Abstract
Although the innate immune function of mast cells in the acute phase of parasitic and bacterial infections is well established, their participation in chronic immune responses to indolent infection remains incompletely understood. In parasitic infection with Trichinella spiralis, the immune response incorporates both lymphocyte and mast cell-dependent effector functions for pathogen eradication. Among the mechanistic insights still unresolved in the reaction to T. spiralis are the means by which mast cells respond to parasites and the mast cell effector functions that contribute to the immunologic response to this pathogen. We hypothesized that mast cell elaboration of tryptase may comprise an important effector component in this response. Indeed, we find that mice deficient in the tryptase mouse mast cell protease-6 (mMCP-6) display a significant difference in their response to T. spiralis larvae in chronically infected skeletal muscle tissue. Mechanistically, this is associated with a profound inability to recruit eosinophils to larvae in mMCP-6-deficient mice. Analysis of IgE-deficient mice demonstrates an identical defect in eosinophil recruitment. These findings establish that mast cell secretion of the tryptase mMCP-6, a function directed by the activity of the adaptive immune system, contributes to eosinophil recruitment to the site of larval infection, thereby comprising an integral link in the chronic immune response to parasitic infection.
The role of mast cells as sentinels in innate immune responses acting acutely against infectious pathogens is well established (1, 2). They respond rapidly to a variety of stimuli and organisms via recognition by pathogen-associated molecular pattern receptors, including Toll-like receptors, mannosyl receptors, and others (reviewed in Ref. 3). In this context, mast cells participate in rapid mobilization of the innate immune response by elaborating leukocyte chemoattractants and can participate in stimulating initial adaptive immune responses either by direct Ag presentation or by inducing the migration of dendritic cells or Langerhans cells to draining lymph nodes (4–6).
What remains less clear is the mast cell participation in chronic responses to pathogens whose infections are long term and whose clearance requires orchestration by the adaptive immune system. Are mast cells members of the “orchestra” directed by the adaptive immune response to chronic infection? Mast cells are theoretically capable of responding to signals from the adaptive immune system through stimulation of cytokine receptors (IL-1R, IL-10R, IL-12R, IFN-γR) (reviewed in Ref. 3) or by activation via Ig receptors such as FcεRI or FcγRIII (7). Indeed, the clearest examples of mast cell responses directed by adaptive immunity are their involvement in allergic disease via stimulation by IgE (reviewed in Ref. 8) and their participation in autoantibody-driven autoimmune diseases (9, 10). Aside from these pathologic states, very little else is known about the use of mast cells in the adaptive immune system directed toward clearance of chronic infections.
Chronic parasitic infections elicit robust humoral responses from the adaptive immune system, and this suggests a role for mast cells in the coordinated immune response required for pathogen clearance. A well-established model of parasitic infection is that of the intestinal nematode Trichinella spiralis (11–13). Acute infection by T. spiralis proceeds from ingestion of larvae, which mature and move into the small intestine and burrow into the intestinal mucosa. There, they produce larvae that penetrate the intestine and disseminate to muscle. In this chronic phase of infection, larvae infect muscle tissue, forming nurse cells (14). Active muscles, such as diaphragm and tongue, typically display the highest levels of chronic parasite burden. Within 2 wk of infection, high titers of serum IgE are present and a T cell-dependent intestinal mastocytosis is prominent (15–18). Previous studies have demonstrated a functional contribution from both lymphocytes and mast cells in the clearance of adult parasites from the intestine. Mast cell-deficient mice or wild-type mice whose mast cells have been depleted by anti-c-kit Abs display profoundly delayed expulsion of the parasite (19–24). Similarly, mice lacking lymphocytes also exhibit a significant inability to resolve the primary gastrointestinal infection by T. spiralis (24). Furthermore, the exogenous addition of the mast cell growth factor IL-3 or the Th2 cytokine IL-4 increases the rate of intestinal pathogen clearance (24, 25).
The mast cell population present within the infected intestinal tissues is not homogenous. Rather, mast cells from both the mucosal mast cell (MMC)3 subpopulation (that express mouse mast cell protease (mMCP)-1 and mMCP-2)) and the connective tissue-type mast cell (CTMC) subpopulation (that express mMCP-4, mMCP-5, mMCP-6, and mMCP-7) are present. The MMC subset undergoes massive hyperplasia in the infected intestinal mucosa, while the CTMC population remains sparse and located predominantly in the intestinal serosal region. Mechanistically, mast cells have been shown to participate in intestinal expulsion of Trichinella by elaboration of the chymase mMCP-1 (26, 27). This observation was consistent with the functional importance of the expanded MMC subpopulation in this tissue in the initial responses to this early stage of T. spiralis infection.
Unlike the intestinal phase of T. spiralis infection, little is known about the mechanisms of host immune response in the chronic, skeletal muscle stage of disease. Histologically, clearance of the larvae is signified by patchy necrotic lesions characterized by infiltration of eosinophils, lymphocytes, and macrophages, which later turn into calcified deposits (28, 29). Eosinophils have been implicated in newborn larval cytotoxicity by both in vitro and in vivo studies (30–32). This has been further supported by more recent in vivo studies. In chemokine receptor CCR3-deficient mice, there is little eosinophil influx around the larvae infecting skeletal muscle, unlike in wild-type mice, and there is decreased immune response to these larvae (28). IgE also contributes to the immune response to larval presence through a mechanism not fully understood, although activation of mast cells found in the skeletal muscle has been hypothesized (29). Mouse eosinophils are not thought to express the high-affinity IgE receptor; however, eosinophil activation via antibody-dependent cytotoxicity is not excluded and has been suggested by in vitro studies (31).
Skeletal muscle is populated exclusively with the CTMC subpopulation (33) that expresses both the chymotryptic-like serine proteases called chymases and the tryptic-like serine proteases called tryptases. Previous in vivo analyses with recombinant tryptases mMCP-6 and mMCP-7 have demonstrated potent leukocyte chemoattractant properties for these neutral proteases (34, 35). Knowing that degranulation and release of granule proteases are among the effector functions elicited by IgE stimulation, we hypothesized that IgE-stimulated release of mast cell tryptase comprises an important mediator in the immunologic response to parasites in skeletal muscle. Herein, using a newly generated strain of mMCP-6-deficient mice, we demonstrate that this tryptase functions to direct the immune response to T. spiralis-infected skeletal muscle via the recruitment of eosinophils. Furthermore, we demonstrate the same functional immunologic deficit in mice lacking IgE. Taken together, these observations demonstrate that mast cell activation, directed by the adaptive immune system, comprises an important facet of immune system function in response to chronic pathogen infection.
Materials and Methods
Generation of mMCP-6−/− mice
The mMCP-6 gene locus was knocked out using a transgenic construct transfected to 129/Sv strain embryonic stem cells (Fig. 1). Sequence of the mMCP-6 gene was obtained by probing both National Institutes of Health and Celera mouse genome sequences. The 5′ and 3′ arms of the targeting construct were obtained by using a PCR cloning strategy. These genomic sequences were cloned into recipient plasmids (pCR-TOPO, Invitrogen) and subcloned into pBlueScript (Stratagene) along with a loxP-flanked neomycin selection marker from pLNTK. The construct was cloned into a diphtheria toxin expressing polylinker 915 vector. 129/Sv embryonic stem cells were electroporated with 10 μg of vector and screened for homologous recombination with Southern blot hybridization. Positive clones were injected into C57BL/6 blastocysts, and chimeric males were mated to C57BL/6 females. Germline transmission was assayed by Southern blot of tissue from the F1 offspring. Heterozygous mice were first mated to the Cre-expressing strain B6.FVB-Tg(EIIa-cre)C5379Lmgd/J (36), and deletion of the loxP-flanked PGK-neor was confirmed by PCR. These offspring were further backcrossed to C57BL/6J mice for five generations (N5), and then intercrossed to generate N5 homozygous mMCP-6−/− and control wild-type mice.
FIGURE 1.
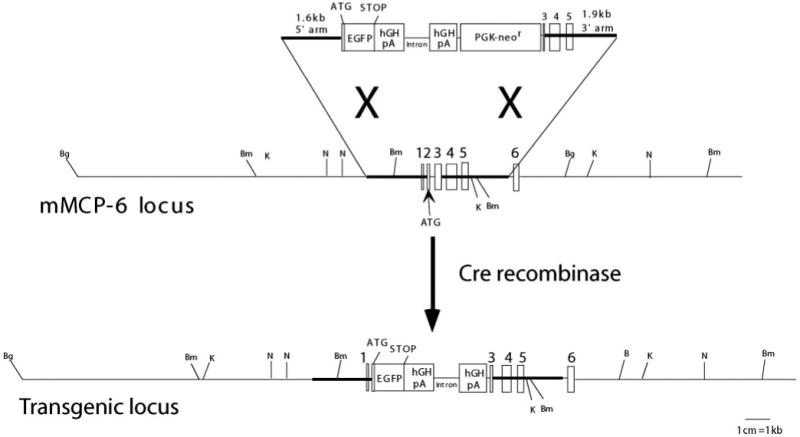
Map of mMCP-6 gene and targeting construct. After homologous recombination, the final product deletes exon 2 after the initial fMet codon through the proximal portion of exon 3. Stop codons in all frames are introduced after exon 2 into the mMCP-6 locus. Founder mice were mated with the Cre-expressing strain B6.FVB-Tg(EIIa-cre)C5379Lmgd/J (36) to delete the PGK-neor selecting element before backcrossing (Bg indicates BglII; Bm, BamHI; K, KpnI; N, NheI).
Mice infected with T. spiralis
In addition to mMCP-6−/− mice, we used IgE-deficient mice backcrossed through 10 generations onto the BALB/cAnTac (Taconic Farms) background (29, 37). BALB/cAnTac mice were used as controls. All mice were 9–12 wk of age when infected with T. spiralis. All experiments were conducted with the approval of the Dana-Farber Cancer Institute Animal Care and Use Committee.
T. spiralis infection and worm burden analysis
Mice were infected orally with freshly isolated T. spiralis larvae, as previously described (38). With a gastric gavage, ∼450 larvae were injected to each mouse. Five mice per group were selected for determination of the intestinal worm burden at each time point. The small intestine was sectioned into 1–2-cm pieces in a 100-mm petri dish containing 20 ml of PBS. After rocking the dish slowly for 3 h, adult worms were counted under an inverted microscope (Nikon). To evaluate worm burdens histomorphometrically, 9–10 mice per group per experiment were infected with T. spiralis larvae and euthanized at 5 wk. Tongue tissues were obtained and worm burdens were histomorphometrically quantified as previously described (28, 29). To quantify skeletal worm burdens via isolation of parasites from tissue, tongues from experimental mice were weighed and then digested with 1% pespsin, 0.1 N HCl for 4 h, and the total numbers of larvae per digested tongue were enumerated via inverted microscopy (29).
Histology
Organs including ear skin, stomach, lung, spleen, tongue, and a small piece (∼2 cm) of jejunum of both healthy and T. spiralis-infected mice were fixed in 4% paraformaldehyde in PBS for 24 h, and then embedded in paraffin. Tissue was cut into 3-μm sections. Slides were deparaffinized in xylene for 5 min twice and then hydrated in graded ethanol. For toluidine blue staining, a working solution of 0.1% toluidine blue O (Sigma-Aldrich) in water was prepared. Tissue sections were stained for 30 s, rinsed in distilled water, next dehydrated in graded alcohol, and mounted with Cytoseal 60 (Richard-Allan Scientific). H&E staining and Congo red staining were performed as previously described (28).
Immunohistochemistry
Immunohistochemistry of mast cell proteases mMCP-1, mMCP-4, mMCP-5, mMCP-6, and mMCP-7 was performed with Vectastain ABC-alkaline phosphatase kit (Vector Laboratories) as previously described (39). After a final series of washes in PBS, 200 μl of Fast Red (1 mg/ml) (Sigma-Aldrich) substrate was applied for 25 min. Tissue was subsequently counterstained with Gill II hematoxylin and mounted with Crystal/Mount (Biomeda). Immunohistochemistry of IgE was done with Vectastain ABC-peroxidase kit (Vector Laboratories) using anti-IgE (Novus Biologicals) as previously described (29).
Histomorphometric analysis
Mast cell density in tongue or intestine was determined by the average number of toluidine blue-positive cells in five low power fields (LPF) under a light microscope (Leica Microsystems). Necrotic larvae in the tongue were defined by lesions with heavy infiltration of inflammatory cells invading the nurse cell around the parasite as previously described (28, 29). To increase our sampling accuracy, sections were obtained at three depths separated by at least 100 μm for each tongue. The percentage of necrotic larvae was enumerated at each depth in each tongue section. The number of live larvae in five LPF was also calculated in all sections. For the eosinophil density around the larva, an eyepiece reticule (Leica) was used to define a square unit of 0.04 mm2 encompassing individual larva. Congo red-positive eosinophils inside the unit area were enumerated in 40 larvae per experimental group. Separately, serial sections within a single depth were evaluated to confirm consistency of enumeration for individual larvae. Other cell lineages were enumerated as described (40). Observers were blinded to the experimental condition and genotype in all analyses.
Western blot analysis
Bone marrow-derived mast cells were cultured from both N5 mMCP-6−/− and wild-type mice. Briefly, cells obtained from femur flushes with DMEM (Life Technologies) were cultured in media with 10 ng/ml stem cell factor (PeproTech) and 10 ng/ml recombinant IL-3 (Pierce) at 10% CO2, 37°C for 5 wk. Media was changed weekly and added with fresh cytokines. Cells were lysed with Triton X-100-based buffer supplemented with a protease inhibitor cocktail (Sigma-Aldrich). Proteins were blotted onto a PolyScreen membrane (0.45 μm pore size, PerkinElmer) and then the membrane was blocked with 1% milk in TBS containing 0.05% Tween 20 (Sigma-Aldrich) for 2 h. After incubation with mMCP-6 or mMCP-7 Ab diluted 1/10,000 in TBS/Tween 20, membrane was washed and then incubated with HRP-conjugated donkey anti-rabbit Ab (Jackson ImmunoResearch) diluted 1/10,000 with TBS/Tween 20. Western Lighting (PerkinElmer) was used as the substrate for development.
ELISA
Serum IgE levels were determined as previously described (29). Briefly, 96-well plates were coated with purified anti-IgE (BD Pharmingen) at 1 μg/ml overnight at 4°C. The next day, plates were washed twice with PBS containing 0.05% Tween 20. Serum was added at dilutions ranging from 1/500 to 1/1500. Standards and sera were diluted in PBS/1% BSA (Sigma-Aldrich) and left on plates for 2 h. Plates were then washed and incubated for 45 min with biotinylated anti-IgE at 1 μg/ml. After washes, plates were given avidin conjugated to HRP (Zymed Laboratories) and incubated for 30 min. Plates were washed before adding substrate, 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (Zymed Laboratories). OD405 was measured 10–20 min after addition of substrate. Specific IgG against T. spiralis was measured using a LMD Trichinella serology microwell ELISA kit (Remel) per the manufacturer's protocol.
Statistical analysis
Data were analyzed using Prism software v4.0 (GraphPad Software). All mean values are presented as the means ± SE. For comparison of the mean values, Student's t test (two-tailed) was performed. p values <0.01 were considered significant.
Results
mMCP-6−/− mice
We generated tryptase mMCP-6−/− mice by disrupting exons 1 and 2 via homologous recombination in 129/Sv ES cells (Fig. 1). mMCP-6−/− mice are viable, fertile, devoid of any opportunistic infection in specific pathogen-free housing conditions, and lack detectable mMCP-6 protein in tissue mast cells and in bone marrow-derived cultured mast cells (Fig. 2A). We backcrossed the 129/Sv strain founder mice for five generations onto the C57BL/6 strain for these studies. To exclude aberrant mast cell protease expression in vivo, we stained tongue CTMC with protease-specific Abs and found expression of the chymases mMCP-4 and mMCP-5 intact in mMCP-6−/− mice (Fig. 2B). Since the mMCP-7 gene locus resides immediately adjacent to the mMCP-6 locus, and since 129/Sv strain mice have intact mMCP-7 while C57BL/6 mice contain a null mMCP-7 gene (41), we also examined mMCP-7 expression in tongue CTMC. As expected, mMCP-7 was expressed in mMCP-6−/− CTMCs, in contrast to wild-type littermates (Fig. 2). Our data indicate that mMCP-6 is not essential for the expression of these CTMC proteases.
FIGURE 2.
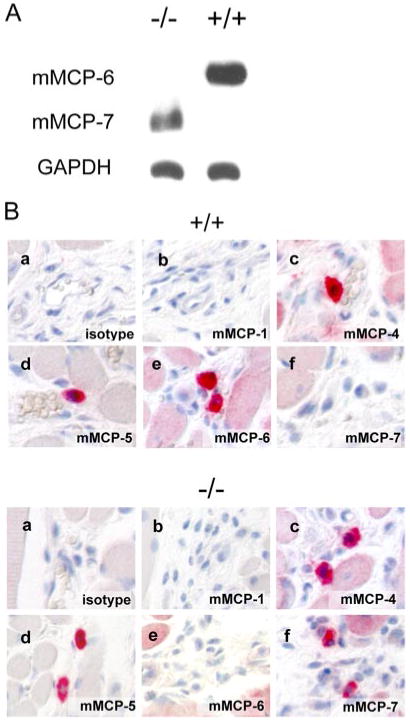
Absence of mMCP-6 protein expression in mMCP-6−/− mice. A, Western blots of wild-type and mMCP-6−/− bone marrow-derived mast cell (BMMC) lysates demonstrate lack of detectable mMCP-6 protein. Note that in contrast to C57BL/6 wild-type BMMCs, mMCP-6−/− BMMCs express mMCP-7. B, Immunohistochemistry of tongue connective tissue mast cells from wild-type and mMCP-6−/− mice demonstrate lack of mMCP-6 expression in vivo as well as intact mMCP-4 and mMCP-5 expression. Note mMCP-7 expression in the wild-type strain (a, isotype control; b, anti-mMCP-1; c, anti-mMCP-4; d, anti-mMCP-5; e, anti-mMCP-6; f, anti-mMCP-7; magnification of ×200).
This finding contrasts with some secretory granule proteases whose absence perturbs expression of other constituents of the secretory granule (35, 42).
mMCP-6 is not required for intestinal T. spiralis expulsion
To explore a role for tryptase mMCP-6 in the response to parasitic infection, we first investigated the contribution of mMCP-6 in the acute stage of T. spiralis infection. Previous studies identified a role for the intestinal mucosal cell subpopulation through their elaboration of mMCP-1, a population that does not express mMCP-6 to any significant degree (26, 27). Herein we find no difference in the worm expulsion kinetics between mMCP-6−/− and wild-type mice (Fig. 3). Worm expulsion in both groups was complete by day 18, and similar numbers of adult worms were found in the intestine of the two strains at various earlier time points. Thus, although mMCP-6-positive CTMCs are present in the jejunal lamina propria after T. spiralis infection (18), mMCP-6 is dispensable for the intestinal expulsion of the parasite.
FIGURE 3.
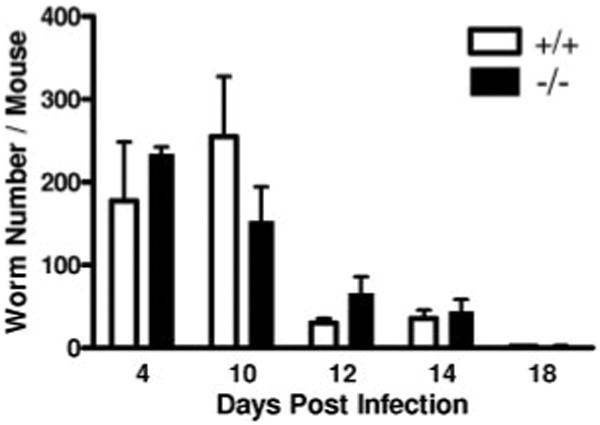
Kinetics of T. spiralis expulsion from small intestine. Mice acutely infected with T. spiralis were analyzed for intestinal worm burden. Each time point shows the mean number of worms (with SE) recovered per mouse from two independent experiments with 5 mice per group in each experiment (p = NS).
mMCP-6 enhances the immune response to T. spiralis
We next examined whether there may be a role for mMCP-6 in the immune response during the chronic stages of T. spiralis infection. After 5 wk of infection, we confirmed large numbers of larvae in the diaphragm, quadriceps, and tongue (28, 29). Tongue mast cell density at baseline was comparable between the two groups (wild-type vs mMCP-6−/−, 29.0 ± 3.1/LPF vs 26.0 ± 3.5/LPF; p = 0.54). Moreover, the tissue mastocytosis after larval invasion was indistinguishable in the absence of mMCP-6 (48.9 ± 4.1/LPF vs 57.7 ± 2.6/LPF, respectively; p = 0.21). Analysis of tongue tissue shows degranulating mast cells located around larvae in both wild-type and mMCP-6−/− mice (Fig. 4, A and B). Despite the similarity in tongue mast cell numbers, examination of necrosis of larvae revealed significant decreases in mMCP-6−/− mice (Fig. 4, C and D). More specifically, there was a significant decrease in the percentage of necrotic larvae in the tongue of mMCP-6−/− mice in comparison to wild-type mice infected in parallel with a concomitant increase in intact larvae (Fig. 4, E and F, p < 0.001). We further quantified muscle larvae burden at 5 wk after infection. Although we found a trend toward higher average parasite burden in the mMCP-6−/− mice, the differences between the two strains does not achieve statistical significance (3433 ± 428/g vs 2953 ± 348/g, p = 0.3872).
FIGURE 4.
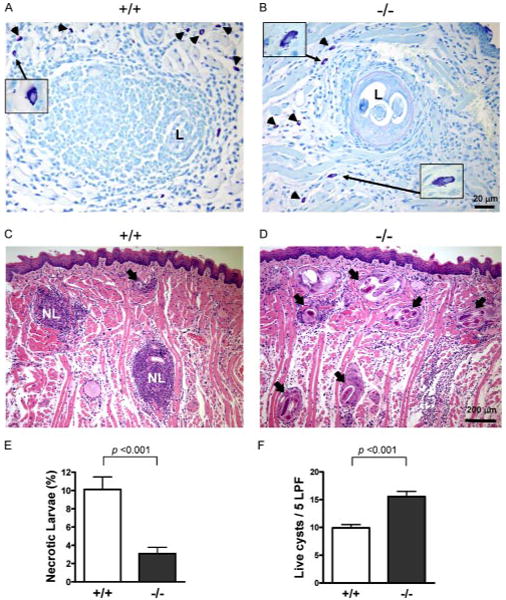
Chronic phase elimination of T. spiralis from skeletal muscle. A and B, Mast cells (arrowheads) locate at the periphery of larvae in both wild-type and mMCP-6−/− mice. Insets, Degranulating mast cells in higher magnification (×630). C and D, H&E stain of tongue skeletal muscle from chronically infected wild-type (C) and mMCP-6−/− (D) mice (NL indicates necrotic larva; arrow, live larva). E and F, Histomorphometric quantification of necrotic and live larvae in tongue skeletal muscle tissue sections from 4 mice per group pooled from two independent experiments. Values are means ± SE.
Tissue eosinophil recruitment by mMCP-6
Since leukocyte recruitment is thought to comprise one of the primary functions of tryptase (34, 43), we hypothesized that a defect in leukocyte recruitment may be evident in the immune response to chronic T. spiralis infection of mMCP-6−/− skeletal muscle. We therefore quantified leukocyte populations around T. spiralis larvae (Fig. 5C). We found that eosinophils, a major infiltrate in the leukocytic population surrounding infecting parasites (28), were markedly decreased in the mMCP-6−/− mice (Fig. 5, p = 0.0091). We did not note decreases in the fraction of other leukocyte populations infiltrating skeletal muscle larvae.
FIGURE 5.
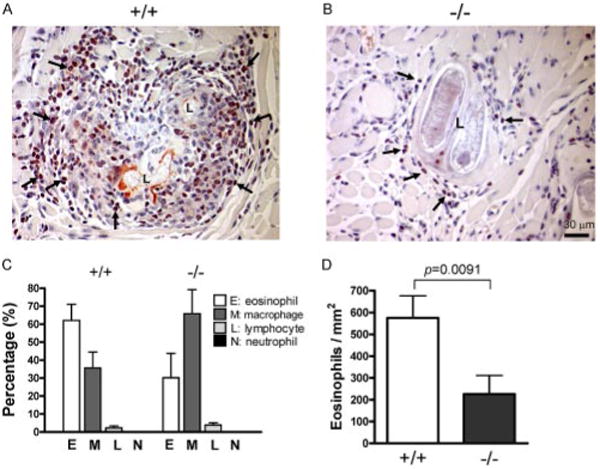
Eosinophil recruitment to larvae in mMCP-6−/− mice. A and B, Representative tongue skeletal muscle sections containing larva from wild-type (A) and mMCP-6−/− (B) mice stained with Congo red to identify eosinophils (arrows point to examples). C and D, Histomorphometric quantification of (C) different cell lineages and (D) eosinophil density in the perilarval space. Values are means ± SE.
Since we had previously noted a role for IgE in the immune response to chronic T. spiralis infection (29), it was possible that mMCP-6−/− mice displayed a lesion in their capacity to mount an effective adaptive immune response, especially production of IgE. We measured serum IgE levels 2 wk post infection and found no defect in IgE production by mMCP-6−/− mice (mMCP-6−/− vs wild-type mice, 260.6 ± 30.9 vs 134.7 ± 14.5 μg/ml). We further assessed IgE binding to the infecting parasite in vivo via immunohistochemistry (29) and found indistinguishable, robust IgE coating of T. spiralis larvae in both strains of mice (Fig. 6). Additionally, we also quantified the anti-T. spiralis humoral response via ELISA (44) and found no difference in IgG levels between mMCP-6−/− and wild-type mice (OD 0.085 ± 0.01 vs 0.082 ± 0.01, n = 10 mice/group, p = 0.7304). These data provide evidence that the mast cell tryptase mMCP-6 contributes to the eosinophil-rich immune response to T. spiralis in skeletal muscle in the context of an intact adaptive immune response to parasite.
FIGURE 6.
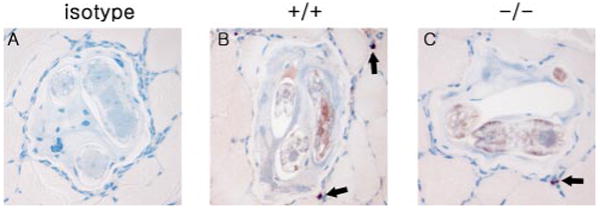
IgE immunostaining of larvae in mMCP-6−/− mice. A, Wild-type mouse stained with isotype control. Representative skeletal muscle sections containing larva from wild-type (B) and mMCP-6−/− (C) mice were stained with anti-IgE Ab (arrows point to IgE-positive mast cells) (magnification of ×400).
IgE-deficient mice demonstrate defective skeletal muscle tissue eosinophilia
Previous analyses in IgE-deficient mice also exhibited an altered immune response to skeletal muscle T. spiralis infection, highlighting the importance of the adaptive immune system response in chronic T. spiralis infection and implicating mast cells in this process (29). Having observed the identical phenotype in mMCP-6−/− mice, we hypothesized that the phenotype in IgE−/− mice resulted from their inability to stimulate mast cell mMCP-6 release. This led us to predict that eosinophil infiltration of T. spiralis larvae would also be decreased in IgE−/− mice. Indeed, histomorphometric quantification of leukocyte populations infiltrating larval structures in skeletal muscle revealed markedly depressed eosinophil numbers in infected IgE-deficient mice compared with infected wild-type mice (Fig. 7). Thus, IgE−/− and mMCP-6−/− mice both demonstrate prominent defects in their immune response to skeletal muscle T. spiralis infection via a decrease in the eosinophil-rich leukocytic infiltrate.
FIGURE 7.
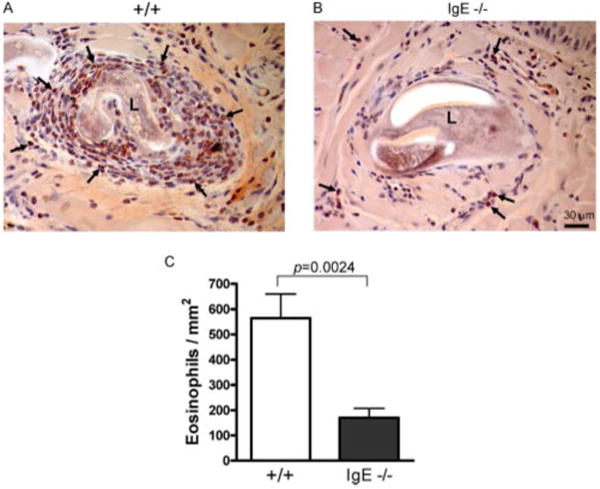
Eosinophil recruitment to larvae in IgE−/− mice. A and B, Representative tongue skeletal muscle sections containing larva from wild-type (A) and IgE−/− (B) mice stained with Congo red to identify eosinophils (arrows point to examples). C, Histomorphometric quantification of perilarval eosinophil density in tongue tissue from 4 mice per group pooled from two independent experiments. Values are means ± SE.
Discussion
As a mast cell-, lymphocye-, and IgE-dependent infection model with distinct acute (intestinal) and chronic (skeletal muscle) stages of infection, T. spiralis is an ideal pathogen to investigate immunologic mechanisms of parasite elimination. Infection of both humans and rodents has long been known to elicit prominent IgE responses and tissue mast cell hyperplasia (45, 46). Although previous studies in mast cell-deficient mice and in mMCP-1-deficient mice have provided insight into the role of mast cells in the intestinal phase of infection, little was known about the role of mast cells in the chronic response to this pathogen in skeletal muscle (29). In this study we demonstrate that mast cells participate in the immune response to chronic T. spiralis infection via elaboration of the tryptase mMCP-6. Furthermore, we confirm that leukocyte recruitment, particularly eosinophil recruitment, is an important physiologic role for mast cells and mMCP-6 in vivo.
More broadly, these studies highlight that the effector function of the mast cell is an integral aspect of the responses orchestrated by the adaptive immune system in reaction to an important class of infectious pathogens. Previous analyses in IgE-deficient mice and in IgE-depleted rats demonstrated the importance of the adaptive immune response in clearance of T. spiralis from skeletal muscle (29, 47). Because the mouse eosinophil does not express the high-affinity IgE receptor, this dependence implicated either mast cells in this process based on their expression of the high-affinity IgE receptor (although no direct assessment of mast cells around the lesion was performed) or antibody-dependent cytotoxicity by the eosinophil based on killing of newborn larvae in vitro (31). We herein extend those studies and show that mast cells are recruited to the site of larval infection, and that mMCP-6−/− mice, like IgE−/− mice, exhibit profound decreases in eosinophil recruitment to T. spiralis larvae despite an intact humoral IgE response. These observations, combined with previous studies demonstrating IgE binding to skeletal muscle larvae (29) and the well-documented ability of IgE to cause mast cell degranulation, suggest a chain of events by which the adaptive immune system directs mast cell and eosinophil participation in chronic T. spiralis infection. Initially, the adaptive immune response produces Ag-specific IgE that binds to T. spiralis larvae in skeletal muscle; the T. spiralis Ag-binding IgE activates CTMC expressing mMCP-6, causing release of this tryptase, which leads to eosinophil recruitment.
Although the significant decrease in necrotic larvae and increase in infected muscle cells are consistent with less efficient killing of T. spiralis larvae in mMCP-6−/− mice, our studies do not directly demonstrate increased parasitic cytotoxicity or increased parasite burden. Previous studies have demonstrated that eosinophils are capable of killing T. spiralis newborn larvae (29–32); however, definitive studies demonstrating this activity on mature larvae, such as those present in chronically infected muscle tissue, are lacking. It is thus possible that mast cells contribute to the immunologic response to dead larvae in these studies via elaboration of mMCP-6.
Our results provide further evidence for distinct functional activities of discrete mast cell subpopulations in T. spiralis infection. Numerous previous studies have identified a role for the intestinal MMC subpopulation, in part through their elaboration of mMCP-1, to direct rejection of the adult worms from the small intestine (26, 27). The defects in mMCP-6-deficient mice now provide evidence for participation of the CTMC subpopulation in a temporally and anatomically distinct phase of the immunologic reaction to this parasite from that of the MMC population. As such, we think that our data provide the first definitive example of a primary immunologic contribution from the connective tissue mast cell population in a chronic parasitic infection.
Previous studies on the role of mast cells in tissue eosinophilia have focused primarily on cytokine production. Mast cell elaboration of cytokines and chemokines such as IL-3, IL-5, GM-CSF, and eotaxin (48–50) are thought to recruit eosinophils and may potentiate eosinophil survival and function (51). The observations in this study add the mast cell tryptase mMCP-6 to this list of molecules recruiting eosinophils and extend previous observations regarding the roles of the mast cell tryptases mMCP-6 and mMCP-7 in leukocyte recruitment.
More specifically, experiments using recombinant mMCP-6 demonstrated a neutrophil influx after intraperitoneal injection, whereas recombinant mMCP-7 induced eosinophil infiltration inside the peritoneum (34, 43, 52). Herein, we find a profound decrease in eosinophil recruitment in mMCP-6-deficient, mMCP-7-sufficient mice while eosinophil recruitment remains intact in mMCP-7-deficient C57BL/6 (41) control mice. Thus, our results suggest that in the skeletal muscle, unlike in the peritoneum, mMCP-6 rather than mMCP-7 is critical to eosinophil recruitment. Furthermore, our findings underscore the importance of mast cell tryptases in leukocyte recruitment and provide evidence that mast cell protease function may be anatomically or stimulation context-specific. These observations have a broader context in that mast cell neutral protease function may be of importance in chronic inflammation in other tissues such as the asthmatic lung, where eosinophilic recruitment is implicated in pathobiology (53); the molecular basis for these distinctions awaits further experimental insight.
In summary, we have delineated that the tryptase mMCP-6, produced by CTMCs, modulates the immunologic response to the parasite T. spiralis, specifically in the chronic, skeletal muscle colonization phase. Moreover, tryptase-dependent eosinophil recruitment is a plausible effector mechanism by which CTMC contributes to adaptive immunity-driven (IgE-dependent) elimination of this parasite. This demonstrates the first example of a contribution by tryptase in the immunologic response to a chronic parasitic pathogen. Furthermore, our study highlights the discrete functional contributions of mast cell subsets as well as the functional diversity of the responses, driven by either adaptive immunity or by innate stimuli, of the mast cell lineage.
Acknowledgments
We thank Teresa Bowman for expert histotechnology input.
Footnotes
This study was supported by National Institutes of Health Grants AI059746-01, HL036110, AI031599, and AI05447, an Arthritis Foundation postdoctoral fellowship (100972), and the Cogan Family Foundation. A.D.P. was funded by the Veterinary Training Research Initiative (VT0102).
Abbreviations used in this paper: MMC, mucosal mast cell; CTMC, connective tissue-type mast cell; mMCP, mouse mast cell protease; LPF, low power field.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 2.Maurer M, Echtenacher B, Hultner L, Kollias G, Mannel DN, Langley KE, Galli SJ. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. J Exp Med. 1998;188:2343–2348. doi: 10.1084/jem.188.12.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–799. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- 4.Malaviya R, Twesten NJ, Ross EA, Abraham SN, Pfeifer JD. Mast cells process bacterial Ags through a phagocytic route for class I MHC presentation to T cells. J Immunol. 1996;156:1490–1496. [PubMed] [Google Scholar]
- 5.Bryce PJ, Miller ML, Miyajima I, Tsai M, Galli SJ, Oettgen HC. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE. Immunity. 2004;20:381–392. doi: 10.1016/s1074-7613(04)00080-9. [DOI] [PubMed] [Google Scholar]
- 6.Jawdat DM, Albert EJ, Rowden G, Haidl ID, Marshall JS. IgE-mediated mast cell activation induces Langerhans cell migration in vivo. J Immunol. 2004;173:5275–5282. doi: 10.4049/jimmunol.173.8.5275. [DOI] [PubMed] [Google Scholar]
- 7.Nigrovic PA, Lee DM. Mast cells in inflammatory arthritis. Arthritis Res Ther. 2005;7:1–11. doi: 10.1186/ar1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oettgen HC, Geha RS. IgE regulation and roles in asthma pathogenesis. J Allergy Clin Immunol. 2001;107:429–440. doi: 10.1067/mai.2001.113759. [DOI] [PubMed] [Google Scholar]
- 9.Chen R, Ning G, Zhao ML, Fleming MG, Diaz LA, Werb Z, Liu Z. Mast cells play a key role in neutrophil recruitment in experimental bullous pemphigoid. J Clin Invest. 2001;108:1151–1158. doi: 10.1172/JCI11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nigrovic PA, Binstadt BA, Monach PA, Johnsen A, Gurish M, Iwakura Y, Benoist C, Mathis D, Lee DM. Mast cells contribute to initiation of autoantibody-mediated arthritis via IL-1. Proc Natl Acad Sci USA. 2007;104:2325–2330. doi: 10.1073/pnas.0610852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruitenberg EJ, Elgersma A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature. 1976;264:258–260. doi: 10.1038/264258a0. [DOI] [PubMed] [Google Scholar]
- 12.Briggs NT. Immunological injury of mast cells in mice actively and passively sensitized to antigens from Trichinella spiralis. J Infect Dis. 1963;113:22–32. doi: 10.1093/infdis/113.1.22. [DOI] [PubMed] [Google Scholar]
- 13.Keller R, Cottier H, Hess MW. Mast cell responses in mesenteric lymph nodes to infection of rats with the nematode, Nippostrongylus brasiliensis. Immunology. 1974;27:1039–1044. [PMC free article] [PubMed] [Google Scholar]
- 14.Ko RC, Fan L, Lee DL, Compton H. Changes in host muscles induced by excretory/secretory products of larval Trichinella spiralis and Trichinella pseudospiralis. Parasitology. 1994;108:195–205. doi: 10.1017/s0031182000068293. [DOI] [PubMed] [Google Scholar]
- 15.Ghildyal N, McNeil HP, Stechschulte S, Austen KF, Silberstein D, Gurish MF, Somerville LL, Stevens RL. IL-10 induces transcription of the gene for mouse mast cell protease-1, a serine protease preferentially expressed in mucosal mast cells of Trichinella spiralis-infected mice. J Immunol. 1992;149:2123–2129. [PubMed] [Google Scholar]
- 16.Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, Nawa Y, Dranoff G, Galli SJ. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 17.Madden KB, Urban JF, Jr, Ziltener HJ, Schrader JW, Finkelman FD, Katona IM. Antibodies to IL-3 and IL-4 suppress helminth-induced intestinal mastocytosis. J Immunol. 1991;147:1387–1391. [PubMed] [Google Scholar]
- 18.Friend DS, Ghildyal N, Gurish MF, Hunt J, Hu X, Austen KF, Stevens RL. Reversible expression of tryptases and chymases in the jejunal mast cells of mice infected with Trichinella spiralis. J Immunol. 1998;160:5537–5545. [PubMed] [Google Scholar]
- 19.Ha TY, Reed ND, Crowle PK. Delayed expulsion of adult Trichinella spiralis by mast cell-deficient W/Wv mice. Infect Immun. 1983;41:445–447. doi: 10.1128/iai.41.1.445-447.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donaldson LE, Schmitt E, Huntley JF, Newlands GF, Grencis RK. A critical role for stem cell factor and c-kit in host protective immunity to an intestinal helminth. Int Immunol. 1996;8:559–567. doi: 10.1093/intimm/8.4.559. [DOI] [PubMed] [Google Scholar]
- 21.Newlands GF, Miller HR, MacKellar A, Galli SJ. Stem cell factor contributes to intestinal mucosal mast cell hyperplasia in rats infected with Nippostrongylus brasiliensis or Trichinella spiralis, but anti-stem cell factor treatment decreases parasite egg production during N. brasiliensis infection. Blood. 1995;86:1968–1976. [PubMed] [Google Scholar]
- 22.Grencis RK, Else KJ, Huntley JF, Nishikawa SI. The in vivo role of stem cell factor (c-kit ligand) on mastocytosis and host protective immunity to the intestinal nematode Trichinella spiralis in mice. Parasite Immunol. 1993;15:55–59. doi: 10.1111/j.1365-3024.1993.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 23.Alizadeh H, Murrell KD. The intestinal mast cell response to Trichinella spiralis infection in mast cell-deficient w/wv mice. J Parasitol. 1984;70:767–773. [PubMed] [Google Scholar]
- 24.Urban JF, Jr, Schopf L, Morris SC, Orekhova T, Madden KB, Betts CJ, Gamble HR, Byrd C, Donaldson D, Else K, Finkelman FD. Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell- and T cell-dependent mechanism. J Immunol. 2000;164:2046–2052. doi: 10.4049/jimmunol.164.4.2046. [DOI] [PubMed] [Google Scholar]
- 25.Korenaga M, Abe T, Hashiguchi Y. Injection of recombinant interleukin 3 hastens worm expulsion in mice infected with Trichinella spiralis. Parasitol Res. 1996;82:108–113. doi: 10.1007/s004360050079. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence CE, Paterson YY, Wright SH, Knight PA, Miller HR. Mouse mast cell protease-1 is required for the enteropathy induced by gastrointestinal helminth infection in the mouse. Gastroenterology. 2004;127:155–165. doi: 10.1053/j.gastro.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000;192:1849–1856. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurish MF, Humbles A, Tao H, Finkelstein S, Boyce JA, Gerard C, Friend DS, Austen KF. CCR3 is required for tissue eosinophilia and larval cytotoxicity after infection with Trichinella spiralis. J Immunol. 2002;168:5730–5736. doi: 10.4049/jimmunol.168.11.5730. [DOI] [PubMed] [Google Scholar]
- 29.Gurish MF, Bryce PJ, Tao H, Kisselgof AB, Thornton EM, Miller HR, Friend DS, Oettgen HC. IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis. J Immunol. 2004;172:1139–1145. doi: 10.4049/jimmunol.172.2.1139. [DOI] [PubMed] [Google Scholar]
- 30.Bell RG, Wang CH. The Trichinella spiralis newborn larvae: production, migration, and immunity in vivo. Wiad Parazytol. 1987;33:453–478. [PubMed] [Google Scholar]
- 31.Gansmuller A, Anteunis A, Venturiello SM, Bruschi F, Binaghi RA. Antibody-dependent in-vitro cytotoxicity of newborn Trichinella spiralis larvae: nature of the cells involved. Parasite Immunol. 1987;9:281–292. doi: 10.1111/j.1365-3024.1987.tb00508.x. [DOI] [PubMed] [Google Scholar]
- 32.Grove DI, Mahmoud AA, Warren KS. Eosinophils and resistance to Trichinella spiralis. J Exp Med. 1977;145:755–759. doi: 10.1084/jem.145.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurish MF, Austen KF. The diverse roles of mast cells. J Exp Med. 2001;194:F1–F5. doi: 10.1084/jem.194.1.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C, De Sanctis GT, O'Brien PJ, Mizgerd JP, Friend DS, Drazen JM, Brass LF, Stevens RL. Evaluation of the substrate specificity of human mast cell tryptase βI and demonstration of its importance in bacterial infections of the lung. J Biol Chem. 2001;276:26276–26284. doi: 10.1074/jbc.M102356200. [DOI] [PubMed] [Google Scholar]
- 35.Huang C, Sali A, Stevens RL. Regulation and function of mast cell proteases in inflammation. J Clin Immunol. 1998;18:169–183. doi: 10.1023/a:1020574820797. [DOI] [PubMed] [Google Scholar]
- 36.Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oettgen HC, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 38.Friend DS, Ghildyal N, Austen KF, Gurish MF, Matsumoto R, Stevens RL. Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J Cell Biol. 1996;135:279–290. doi: 10.1083/jcb.135.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin K, Gurish MF, Friend DS, Pemberton AD, Thornton EM, Miller HR, Lee DM. Lymphocyte-independent connective tissue mast cells populate murine synovium. Arthritis Rheum. 2006;54:2863–2871. doi: 10.1002/art.22058. [DOI] [PubMed] [Google Scholar]
- 40.Friend DS, Gurish MF, Austen KF, Hunt J, Stevens RL. Senescent jejunal mast cells and eosinophils in the mouse preferentially translocate to the spleen and draining lymph node, respectively, during the recovery phase of helminth infection. J Immunol. 2000;165:344–352. doi: 10.4049/jimmunol.165.1.344. [DOI] [PubMed] [Google Scholar]
- 41.Hunt JE, Stevens RL, Austen KF, Zhang J, Xia Z, Ghildyal N. Natural disruption of the mouse mast cell protease 7 gene in the C57BL/6 mouse. J Biol Chem. 1996;271:2851–2855. doi: 10.1074/jbc.271.5.2851. [DOI] [PubMed] [Google Scholar]
- 42.Feyerabend TB, Hausser H, Tietz A, Blum C, Hellman L, Straus AH, Takahashi HK, Morgan ES, Dvorak AM, Fehling HJ, Rodewald HR. Loss of histochemical identity in mast cells lacking carboxypeptidase A. Mol Cell Biol. 2005;25:6199–6210. doi: 10.1128/MCB.25.14.6199-6210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang C, Friend DS, Qiu WT, Wong GW, Morales G, Hunt J, Stevens RL. Induction of a selective and persistent extravasation of neutrophils into the peritoneal cavity by tryptase mouse mast cell protease 6. J Immunol. 1998;160:1910–1919. [PubMed] [Google Scholar]
- 44.Marva E, Markovics A, Gdalevich M, Asor N, Sadik C, Leventhal A. Trichinellosis outbreak. Emerg Infect Dis. 2005;11:1979–1981. doi: 10.3201/eid1112.050461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morakote N, Sukhavat K, Khamboonruang C, Siriprasert V, Suphawitayanukul S, Thamasonthi W. Persistence of IgG, IgM, and IgE antibodies in human trichinosis. Trop Med Parasitol. 1992;43:167–169. [PubMed] [Google Scholar]
- 46.Alizadeh H, Urban JF, Jr, Katona IM, Finkelman FD. Cells containing IgE in the intestinal mucosa of mice infected with the nematode parasite Trichinella spiralis are predominantly of a mast cell lineage. J Immunol. 1986;137:2555–2560. [PubMed] [Google Scholar]
- 47.Dessein AJ, Parker WL, James SL, David JR. IgE antibody and resistance to infection: I. Selective suppression of the IgE antibody response in rats diminishes the resistance and the eosinophil response to Trichinella spiralis infection. J Exp Med. 1981;153:423–436. doi: 10.1084/jem.153.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bressler RB, Lesko J, Jones ML, Wasserman M, Dickason RR, Huston MM, Cook SW, Huston DP. Production of IL-5 and granulocyte-macrophage colony-stimulating factor by naive human mast cells activated by high-affinity IgE receptor ligation. J Allergy Clin Immunol. 1997;99:508–514. doi: 10.1016/s0091-6749(97)70078-2. [DOI] [PubMed] [Google Scholar]
- 49.Hogaboam C, Kunkel SL, Strieter RM, Taub DD, Lincoln P, Standiford TJ, Lukacs NW. Novel role of transmembrane SCF for mast cell activation and eotaxin production in mast cell-fibroblast interactions. J Immunol. 1998;160:6166–6171. [PubMed] [Google Scholar]
- 50.Wallaert B, Desreumaux P, Copin MC, Tillie I, Benard A, Colombel JF, Gosselin B, Tonnel AB, Janin A. Immunoreactivity for interleukin 3 and 5 and granulocyte/macrophage colony-stimulating factor of intestinal mucosa in bronchial asthma. J Exp Med. 1995;182:1897–1904. doi: 10.1084/jem.182.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levi-Schaffer F, Piliponsky AM. Tryptase, a novel link between allergic inflammation and fibrosis. Trends Immunol. 2003;24:158–161. doi: 10.1016/s1471-4906(03)00058-9. [DOI] [PubMed] [Google Scholar]
- 52.Hallgren J, Karlson U, Poorafshar M, Hellman L, Pejler G. Mechanism for activation of mouse mast cell tryptase: dependence on heparin and acidic pH for formation of active tetramers of mouse mast cell protease 6. Biochemistry. 2000;39:13068–13077. doi: 10.1021/bi000973b. [DOI] [PubMed] [Google Scholar]
- 53.Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323:1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]


