik et al., 1997; Ahsan and Shah, 2006).
Little is known about the risk factors for PML. Low CD4 T-cell counts and increasing age are associated with greater risk for PML, but only a small fraction of persons with immunosuppressive conditions will develop the disease (Richardson, 1988; Weber et al., 2001; Ahsan and Shah, 2006). Some anti-inflammatory therapies have also been linked to PML, most notably natalizumab (Tysabri), an alpha-4 integrin inhibitor that showed promise as a treatment for relapsing multiple sclerosis (MS) in clinical trials (Garcia-Suarez et al., 2005; Kiewe et al., 2003; Vidarsson et al., 2002; Yousrey et al., 2006). The role that anti-inflammatory therapies play in the development of PML is not yet clear.
The mechanisms that result in JCV infection of oligodendrocytes in the central nervous system (CNS) and subsequent PML disease are also not well known. A study of humoral immune responses in patients with PML showed high levels of anti-JCV IgG rather than IgM, suggesting that PML is most likely a result of a reactivation of latent virus rather than a pathological consequence associated with primary infection (Weber et al., 1997). It has not yet been established if and what latent sites play important roles in the pathogenesis of JCV. At present, it is not known whether PML is caused by reactivation of JCV in the brain or, alternatively, JCV that is reactivated in the peripheral tissues, such as the kidney or blood, and then traffics to the CNS.
Previous studies have provided direct evidence for JCV infection in the kidney (Bolderini et al., 2005; Chesters et al., 1983) and have shown that reactivation of JCV occurs in the kidney resulting in an accumulation of virus in the urogenital tract and the excretion of virus and epithelial cells containing virus in the urine (Dorries, 2006). Virus has been detected in the urine of both healthy and immunosuppressed individuals in similar proportion (Lednicky et al. 2003; Behzad-Behbahani et al., 2004). The archetype or non-rearranged form of JCV DNA is the viral type that is most commonly detected in the urine of both PML patients and healthy and immunosuppressed persons who do not have PML (Azzi et al., 1996; Delbue et al., 2005). In addition to archetype virus, JCV with various deletions and duplications in the transcriptional control region also have been detected in blood and brain of PML patients (Azzi et.al, 1996).
Studies specifically examining the relationship between reactivation of JCV in the kidney and PML suggest that JCV reactivation in the kidney may not be related to PML, (Ferrante et al., 1997; Koralnick et al., 1999), however these studies utilized specimens collected at the time or following the diagnosis of PML, and therefore do not exclude the possibilities that disease status affects JCV viruria or that replication in the kidney prior to PML is related to disease development.
We present here the results of a nested case control study conducted within the Multicenter AIDS Cohort Study (MACS) examining the associations between JCV DNA in the urine and serum and IgG antibody to JCV capsid in the serum with PML. Specimens were obtained from 28 incident cases of PML prior to the diagnosis of PML and 26 matched HIV-positive and 50 HIV-negative men who never developed PML. Our analysis focused primarily on the examination of JCV specific virological markers as predictors of future PML, although other markers and risk factors, including BK virus (BKV) viruria, were also studied. Given the limited size of the case group, our analysis was limited in power and may be considered more of a hypothesis-generating study.
METHODS
Study Population
We used a case-control design nested within the Multicenter AIDS Cohort Study (MACS) to examine archived urine and serum specimens from 28 cases of PML and from similarly immunosuppressed and non-immunosuppressed men who did not develop PML. The MACS is an ongoing prospective cohort study of the natural and treated histories of HIV/AIDS in homosexual and bisexual men in the United States (Kaslow et al., 1987), with four sites; Baltimore, Chicago, Los Angeles, and Pittsburgh. Initial recruitment for MACS began in April of 1984. As of May 2007, a total of 6,972 HIV-negative and HIV-positive men have been enrolled and have contributed over 75,000 person-years of observation.
Study visits for MACS participants occur semiannually, and at each visit, demographic and clinical data are collected through detailed interview and computer-administered questionnaires, neuropsychological screenings, and physical examination. Plasma, serum, and peripheral blood mononuclear cells (PBMC) are also collected and stored at each visit. Urine specimens were collected for the first three visits (1.5 years) only, and therefore, the present study was limited to the analysis of urine and serum specimens collected from cases and controls during these visits. Calendar dates at which the urine specimens were collected ranged from April of 1984 to June of 1986 and corresponded to time intervals of 5 months to 12 years prior to PML diagnosis.
PML Cases
Thirty-two cases of PML have been diagnosed in MACS since 1984, of which 28 had urine specimens available for this study. Case diagnoses were made on the basis of brain biopsy (40%), necropsy (14%), a combination of radiographic and clinical diagnosis (28%), or clinical diagnosis alone (18%). All of the cases were HIV-1 seropositive. Cases with urine specimens had either one (n = 5), two (n = 21), or three (n = 2) urine specimens. Corresponding serum specimens were obtained for cases for the same visits as for the urine specimens. One case did not have serum specimens available. Cases were diagnosed with PML between June of 1985 and July of 1996, and urine specimens were collected from 0.38 (approximately 5 months) to 12 years prior to the date of PML diagnosis. Of the 50 urine specimens analyzed, 9 specimens were collected between 0 and 3 years, 15 between 3 years (inclusive) and 6 years, 15 between 6 years (inclusive) and 9 years, and 11 between 9 (inclusive) and 12 years prior to diagnosis of PML. The median time from sample collection to date of PML diagnosis was 6.4 years (IQR: 4.1–8.4 years). The date of PML diagnosis was defined as the clinical date of PML diagnosis and was obtained from the patients' medical records.
PML was the AIDS-defining event in 18 of the 28 cases in this study. Other AIDS-defining events included Kaposi's sarcoma (n = 3), pneumocystis pneumonia (n = 4), non-Hodgkin's lymphoma (n = 1), and candida esophagitis (n = 2).
At the time the urine specimens were collected, no cases or HIV-positive controls were receiving antiretroviral therapies.
HIV-positive controls
Each case was matched to a HIV-1 seropositive MACS participant who did not develop PML, according to (1) baseline (MACS entry visit) CD4 T-cell count (+/− 50 cells per μl) to account for duration of HIV-infection prior to entry into the study (Munoz et al., 1988) and (2) PML-free time from baseline. Cases for which PML was the AIDS defining event were further matched to HIV-positive controls on rate of CD4 T-cell decline from baseline (+/− 50 cells per ul year). If PML was not the AIDS-defining event of a case, an HIV-positive control was selected who had an AIDS-defining event within one calendar year of the AIDS-defining event of the case and comparable PML-free time from AIDS. Using these matching criteria, one PML case was matched to a HIV-positive control who was diagnosed with PML at a later date. The dates of PML diagnoses between these men differed by 0.35 years (127 days) only. Since the possibility of sub-clinical PML disease in the matched control at the time of PML diagnosis of the case could not be ruled out, both men were excluded from the matched analyses. The urine and serum from these men, however, were tested for the presence of JCV DNA and anti-JCV capsid antibody, and their results were incorporated into our unmatched statistical analyses, including the overall prevalence estimates for JCV viruria among cases. Urine and serum specimens were obtained from controls for the same study visits at which they were obtained from their corresponding case. One control did not have urine specimens available for two visits of its corresponding case. A total of twenty-six cases (fifty urine specimens) and twenty-six controls (forty-eight urine specimens) were included in the final matched analysis.
Of the twenty-six HIV-positive controls in the study, sixteen were diagnosed with an AIDS-defining event, including: Kaposi's sarcoma (n=4), pneumocystis pneumonia (n = 5), cryptosporidiosis (n = 1), CMV retinitis (n = 2), dementia (n = 2), cryptococcal infection (n = 1), and pulmonary TB (n = 1).
HIV-negative controls
Fifty unmatched HIV-negative men were used as a second set of controls. Samples were obtained from a single study visit, except for one man for whom urine and serum samples were obtained at two visits. Urine and serum specimens from HIV-negative controls were frequency matched on the number of urine and serum specimens obtained per MACS study visit for cases.
Laboratory Methods
Specimen storage and extraction/purification of DNA
All urine and serum specimens were stored at − 80 °C before testing. DNA was extracted from 200 μl of urine and 100 μl of serum by use of the QIAmp RNA mini kit (Qiagen) according to the manufacturer's instructions. Urine specimens were spun at 1500 × g for 10 min prior to extraction. JCV whole genome inserted into pBR322 plasmids in a background of herring sperm DNA (70 ng/ml) was used as a positive extraction control for the urine. Total nucleic acid from urine and serum were eluted in 50 and 30 μl quantities respectively.
JCV and BKV quantitative PCR
Taqman real-time quantitative PCR assays were performed to detect and quantify JCV DNA in urine and serum and BKV DNA in urine using an ABI 7300 sequence detection system (Applied Biosystems) as described (Ryschkewitsch et al., 2004; Rollison et al., 2006). For detection of JCV DNA, JCT-1 (5'- AGA GTG TTG GGA TCC TGT GTT TT - 3') and JCT-2 (5'- GAG AAG TGG GAT GAA GAC CTG TTT - 3') forward and reverse primers were used. JCT 1.1 (5'-FAM- TCA TCA CTG GAC AAC AAT TCT TCA TGG C –TAMRA-3') was used as an internal probe.
The assay parameters differed for JCV PCR of urine and serum extractions slightly. For quantification of JCV DNA in the urine, the PCR reaction volume was 50 μl and contained Taqman Universal PCR Master Mix (Roche), 5 μl of purified sample DNA, 0.2 μmol of JCT 1.1 probe, and 0.3 μmol of each primer. PCR was performed at the following conditions: 50°C for 2 min (1 cycle), 95°C for 10 min (1 cycle), and 95°C for 15 sec and 57°C for 60 seconds (40 cycles). For serum specimens, real-time reactions were run using 8 μl of eluted sample DNA, 0.3 μl of each primer and 0.2 μl of probe, in a total volume of 20 μl. The real-time PCR program parameters for serum were as follows: 50°C for 2 min (1 cycle), 95°C for 10 min (1 cycle), and 95°C for 15 sec and 60°C for 60 seconds (40 cycles).
For detection of BKV DNA, the primers were VpF (5'-AGT GGA TGG GCA GCC TAT GTA -3') and VPr (5'-TCA TAT CTG GGT CCT CCC CTG GA - 3'). A BKV probe (5'-FAM- AGG TAG AAG AGG TTA GGG TGT TTT GAT GGC ACA G - BHQ- 3') was used as the internal probe. The BKV PCR was performed in 50 μl using 5 μl of DNA extracted from urine, 0.2 μmol of BKV probe, 0.2 μmol of VPf primer, and 0.4 μmol of VPr primer. Reaction conditions were the same as that for detection of JCV DNA in urine except the annealing and extension reactions were performed at 63°C for 60 seconds.
Five-fold serial dilutions of JCV and BKV plasmid DNA in a background of human placental DNA (40 ng/ml) were run as external standard curves and included in duplicate on each plate for both JCV and BKV PCR experiments for the urine extractions. Linear regression analyses were used to determine the number of JCV and BKV copies in each reaction mixture relative to the standard curve. The sensitivity of the assays was approximately 50 copies of JCV or BKV genome per 1 ml of urine.
For the JCV PCR with serum, the standard curve was prepared from the JCV Mad-1 pM1TC plasmid using amounts of DNA ranging from 106 copies to 1 copy in decreasing 1:10 serial dilutions and amounts of 50 and 5 copies. Results were reported as the mean number of copies per ml of serum from duplicate determinations. This sensitivity of the PCR was 40 copies of JCV genome per 1 ml of serum.
JCV Serology
A VLP-based ELISA assay was performed to detect IgG antibody to JCV capsid (de Sanjose et al., 2003; Viscidi et al., 2003). This assay has been validated and compared to the hemagglutination inhibition assay previously (Hamilton et al., 2000). Briefly, wells of Polysorp microtiter plates (Nunc, Naperville IL) were coated with 30 ng of JCV VP1 VLP protein, blocked with 0.5% (wt/vol) polyvinyl alcohol, MW 30,000–70,000 (Sigma, St. Louis MO), and incubated overnight. Serum samples diluted to a concentration of 1:400 were added to JCV-VLP antigen coated plates. Antigen-antibody complexes were detected using peroxidase-conjugated goat antibodies against human IgG. To initiate color development, a 2, 2'-azino-di-(3-ethylbenzthiazoline-6-sulfonate) hydrdogen peroxide solution was used (Kirkegaard and Perry, Gaithersburg, MD). Optical density (OD) was measured at 405 nm using a reference wavelength of 490 nm and an automated microtiter plate reader (Molecular Devices, Menlo Park CA). The assay was performed in duplicate and the geometric mean of the two measurements was used for statistical analyses. The cutoff value for seropositivity was defined as an OD value greater than the mean plus 3 standard deviations of the reactivity of sera from children, ages 2–5 years, after excluding outliers.
Flow cytometry
Absolute CD4 and CD8 T-lymphocyte cell counts were obtained by flow cytometry from whole blood collected at each semi-annual MACS visit as described previously (Giorgi et al., 1990).
Statistical Analysis
Primary exposure variables examined included presence and level of JCV viruria in the urine and IgG antibody to JCV capsid. Among individuals with JCV viruria, the pattern of viruria was examined. Persistent JCV viruria was defined as the presence of JCV viruria at two or more consecutive visits and transient viruria as JCV viruria at one visit or at non-consecutive visits. BKV viruria was defined and examined similarly. BKV antibody responses were not examined.
Statistical analyses were conducted using STATA version 9.0 (College Station, TX). The Wilcoxon signed rank test for paired data and chi-square test were used to compare levels and prevalence of JCV and BKV DNA in the urine and levels of serum antibody to JCV between PML cases and HIV-positive and HIV-negative controls, respectively. For analysis of JCV viruria as a continuous variable, the number of JCV copies per ml of urine was log10 transformed.
Viral DNA in urine and serum antibody to JCV were analyzed separately for each MACS visit to account for risk set clustering and unknown confounding factors possibly related to calendar time or study visit. No differences in results between visits were observed (data not shown). For simplicity, results from one visit or the baseline visit are presented in some analyses.
To study the association of JCV and BKV DNA in urine, serum antibody to JCV, and baseline demographic and clinical variables with PML, conditional and unconditional logistic regression models were used to compute matched odds ratios (MOR) and unmatched odds ratios (OR), respectively, and 95% confidence intervals.
Unconditional logistic regression was used to analyze the association between JCV DNA in urine and various clinical and demographic variables. The analysis was restricted to individuals who were JCV seropositive in order to exclude the possibility that persons without JCV viruria were not viruric because they had not been infected with JCV.
We also examined if viruria was more likely among cases closer to the date of PML diagnosis and if urinary JCV viral load increased among cases with viruria closer to the date of PML diagnosis. Presence and level of antibody with respect to time to PML were also analyzed. Time to PML was defined as the interval between the date urine or serum specimens were collected and the date of PML diagnosis. For HIV-positive controls, a time interval comparable to that of their matched PML case was used to account for temporal effects of HIV-progression. The linear relationships between JCV DNA in urine and antibody to JCV capsid in serum and time to PML was analyzed by using general estimation equation (GEE) models to obtain estimates for slopes while accounting for risk set clustering (Zeger and Liang, 1986).
RESULTS
Demographic characteristics of cases and of HIV-positive and HIV-negative controls were similar. Ages ranged from 20 to 68 years. The median age was 33 years in cases and HIV-positive controls and 34 years in HIV-negative controls. More than 90% of cases and controls were white and had some college education.
PML cases and HIV-positive controls had similar baseline T-lymphocyte cell counts. The median baseline CD4 T-cell count was 551 cells/μl (IQR: 359–643 cells/μl) and 546 cells/μl (IQR: 405–647 cells/μl) in cases and controls, respectively. Median baseline CD8 T-cell count was similar between the two groups also (Cases: 675 cells/μl (IQR: 530–1017); HIV-positive controls: 668 cells/μl (IQR: 426–853 cells/μl). The average rate of CD4 T-cell decline between cases and HIV-positive controls did not significantly differ either (p = 0.3).
JCV viruria, JCV viremia, and seroreactivity to JCV capsids
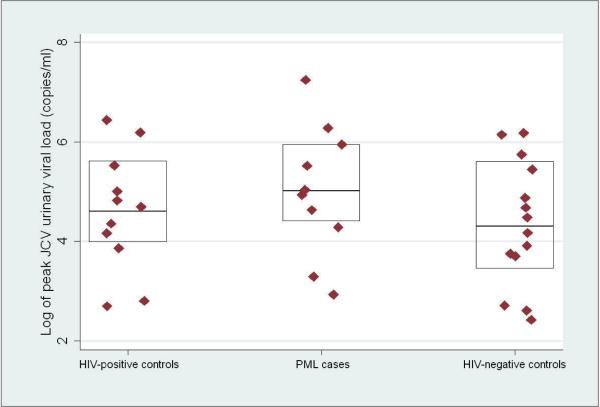
The prevalence of JCV viruria at one or more study visits was 37.0% in cases, 42.3% in HIV-positive controls, and 28.0% in HIV-negative controls (p = 0.43). Figure 1 shows the peak JCV urinary viral loads among cases and controls with JCV viruria. The median peak JCV viral load in cases was 5.1 log10 copies per ml of urine (log cp/ml) (IQR: 4.4 – 5.9 log cp/ml)), compared to 4.6 log cp/ml in HIV-positive controls (IQR: 4.0 –5.6 log cp/ml) and 4.3 log cp/ml in HIV-negative controls (IQR: 3.5 – 5.6 log cp/ml) (Figure 1). Differences in the peak JCV urinary viral load between case and control groups were not statistically significant.
Figure 1.
One-way scatter plot of the log10 pack JCV viral load in HIV-positive controls (n = 11), PML, cases (n = 10), and HIV-negative controls (n = 14) with JCV viruria; boxes indicate medians and 25% and 75% quartiles.
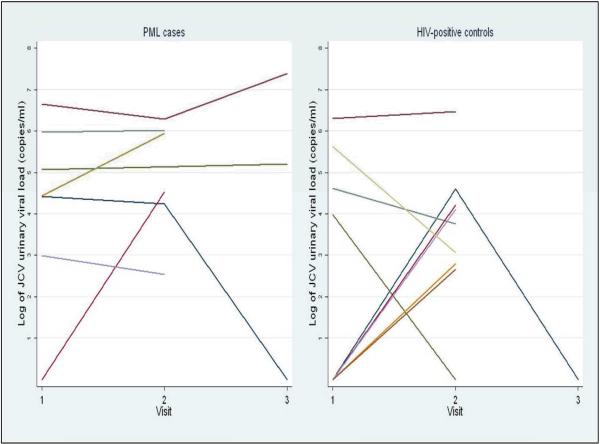
Figure 2 shows the pattern of JCV viruria over visits 1, 2, and 3 for cases (n = 7) and HIV-positive controls (n = 9) who were JCV viruric at any visit and who had urine specimens available for two or more visits. 89% of these e cases were persistently JCV viruric as compared to only 33% of the HIV-positive controls (p = 0.016).
Figure 2.
Pattern of JCV viruria in PML cases and HIV-positive controls; log10 JCV cp/ml of urine at visits 1, 2, and 3; figure includes only cases and controls with JCV viruria and with ≥2 urine specimens. No viruria at a visit for persons with transient JCV viruria is represented using a value of 1 jcv copy/ml of urine.
Seroreactivity to JCV capsids at any study visit was detected in 23 (85%) cases, 19 (79%) HIV-positive controls, and 36 (72%) HIV-negative controls. These differences were not statistically significant. The median OD value for cases with antibody-positive sera at visit 1 was 1.2 compared to 0.78 for HIV-positive controls and 0.95 for HIV-negative controls. These differences were also not statistically significant.
Four cases (14.8%) were JCV seronegative. The sera were collected between 5 months and 8 years before diagnosis of PML. In one such case, large quantities of BKV DNA (8.0 log cp/ml) were detected in the urine 5 months prior to the date of PML diagnosis. This patient was not JCV viruric at any of his study visits. One case and one HIV-positive control were JCV seronegative at the first study visit and became seropositive at a later visit. The case seroconverted approximately 5 years prior to his PML diagnosis at the age of 34.
JCV antibody levels were inversely proportional to baseline CD4 cell counts for persons with counts less than 1000 cells/μl (p=0.04). A similar, but not statically significant, trend was observed for the relationship between JCV antibody level and CD8 T-cell count (p=0.1).
JCV viruria was not predictive of PML when cases were compared to HIV-positive controls by conditional logistic regression analysis (MOR: 0.7, 95% CI: 0.1–2.8) or HIV-negative controls by logistic regression analysis (OR: 1.51, 95% CI: 0.56 – 4.09) (Table I). Seropositivity to JCV was not associated with PML when cases were compared to either of the control groups (relative to HIV-positive controls; MOR: 1.3, 95% CI: 0.3–6.3; relative to HIV-negative controls; OR: 2.2, 95% CI: 0.7 –7.6).
Table I.
Relationships between JCV and BKV viraria and PML.
| (PML cases/HIV-positive controls) |
|||||||
|---|---|---|---|---|---|---|---|
| JCV/BKV outcome | No. pairs | (Nea/Neft) | (Pos/Pos) | (Pos/Neg) | (Nep/Pos) | MOR* (95%CI) | |
| JCV Viruria | |||||||
| Viruria at any visit** | 26 | 11 | 5 | 4 | 6 | 0.7 (0.1 – 2.8) | |
| Transient Viruria† | 26 | 20 | 1 | 0 | 5 | - | |
| Persistent Viruria† | 26 | 17 | 1 | 6 | 2 | 3 (0.5 – 30.4) | |
| BKV Viruria | |||||||
| Viruria at any visit** | 26 | 18 | 0 | 3 | 5 | 0.6 (0.1 – 3.1) | |
Matched odds ratio and 95% confidence interval
JCV and BKV viruria at any visit defined as presence of JCV or BKV viruria at either visits 1,2 or 3
Persistent viruria defined as two or more consecutive visits with JCV Viruria; transient viruria defined as Viruria at one or more non-consecutive visits.
Of the 149 sera analyzed, JCV DNA was detected in a single serum (70 copies/ml) of one HIV-positive control. This man was also JCV viruric (40,668 copies/ml) at the same visit. He was JCV antibody positive on the visit prior to detection of viruria and viremia.
Risk factors for PML
Cases and matched HIV-positive controls were similarly distributed by age, baseline education, and steroid use. However, smoking was much more common in the cases than in the matched controls. Specifically, there were 9 pairs in which the case smoked but the control did not, while the reverse was true in only 1 pair (MOR: 9.0, 95% CI: 1.2–394.5). Smoking was also more common in the cases than in the unmatched HIV-negative controls (12/26 = 46% vs. 15/50=30%, respectively), but this difference was of only borderline significance (OR: 2.3, 95% CI: 0.99–5.2).
Correlates of JCV viruria
Table II shows risk factors for JCV viruria. Baseline CD4 and CD8 T-cell counts and CD4/CD8 T-cell ratios were examined among persons with antibodies to JCV and HIV only. Persons who were JCV viruric had higher baseline CD8 T-cell counts (median 695 cells/mm3, IQR: 530–945) than those who were not viruric (median 504 cells/mm3, IQR: 412–704; p = 0.05). Baseline CD4 T-cell counts and baseline CD4/CD8 T-cell ratios, however, were similar between persons with viruria and without viruria.
Table II.
Associations between JCV viruria and selected baseline laboratory and demographic variables; JCV seropositive patients only
| Shedding (n = 32) No. (%) | No Shedding (n = 46) No. (%) | OR (95% CI) | |
|---|---|---|---|
| CD4 (cells/mm3) † | |||
| HIV-positive patients only | |||
| ≤200 | 2 (66.7) | 1 (33.3) | 2.5 (0.2 – 30.3) |
| 200–500 | 3 (30.0) | 7 (70.0) | 0.5 (0.1 – 2.4) |
| ≥500 | 13 (44.8) | 16 (55.2) | 1 (Ref.) |
| CDS (cells/mm3) † | |||
| HIV-positive patients only | |||
| < 500 | 7 (63.6) | 4 (36.4) | 3.2 (0.76 – 13.3) |
| ≥500 | 11 (35.5) | 20 (64.5) | 1 (Ref.) |
| CD4/CD8 ratio † | |||
| HIV-positive patients only | |||
| <1.5 | 11 (61.1) | 22 (91.7) | 0.1 (0.28 – 0.81)** |
| ≥1.5 | 7 (38.9) | 2 (8.3) | 1 (Ref.) |
| Rate of CD4 decline (cells/mm3 per year) † | |||
| HIV-positive patients only | |||
| ≤ − 50 | 10 (55.6) | 11 (55.0) | 1.0 (0.28 – 3.7) |
| >−50 | 8 (44.4)) | 9 (45.0) | 1 (Ref.) |
| IgGO.D. ‡ | |||
| < 1.0 | 6 (18.7) | 24 (52.2) | 1 (Ref.) |
| ≥ 1.0 | 26 (81.3) | 22 (47.8) | 4.7 (1.6 – 13.6)*** |
| BKV Viruria | |||
| No viruria | 31 (96.9) | 41 (89.1) | 1 (Ref.) |
| Viruria | 1 (3.1) | 5 (10.9) | 0.3 (0.03 – 2.4) |
| Age | |||
| ≤30 | 9 (28.1) | 13 (20.3) | 1 (Ref.) |
| 30–40 | 14 (43.8) | 20 (46.5) | 1.01 (0.3 – 3.0) |
| ≥40 | 9 (28.1) | 20 (23.3) | 1.30 (0.4 – 4.5) |
| Smoke | |||
| No | 19 (59.4) | 29 (60.4) | 1 (Ref.) |
| Yes | 12 (37.5) | 17 (36.9) | 1.1 (0.4 – 2.8) |
| Education | |||
| Less than College | 3 (9.4) | 2 (4.6) | 1 (Ref.) |
| college | 16 (50.0) | 26 (60.5) | 0.57 (0.10 – 3.17) |
| Post-college | 13 (40.6) | 15 (34.9) | 0.87 (0.14 – 5.06) |
| Steroid Use * | |||
| No | 28 (87.5) | 35 (76.1) | 1 (Ref.) |
| Yes | 4 (12.5) | 11 (23.9) | 0.5 (0.1 – 1.6) |
Analysis restricted to HIV-positive patients (patients with JCV Viruria (n = 18), patients without JCV Viruria (n = 24))
Anti-JCV IgG optical density from visit 1 only
Steroid use includes use of oral, applied, or injected steroids
p < .05
p < .0001
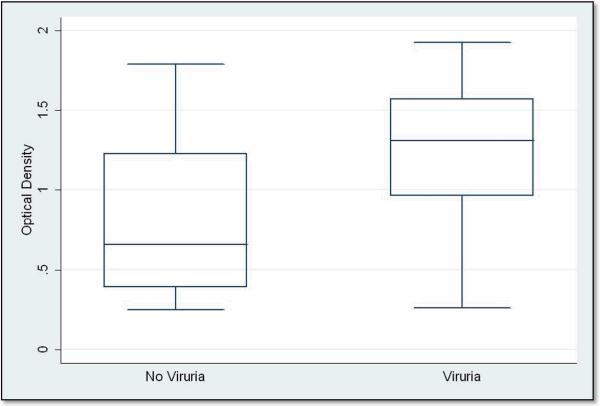
Antibody level to JCV capsid was also analyzed among cases and controls with viruria and without viruria. Cases and controls with higher anti-JCV IgG antibody levels had almost 5 times the odds of viruria than those with lower antibody levels (OR: 4.72, 95% CI: 1.64, 13.64). For MACS visit 1, the anti-JCV IgG O.D. value in persons with viruria was 1.3 as compared to only 0.66 in persons without viruria (p = 0.002) (Figure 3).
Figure 3.
Box plots of anti-JCV IgG OD values for patients with JCV viruria (n = 18) and without JCV viruria (n = 37) in HIV-positive and HIV-negative men with antibody to JCV capsid at visit 1.
In this analysis neither HIV-serostatus nor PML affected the relationship between JCV viruria and any of the variables assessed (data not shown).
Time to PML
To determine if likelihood of viruria was temporally related to the development of PML, we examined if cases were more likely to be viruric closer to their date of PML diagnosis using the last available visit for cases. No temporal trends in prevalence of JCV viruria were found (OR: 1.0, 95% CI: 0.8 –1.4).
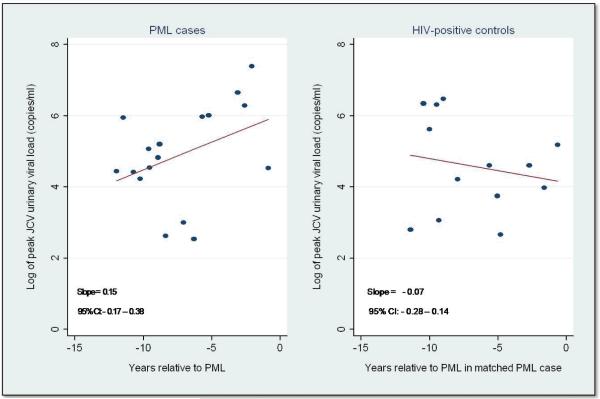
Time trends in magnitude of JCV viruria and antibody to JCV capsid among cases and controls were also examined. The number of JCV copies per ml of urine increased with increasing proximity to the diagnosis of PML, but this was not seen among HIV-positive controls (Figure 4) (p < 0.00001). To determine if these different trends in viral load were due to variation in immunologic variables, we compared baseline CD4 and CD8 T-cell counts, CD4/CD8 T-cell ratios, and average rate of CD4 T-cell decline between cases and controls with JCV viruria only. Cases and controls did not differ with respect to these variables (data not shown). In addition, no temporal differences in level of anti-JCV capsid antibody between case and control groups were observed.
Figure 4.
Log10 JCV copies per ml urine vs. years relative to PML (PML cases) and year relative to PML in matched PML case (HIV-positive controls) in PML cases and HIV-positive controls with JCV viruria.
BKV shedding
The prevalence of BKV DNA in the urine was 14.3% in cases, 19.2% in HIV-positive controls, and 4.0% in HIV-negative controls over all study visits examined. The median peak BKV viral load in cases was 4.4 log cp/ml (IQR: 3.7 – 6.6 log cp/ml), compared to 5.8 cp/ml in HIV-positive controls (IQR: 4.6 – 5.9 log cp/ml) and 4.0 log cp/ml in HIV-negative controls (IQR: 3.5 – 4.5 log cp/ml)). These differences were not statistically significant. BKV viruria was not associated with PML when cases were compared to HIV-positive controls (MOR: 0.6, 95% CI: 0.3– 6.3) or HIV-negative controls (OR: 4.0, 95% CI: 0.7– 23.4) (Table I).
Of the 46 individuals with either BKV or JCV viruria, only two patients shed both viruses in the urine and only one patient was BKV and JCV viruric simultaneously.
DISCUSSION
In this study, we took advantage of the opportunity to examine risk factors for PML using urine and serum specimens and other data collected prior to the development of PML from twenty-eight incident cases of PML in the MACS cohort. We hypothesized that if reactivation of JCV in the kidney commonly preceded PML, then viruria would be more common in future PML cases and that cases would shed the virus in the urine in larger quantities than HIV-positive men and HIV-negative men who did not develop PML.
Several of our results suggest that reactivation of JCV in the kidney is not a predictor of future PML. First, re-activation was seen in only a minority of the cases. Second, the overall prevalence of JCV viruria was similar between PML cases and HIV-positive and HIV-negative men who did not develop PML. Third, the prevalence of JCV viruria in cases and controls in our study was similar to those reported in cross-sectional analyses of urines from PML cases at the time of PML diagnosis and the results of other studies conducted among HIV-positive and HIV-negative persons (Ferrante et al., 1997; Knowles et al., 1997; Behzad-Behbahani et al., 2004). The lack of association between presence of JCV viruria and PML supports the hypothesis that the archetype virus, most commonly found in the urine, is not associated with viral pathogenecity.
Although viruria was seen in only a minority of cases prior to PML and no differences in prevalence of JCV viruria were found, we did find some differences in the pattern and level of JCV viruria between PML cases and HIV-positive men who did not develop PML. Persistent viruria tended to be the rule in cases (89%), but rather an exception in controls (33%). Furthermore, stable or increasing JCV DNA load was observed more frequently in cases. In a stratified analysis of men with JCV viruria, JCV levels in the urine increased over time in the cases but declined over time in the HIV-positive men who did not develop PML. The difference in these slopes was statistically significant suggesting that increasing JCV DNA load in the urine in persons shedding JCV may be predictive of PML.
Unexpectedly, four cases tested JCV-seronegative, including one who was JCV-seronegative only five months prior to being diagnosed with PML. The other three cases were seronegative two, six, and seven years prior to their diagnosis. While it is possible that low levels of JCV antibodies may have been missed by the ELISA assay used, these data suggest the possibility that some cases of PML may result from primary infection of JCV rather than reactivation of latent virus. Consequently, persons who screen JCV seronegative may not necessarily be at less risk for disease than those who are JCV seropositive.
Neither presence nor level of antibody to JCV capsid was associated with the development of PML. Higher levels of antibody to JCV, however, were significantly associated with JCV viruria. These results agree with the findings of a previous study and suggest that high levels of IgG antibodies to JCV are markers of viral replication in the kidney (Engels et al., 2005).
In this study, JCV DNA was not detected in the pre-diagnostic serum samples of PML cases, consistent with a previous report that JCV DNA could not be detected in pre-diagnostic samples of patients with PML associated with natalizumab therapy (Yousry et al., 2006). However, a limitation of our study was the long interval, for many cases, between the time the pre-diagnostic sample was obtained and the date of diagnosis of PML. A low copy number of virus, however, was detected in one HIV-positive man who did not develop PML. This individual was also JCV viruric at the same time, but no JCV DNA was detected in urine or serum at the next visit.
BKV DNA was detected in only a small proportion of the urines from both HIV-positive and HIV-negative men, as has been reported (Ahsan and Shah, 2006; Behzad-Behbahani et al., 2004). Although JCV and BKV are biologically and genetically similar, no association between JCV and BKV viruria was found.
It is of interest that the PML case who was JCV seronegative five months prior to his PML diagnosis also had the highest BKV viral load. A diagnosis of PML for this case was made on the basis of a pathologic examination of a brain biopsy sample and clinical assessment. However, tissue was not available for further analysis. Although BKV has never been associated with PML, it is conceivable BKV may occasionally be associated with PML; nephropathy in renal transplant recipients, which is associated most commonly with BKV, is sometimes caused by JCV (Hirsch et al., 2006; Wen et al., 2004).
An intriguing finding was the association between baseline smoking status and PML. This is the first study to observe this association. The relationship between viral reactivation and smoking is not clear, although one study found that increased levels of nicotine in the blood were associated with reactivation of herpes simplex virus- 1 in rabbits (Myles et al., 2003). In our study, smoking was not associated with JCV viruria, suggesting that smoking was not related to reactivation in the kidneys, but leaving open the possibility it may affect this process elsewhere in the body. Future studies of PML should examine this relationship further, particularly given the modifiable nature of the risk factor and its potential implication on the modulation of treatment therapies, such as natalizumab, among patients who smoke.
In an analysis of risk factors for JCV viruria, we found lower levels of CD8 T-cells were associated with presence of JCV in the urine. Conversely, CD4 T-cell count was not associated with viruria. This agrees with the findings of Knowles et al., based on a study of 94 HIV-infected adults (Knowles et al., 1999).
There were several limitations to our study. The relatively small sample size may have limited the power to detect differences between cases and controls. Specimens were collected very early in the study, resulting in a lack of data close to PML diagnosis for many of the cases. The window of time over which JCV viruria was analyzed was narrow and may have been insufficient to assess the patterns of JCV shedding and immunologic responses to JCV accurately. HIV viral load data was not available on most patients so we were unable to determine what effects, if any, HIV-viral load may have had on the risk of subsequent PML. Finally, our study examined JCV viruria and immunologic responses to JCV among HIV-positive PML patients, and so our results are not generalizable to PML patients without HIV.
On the other hand, the study had several strengths. This is among the largest epidemiologic studies of PML to date. Additionally, this study is the first to examine risk factors for PML using data and biological samples collected prior to the diagnosis of PML, and thus not subject to recall or prevalence bias that may happen in traditional case-control studies or those in which samples are obtained after the diagnosis.
In conclusion, we found that the presence of JCV viruria and IgG antibody to JCV capsid were not associated with increased risk for PML. Additionally, we found that neither the presence of viruria nor antibody or antibody level to JCV was related temporally to the diagnosis of PML. However, among persons who were JCV viruric, we found that both persistent JCV viruria and increasing JCV DNA load in the urine were strongly associated with PML. The monitoring of these risk factors in patients receiving therapies associated with PML, such as natalizumab, may be useful in clinical settings. These findings should be evaluated in future studies.
ACKNOWLEDGEMENTS
Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers (Principal Investigators) at The Johns Hopkins University Bloomberg School of Public Health (Joseph B. Margolick, Lisa Jacobson), Howard Brown Health Center and Northwestern University Medical School (John Phair), University of California, Los Angeles (Roger Detels), and University of Pittsburgh (Charles Rinaldo). The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute and the National Heart, Lung and Blood Institute (UO1-AI-35042, 5-MO1-RR-00722 (GCRC), UO1-AI-35043, UO1-AI-37984, UO1-AI-35039, UO1-AI-35040, UO1-AI-37613, UO1-AI-35041).
The serological testing in this study was also supported by grants from the National Cancer Institutes (RO1 CA118348) and the National Institute of Allergy and Infectious Disease (RO1 AI63360).
The investigators also acknowledge Richard Daniel for his assistance with the laboratory aspects of this study.
REFERENCES
- Ahsan N, Shah KV. Polyomaviruses and human diseases. Adv. Exp. Med. Biol. 2006;577:1–18. doi: 10.1007/0-387-32957-9_1. [DOI] [PubMed] [Google Scholar]
- Astrom KE, Mancall EL, Richardson EP. Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin's disease. Brain. 1958;81:93–111. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]
- Azzi A, De Santis R, Ciappi S, Leoncini F, Sterrantino G, Marino N, Mazzotta F, Laszlo D, Fanci R, Bosi A. Human polyomaviruses DNA detection in peripheral blood leukocytes from immunocompetent and immunocompromised individuals. J. Neurovirol. 1996;2:411–416. doi: 10.3109/13550289609146907. [DOI] [PubMed] [Google Scholar]
- Behzad-Behbahani A, Klapper PE, Vallely PJ, Cleator GM, Khoo SH. Detection of BK virus and JC virus DNA in urine samples from immunocompromised (HIV-infected) and immunocompetent (HIV-non-infected) patients using polymerase chain reaction and microplate hybridisation. J. Clin. Virol. 2004;29:224–229. doi: 10.1016/S1386-6532(03)00155-0. [DOI] [PubMed] [Google Scholar]
- Berger JR. Progressive multifocal leukoencephalopathy in acquired immunodeficiency syndrome: explaining the high incidence and disproportionate frequency of the illness relative to other immunosuppressive conditions. J. Neurovirol. 2003;9(Suppl 1):38–41. doi: 10.1080/13550280390195261. [DOI] [PubMed] [Google Scholar]
- Berger JR, Major EO. Progressive multifocal leukoencephalopathy. Semin. Neurol. 1999;19:193–200. doi: 10.1055/s-2008-1040837. [DOI] [PubMed] [Google Scholar]
- Boldorini R, Veggiani C, Barco D, Monga G. Kidney and urinary tract polyomavirus infection and distribution: molecular biology investigation of 10 consecutive autopsies. Arch. Pathol. Lab. Med. 2005;129:69–73. doi: 10.5858/2005-129-69-KAUTPI. [DOI] [PubMed] [Google Scholar]
- Brooks BR, Walker DL. Progressive multifocal leukoencephalopathy. Neurol. Clin. 1984;2:299–313. [PubMed] [Google Scholar]
- Carter JJ, Madeleine MM, Wipf GC, Garcea RL, Pipkin PA, Minor PD, Galloway DA. Lack of serologic evidence for prevalent simian virus 40 infection in humans. J. Natl. Cancer Inst. 2003;95:1522–1530. doi: 10.1093/jnci/djg074. [DOI] [PubMed] [Google Scholar]
- Chesters PM, Heritage J, McCance DJ. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J. Infect. Dis. 1983;147:676–684. doi: 10.1093/infdis/147.4.676. [DOI] [PubMed] [Google Scholar]
- Delbue S, Sotgiu G, Fumagalli D, Valli M, Borghi E, Mancuso R, Marchioni E, Maserati R, Ferrante P. A case of a progressive multifocal leukoencephalopathy patient with four different JC virus transcriptional control region rearrangements in cerebrospinal fluid, blood, serum, and urine. J. Neurovirol. 2005;11:51–57. doi: 10.1080/13550280590900382. [DOI] [PubMed] [Google Scholar]
- de Sanjose S, Shah KV, Domingo-Domenech E, Engels EA, Fernandez de Sevilla A, Alvaro T, Garcia-Villanueva M, Romagosa VE, Gonzalez-Barca E, Viscidi RP. Lack of serological evidence for an association between simian virus 40 and lymphoma. Int. J. Cancer. 2003;104:522–524. doi: 10.1002/ijc.10993. [DOI] [PubMed] [Google Scholar]
- Dorries K, Muelen VT. Progressive multifocal leucoencephalopathy: detection of papovavirus JC in kidney tissue. J. Med. Virol. 1983;2:89–110. doi: 10.1002/jmv.1890110406. [DOI] [PubMed] [Google Scholar]
- Dorries K. Human polyomavirus JC and BK persistent infection. In: Ahsan N, editor. Polyomaviruses and Human Diseases. Springer Science; New York: 2006. pp. 102–111. [DOI] [PubMed] [Google Scholar]
- Elsner C, Dorries K. Evidence of human polyomavirus BK and JC infection in normal brain tissue. Virology. 1992;191:72–80. doi: 10.1016/0042-6822(92)90167-n. [DOI] [PubMed] [Google Scholar]
- Engels EA, Rollison DE, Hartge P, Baris D, Cerhan JR, Severson RK, Cozen W, Davis S, Biggar RJ, Goedert JJ, Viscidi RP. Antibodies to JC and BK viruses among persons with non-Hodgkin lymphoma. Int. J. Cancer. 2005;117:1013–1019. doi: 10.1002/ijc.21277. [DOI] [PubMed] [Google Scholar]
- Ferrante P, Caldarelli-Stefano R, Omodeo-Zorini E, Cagni AE, Cocchi L, Suter F, Maserati R. Comprehensive investigation of the presence of JC virus in AIDS patients with and without progressive multifocal leukoencephalopathy. J. Med. Virol. 1997;52:235–242. doi: 10.1002/(sici)1096-9071(199707)52:3<235::aid-jmv1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Gallia G, Houff SA, Major EO, Khalili K. Review: JC virus infection of lymphocytes--revisited. J. Infect. Dis. 1997;176:1603–1609. doi: 10.1086/514161. [DOI] [PubMed] [Google Scholar]
- Garcia-Suarez J, de Miguel D, Krsnik I, Banas H, Arribas I, Burgaleta C. Changes in the natural history of progressive multifocal leukoencephalopathy in HIV-negative lymphoproliferative disorders: impact of novel therapies. Am. J. Hematol. 2005;80:271–281. doi: 10.1002/ajh.20492. [DOI] [PubMed] [Google Scholar]
- Giorgi JV, Cheng HL, Margolick JB, Bauer KD, Ferbas J. Quality control in the flow cytometric measurement of T-lymphocyte subsets: the multicenter AIDS cohort study experience. The Multicenter AIDS Cohort Study Group. Clin Immunol Immunopathol. 1990;55:173–186. doi: 10.1016/0090-1229(90)90096-9. [DOI] [PubMed] [Google Scholar]
- Hamilton RS, Gravell M, Major EO. Comparison of antibody titers determined by hemagglutination inhibition and enzyme immunoassay for JC virus and BK virus. J Clin Microbiol. 2000;38:105–9. doi: 10.1128/jcm.38.1.105-109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch HH, Drachenberg CB, Steiger J, Ramos E. Polyomavirus-associated nephropathy in renal transplantation: critical issues of screening and management. Adv. Exp. Med. Biol. 2006;577:160–173. doi: 10.1007/0-387-32957-9_11. [DOI] [PubMed] [Google Scholar]
- Holman RC, Torok TJ, Belay ED, Janssen RS, Schonberger LB. Progressive multifocal leukoencephalopathy in the United States, 1979–1994: increased mortality associated with HIV infection. Neuroepidemiology. 1998;17:303–309. doi: 10.1159/000026184. [DOI] [PubMed] [Google Scholar]
- Kaslow RA, Ostrow DJ, Detels R, Phair JP, Polk BF, Rinaldo CR. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am. J. Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- Kiewe P, Seyfert S, Korper S, Rieger K, Thiel E, Knauf W. Progressive multifocal leukoencephalopathy with detection of JC virus in a patient with chronic lymphocytic leukemia parallel to onset of fludarabine therapy. Leuk. Lymphoma. 2003;44:1815–1818. doi: 10.1080/1042819031000116625. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N. Engl. J. Med. 2005;353:369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- Knowles WA, Pillay D, Johnson MA, Hand JF, Brown DW. Prevalence of long-term BK and JC excretion in HIV-infected adults and lack of correlation with serological markers. J. Med. Virol. 1999;59:474–479. [PubMed] [Google Scholar]
- Koralnik IJ, Boden D, Mai VX, Lord CI, Letvin NL. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology. 1999;52:253–260. doi: 10.1212/wnl.52.2.253. [DOI] [PubMed] [Google Scholar]
- Langer-Gould AS, Atlas W, Green AJ, Bollen W, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N. Engl. J. Med. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- Lednicky JA, Vilchez RA, Keitel WA, Visnegarwala F, White ZS, Kozinetz CA, Lewis DE, Butel JS. Polyomavirus JCV excretion and genotype analysis in HIV-infected patients receiving highly active antiretroviral therapy. AIDS. 2003;17:801–807. doi: 10.1097/00002030-200304110-00004. [DOI] [PubMed] [Google Scholar]
- Ling PD, Lednicky JA, Keitel WA, Poston DG, White ZS, Peng R, Liu Z, Mehta SK, Pierson DL, Rooney CM, Vilchez RA, Smith EO, Butel JS. The dynamics of herpesvirus and polyomavirus reactivation and shedding in healthy adults: a 14-month longitudinal study. J. Infect. Dis. 2003;187:1571–1580. doi: 10.1086/374739. [DOI] [PubMed] [Google Scholar]
- Markowitz RB, Thompson HC, Mueller JC, Cohen JA, Dynan WS. Incidence of BK virus and JC virus viruria in human immunodeficiency virus-infected and -uninfected subjects. J. Infect. Dis. 1993;167:13–20. doi: 10.1093/infdis/167.1.13. [DOI] [PubMed] [Google Scholar]
- Munoz A, Carey V, Saah AJ, Phair JP, Kingsley LA, Fahey JL, Ginzburg HM, Polk BF. Predictors of decline in CD4 lymphocytes in a cohort of homosexual men infected with human immunodeficiency virus. J. Acquir. Immune Defic. Syndr. 1988;1:396–404. [PubMed] [Google Scholar]
- Myles ME, Alack C, Manino PM, Reish ER, Higaki S, Maruyama K, Mallakin A, Azcuy A, Barker S, Ragan FA, Thompson H, Hill JM. Nicotine applied by transdermal patch induced HSV-1 reactivation and ocular shedding in latently infected rabbits. J. Ocul. Pharmacol. Ther. 2003;19:121–133. doi: 10.1089/108076803321637654. [DOI] [PubMed] [Google Scholar]
- Richardson EP. Progressive multifocal leukoencephalopathy 30 years later. N. Engl. J. Med. 1988;318:315–317. doi: 10.1056/NEJM198802043180510. [DOI] [PubMed] [Google Scholar]
- Rollison DE, Engels EA, Halsey NA, Shah KA, Viscidi RP, Helzlsouer KJ. Prediagnostic circulating antibodies to JC and BK human polyomaviruses and risk of non-Hodgkin lymphoma. Cancer Epidemiol. Biomarkers Prev. 2006;15:543–550. doi: 10.1158/1055-9965.EPI-05-0728. [DOI] [PubMed] [Google Scholar]
- Rollison DE, Helzlsouer KJ, Alberg AJ, Hoffman S, Hou J, Daniel R, Shah K, Major EO. Serum antibodies to JC virus, BK virus, simian virus 40, and the risk of incident adult astrocytic brain tumors. Cancer Epidemiol. Biomarkers Prev. 2003;12:460–463. [PubMed] [Google Scholar]
- Ryschkewitsch C, Jensen P, Hou J, Fahle G, Fischer S, Major EO. Comparison of PCR-southern hybridization and quantitative real-time PCR for the detection of JC and BK viral nucleotide sequences in urine and cerebrospinal fluid. J. Virol. Methods. 2004;121:217–221. doi: 10.1016/j.jviromet.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Selik RM, Karon JM, Ward JW. Effect of the human immunodeficiency virus epidemic on mortality from opportunistic infections in the United States in 1993. J. Infect. Dis. 1997;176:632–636. doi: 10.1086/514083. [DOI] [PubMed] [Google Scholar]
- Shah KV. Polyomaviruses. In: Fields B, Knipe D, Howley P, editors. Fields Virology. Lippincott-Raven; Philadelphia: 1996. pp. 2027–2043. [Google Scholar]
- Van Assche G, Van Ranst M, Sciot R, Dubois B, Vermeire S, Noman M, Verbeeck J, Geboes K, Robberecht W, Rutgeerts P. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N. Engl. J. Med. 2005;353:362–368. doi: 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- Vidarsson B, Mosher DF, Salamat MS, Isaksson HJ, Onundarson PT. Progressive multifocal leukoencephalopathy after fludarabine therapy for low-grade lymphoproliferative disease. Am. J. Hematol. 2002;70:51–54. doi: 10.1002/ajh.10085. [DOI] [PubMed] [Google Scholar]
- Viscidi RP, Rollison DE, Viscidi E, Clayman B, Rubalcaba E, Daniel R, Major EO, Shah KV. Serological cross-reactivities between antibodies to simian virus 40, BK virus, and JC virus assessed by virus-like-particle-based enzyme immunoassays. Clin. Diagn. Lab. Immunol. 2003;10:278–285. doi: 10.1128/CDLI.10.2.278-285.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F, Goldmann C, Kramer M, Kaup FJ, Pickhardt M, Young P, Petry H, Weber T, Luke W. Cellular and humoral immune response in progressive multifocal leukoencephalopathy. Ann. Neurol. 2001;49:636–642. [PubMed] [Google Scholar]
- Weber T, Trebst C, Frye S, Cinque P, Vago L, Sindic CJ, Schulz-Schaeffer WJ, Kretzschmar HA, Enzensberger H, Hunsmann G, Luke W. Analysis of the systemic and intrathecal humoral immune response in progressive multifocal leukoencephalopathy. J. Infect. Dis. 1997;176:250–254. doi: 10.1086/514032. [DOI] [PubMed] [Google Scholar]
- Wen MC, Wang CL, Wang M, Cheng CH, Wu MJ, Chen CH, Shu KH, Chang D. Association of JC virus with tubulointerstitial nephritis in a renal allograft recipient. J. Med. Virol. 2004;72:675–678. doi: 10.1002/jmv.20037. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Major EO, Ryschkewitsch C, Fahle G, Fischer S, Hou J, Curfman B, Miszkiel K, Mueller-Lenke N, Sanchez E, Barkhof F, Radue EW, Jager HR, Clifford DB. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N. Engl. J. Med. 2006;354:924–933. doi: 10.1056/NEJMoa054693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- ZuRhein GM. Association of papova-virions with human demyelinating disease (progressive multifocal leukoencephalopathy) Prog Med Virol. 1969;11:185–247. [PubMed] [Google Scholar]