Summary
Neurons exhibit rhythmic activity that ultimately affects behavior such as sleep. In living zebrafish larvae, we used time-lapse two-photon imaging of the presynaptic marker synaptophysin (SYP) in hypocretin/orexin (HCRT) neurons to determine the dynamics of synaptic modifications during the day and night. We observed circadian rhythmicity in synapse number in HCRT axons. This rhythm is regulated primarily by the circadian clock but is also affected by sleep deprivation. Furthermore, NPTX2, a protein implicated in AMPA receptor clustering, was found to modulate circadian synaptic changes. In zebrafish, nptx2b is rhythmic gene that is mostly expressed in hypothalamic and pineal gland cells. Arrhythmic transgenic nptx2b overexpression (hcrt:NPTX2b) increases synapse number and abolishes rhythmicity in HCRT axons. Finally, hcrt:NPTX2b fish are resistant to the sleep-promoting effects of melatonin. This behavioral effect is consistent with NPTX2b-mediated increased activity of HCRT circuitry. These data provide real-time in vivo evidence of circadian and homeostatic regulation of structural synaptic plasticity.
Introduction
The molecular mechanisms underlying circadian rhythmicity are well understood at the cellular level. However, it is unclear how this information is integrated to control specific physiological and behavioral outputs such as the timing of sleep (Takahashi et al., 2008). There is close interaction between homeostatic (dependent on duration of wakefulness) and circadian regulation of sleep, which optimizes the distribution of sleep and wake across the 24 hour cycle under natural light-dark (LD) conditions (Dijk and Czeisler, 1994; Achermann and Borbély, 2003; Saper et al., 2005). There are two main hypotheses that attempt to explain the beneficial effect of sleep on brain performance. The “synaptic homeostasis hypothesis” assumes that information encoded during wakefulness increases synaptic load, which is globally downregulated during sleep (Tononi and Cirelli, 2006). The “active system consolidation hypothesis” suggests re-activation of memories during sleep (Wilson and McNaughton, 1994; Ji and Wilson, 2007). These hypotheses therefore predict that there is a great deal of synaptic plasticity associated with sleep cycles. In vertebrates, the rhythmic expression of synaptic and trafficking genes has been postulated to affect behavior by remodeling synapses across day and night (Panda et al., 2002). Additionally, sleep-wake behavior itself has been shown to change synaptic plasticity and the expression of synaptic genes (Cirelli et al., 2004; Terao et al., 2006; Maret et al., 2007; Vyazovskiy et al., 2008). These data suggest that neuronal circuits are plastic over the course of the day. However, rhythmic changes in synaptic density have not been demonstrated in a living vertebrate, and the molecular mechanisms of this type of synaptic plasticity are poorly understood.
The zebrafish, a transparent diurnal vertebrate, is ideally suited to study neuronal anatomy, sleep, and circadian rhythms in a living animal (Zhdanova et al., 2001; Pando and Sassone-Corsi, 2002; Prober et al., 2006; Yokogawa et al., 2007). In zebrafish, the hypocretin (HCRT) neurons regulate sleep and wake, and project throughout the brain (Kaslin et al., 2004; Faraco et al., 2006; Prober et al., 2006; Yokogawa et al., 2007) including to the pineal gland (Appelbaum et al. 2009). In mammals, HCRT neurons are plastic (Rao et al., 2007) and are a major sleep and wake regulatory system (Nishino and Sakurai, 2006; Lu et al., 2006) which, when lost, causes narcolepsy (Lin et al., 1999; Hara et al., 2001).
In this study, we used live two-photon imaging of the presynaptic marker synaptophysin (SYP) to study the dynamic distribution and turnover of synaptic structures in HCRT cells projecting to the pineal gland. SYP is a synaptic vesicle protein that is expressed throughout the developing zebrafish nervous system (Valtorta et al., 2004; Meyer and Smith, 2006). We selected the HCRT-pineal gland circuit because of its functional importance in regulating circadian rhythms and sleep in zebrafish (Appelbaum et al. 2009), as well as the feasibility of imaging HCRT axons located superficially and isolated from other diffuse HCRT projections.
As a candidate regulator of synaptic plasticity, we also studied the role of Neuronal Activity-Regulated Pentraxin (NARP/NPTX2/NP2, Tsui et al., 1996) in regulating synaptic structures in HCRT neurons. The nptx2 gene was of special interest as it is expressed in mammalian HCRT neurons (Reti et al., 2002; Crocker et al., 2005) and is upregulated during wakefulness (Cirelli et al., 2004; Maret et al., 2007). Importantly, it clusters alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors at excitatory synapses in cultured neurons (O’Brien et al., 1999; O’Brien et al., 2002) and mediates synaptic refinement in vivo (Bjartmar et al,. 2006). NPTX2 is secreted in an activity-dependent fashion (Tsui et al., 1996; Reti et al,. 2008a) and has been shown to be important in learning and memory (Johnson et al., 2007; Reti et al., 2008b). Furthermore, NPTX2 can target both pre- and post-synaptic elements in excitatory synapses in a neuronal circuit (O’Brien et al., 1999; O’Brien et al., 2002).
Results
Visualizing synapses in HCRT axons using the presynaptic marker synaptophysin (SYP)
To study the dynamic changes of synaptic structures during day and night, HCRT neurons were labeled with SYP fused to enhanced green fluorescent protein (SYP-EGFP). SYP is a validated marker of presynaptic boutons and synapses in vertebrates including zebrafish (Bamji et al., 2003; Javaherian and Cline, 2005; Meyer and Smith, 2006). We established a stable transgenic line expressing SYP-EGFP in all HCRT cells (hcrt:SYP-EGFP, Figure 1B). In hcrt:SYP-EGFP fish, EGFP is represented in HCRT cell bodies and the axon fibers are strongly labeled with punctuated structures (compare Figure 1A and 1B). A similar pattern of SYP-EGFP puncta distribution was also visualized in HCRT fibers of adult hcrt:SYP-EGFP fish (Figure 1C). To validate that SYP-EGFP puncta correspond to synaptic structures, we performed double immunohistochemistry experiments with other pre- and post-synaptic proteins in zebrafish HCRT cells. First, whole hcrt:SYP-EGFP embryos were labeled with a monoclonal antibody to the synaptic vesicle protein 2 (SV2) and EGFP. Due to high synapse density in the brain, we initially imaged SV2 in regions of the developing spinal cord where SV2 labeling was less dense. We found that SV2 is restricted to presynaptic puncta in spinal motor neurons (Figure 1D and 1E), as was previously shown (Meyer and Smith, 2006). In 32 hours post-fertilization (hpf) embryos, we further demonstrated colocalization of SV2 and SYP-EGFP puncta in HCRT axons located in the hindbrain (Figure 1F, 1G). These data show that SYP-EGFP occupy the same sites in HCRT axons of zebrafish as other vesicular proteins associated with the presynaptic bouton. In addition, using array tomography, a high resolution imaging technique that allows for highly multiplexed immunofluorescent labeling (Micheva and Smith 2007), we show that SYP-EGFP puncta are physically localized in juxtaposition with postsynaptic structures (Figure S1). In adult transgenic hcrt:SYP-EGFP hypothalamus, 21 of 25 (84%) randomly selected SYP-EGFP puncta have at least 1 post synaptic density 95 (PSD95) punctum within 100nm of their location, demonstrating that SYP-EGFP puncta generally represent synaptic structures (Figure S1).
Figure 1. The presynaptic SYP-EGFP fusion protein is a marker of synapses in HCRT axons.
(A, B) Two-photon microscope imaging of transgenic larvae (dorsal views of the midbrain, anterior to the left) expressing EGFP (hcrt:EGFP, A) in all HCRT neurons or the presynaptic marker synaptophysin fused to EGFP (hcrt:SYP-EGFP, B) to specifically target synapses in the axons of HCRT neurons.
(C) Confocal imaging of 100 μm transversal brain sections from a stable hcrt:SYP-EGFP transgenic adult fish. HCRT cell bodies are localized around the ventricle in the hypothalamus and SYP-EGFP puncta are distributed throughout HCRT axons.
(D-G) Confocal imaging (lateral views with anterior to the left) of immunohistochemistry (using SV2 antibody, D, E) and double-immunohistochemistry (using SV2 and EGFP antibodies, F, G) in 32 hours post-fertilization (hpf) embryo. (E) and (G) are close-ups of the white frames of (D) and (F), respectively. Of note, red (SV2) and green (SYP-EGFP) colocalization is marked with yellow. Abbreviation: FB, forebrain; MB, midbrain; HB, hindbrain; SC, spinal cord. White and yellow arrows indicate HCRT cell bodies and SV2 presynaptic clusters in motor neurons, respectively.
Rhythmic synaptic plasticity in HCRT axons
To visualize progressive changes in synapse morphology across the day-night cycle, hcrt:SYP-EGFP fish were synchronized to 14h light/10h dark (LD) cycles for 6 days to entrain their molecular oscillators. At 7 days post fertilization (dpf), each individual was imaged every 3 hours under LD or constant dark conditions (DD). Imaging was conducted after maturation of HCRT axons projecting toward the pineal gland, thus avoiding confounding developmental effects (Figure 2A). All images were normalized for intensity, and punctum counting was performed blindly (Figures 2B–2K, see Supplementary Experimental Procedures). Data was collected in living fish. Therefore, for each individual we normalized the number in each time point to the mean value across the entire experiment. In LD, punctum number varied over the course of a 24-hour cycle, with the highest value occurring during the day, and reduced at nighttime (Figure 2L, 2B–2F, amplitude of 38.6 ± 12.2% SEM, n=12). Interestingly, the greatest rate of change of punctum numbers occurred at dark to light transition (30.9±9.24% SEM, Figure 2L). LD transitions are known to be periods of maximal activity and wakefulness in zebrafish (Prober et al., 2006; Yokogawa et al., 2007). These data also concur with recent reports that the effect of HCRT is most relevant during the transitions between sleep and wake in mammals and zebrafish (Adamantidis et al., 2007; Naumann et al., 2010).
Figure 2. Real-time in vivo analysis of rhythmic synaptic density in HCRT axons.
(A) Two-photon imaging of a 7dpf stable transgenic hcrt:SYP-EGFP larva (dorsal view with head to the left). White arrows indicate HCRT cell bodies, and areas within the white frames in the midbrain and in the hindbrain are expended in (B-K) and Figure S2, respectively.
(B-K) Time-lapse imaging of HCRT axons located by the pineal gland (close-ups of the white box area shown in B) in two representative hcrt:SYP-EGFP individuals [B-F under 14 light:10 dark (LD) and G-K under constant dark conditions (DD)]. Examples of lost and new puncta are marked with red and green arrows, respectively. (L, M) Quantification of SYP-EGFP punctum number under LD (L, n=12, LD represented by white and black bars) and DD (M, n=9, gray bars represent subjective day) across 24 hours are presented. Numbers of puncta are rhythmic under both LD (p<0.01) and DD (p<0.01) conditions, with higher levels during the daytime. In each individual, punctum number was normalized at each time point to the mean value across the entire experiment. Repeated measure ANOVA indicated a significant effect of time on these parameters. Each value represents normalized mean ± SEM.
Rhythmic synaptic plasticity in HCRT axons is primarily regulated by the circadian clock
Observed rhythmicity in LD may be driven directly by light, independently of the circadian clock. To address this question, the number of puncta was measured under DD. As in LD, synapse number was rhythmic and peaked during subjective day (amplitude, 20.4% ± 3.08% SEM, n=9, Figure 2G–2K, 2M), suggesting a circadian control in the absence of light entrainment. Furthermore, the biggest change was observed during the dark to subjective light transition (16.1% ± 6.31% SEM, Figure 2M), as in LD.
To assess whether the number of puncta is rhythmic in other HCRT axons, we used continuous imaging to study HCRT axons projecting into the hindbrain in 7dpf hcrt:SYP-EGFP transgenic fish under DD. In this region, HCRT axons are divided from the main caudal projection tract (Figure 2A) and can be efficiently distinguished within the same individual at different time points and across individuals. Punctum number varied over the course of a 24-hour cycle, such that the highest value occurred late during the day and early during the night (CT8-CT17) and was reduced toward the morning and the beginning of the day (CT20-CT5, Figure S2, amplitude of 16.24 ± 3.7% SEM, n=13). Interestingly, under DD the amplitude of the rhythm of synapse number is similar in HCRT axons projecting to the pineal gland and hindbrain. However, the phase of the rhythm is different and almost opposite suggesting diverse pre- and likely post-synaptic regulation. Taken together, these results indicate that intrinsic circadian oscillators drive rhythmic synaptic plasticity in HCRT axons that differ in a region specific manner.
Synaptic plasticity in HCRT axons is also regulated by sleep-dependent homeostatic processes
We next investigated whether the number of SYP-EGFP puncta in HCRT axons is regulated by sleep-dependent homeostatic processes by performing sleep deprivation (SD) experiments. Control of punctum number by sleep is an intriguing possibility because HCRT regulates sleep and wake in zebrafish, and the amplitude of daily changes in punctum number under DD was reduced by nearly 50% compared with LD (Figure 2). 7dpf larvae were kept under LD for 6 days, and then transferred into DD conditions. The subjective day (active period) was then extended for 3 hours (CT14-CT17, Figure 3A) using gentle vibration and tapping (protocol previously established by Zhdanova et al. 2001). Larvae were forced to maintain activity while sibling control larvae were allowed to sleep (both in DD, n=12 for each treatment). Puncta located adjacent to the pineal gland were measured prior to SD, immediately after SD, and 3 hours later in the same individuals (Figure 3A, 3B). Punctum number was normalized to baseline (before SD, CT14) for each individual. In both control and SD larvae, punctum number was reduced (p<0.01 for control and p<0.05 for SD) at CT20, confirming that synapse number decreased during the night (Figure 3 and Figure S3A for more details). However, no differences were found in the number of puncta between control and SD larvae following 3 hours of SD (CT17) and recovery (CT20, Figure 3B). These results suggest that 3 hours of SD has no effect on synapse number, and that longer periods of SD may be required in zebrafish larvae as is the case in flies (Shaw et al., 2000, Gilestro et al., 2009, Donlea et al., 2009). We therefore sleep deprived 7dpf larvae for a longer period (6 hours, CT14-CT20, Figure 3A, n=13) while sibling larvae (n=8) were allowed to sleep. We also increased recovery time to 6 hours, and the experiment was performed under similar conditions as the 3 hour SD experiment (Figure 3A, Figure 3C). The rhythm of punctum number (Figure 3C and Figure S3B) was consistent with the finding shown above (Figure 3B and Figure S3A) in control (p<0.01) and sleep deprived (p<0.05) larvae. Importantly, synapse number was increased (17%, p<0.05) in sleep deprived larvae at CT20 when compared to controls (Figure 3C). After recovery (CT2), punctum numbers in SD and control larvae were similar.
Figure 3. Punctum number in HCRT axons increases following 6 hours of sleep deprivation (SD).
(A-C) A schematic illustration of the SD experiments (A). 7dpf larvae were sleep deprived for 3 (B) or 6 (C) hours at the beginning of the night (CT14). SYP-EGFP punctum number were quantified before, (CT14 in B and C), immediately after SD (CT17 in B or CT20 in C) and after recovery (CT20 in B or CT2 in C). All experiments were performed under dark conditions (DD) and in living larvae. The number of puncta at each time point was normalized to the number recorded at the first time point (CT14) within the same individual. In all cases, with or without SD, punctum number was lower at night (see also Figure S3). 6 but not 3 hours of SD significantly (*p<0.05) increased the number of puncta. Statistical comparisons were performed using t-tests and ANOVAs. Each value represents mean ± SEM.
(D, E) Brain activity and wake is indicated by expression of c-fos mRNA in the brains of control (D) and sleep deprived (E) larvae. All images are dorsal, with heads pointing to the top (three in each image). OB (olfactory bulb), Tel (telencephalon), Di (diencephalon), Rh (rhombencephalon). Similar results were obtained after 3 and 6 hours of SD. Note the efficiency of the SD procedure (D versus E). All sleep deprived fish showed strong increased in expression.
At the end of SD (CT17 or CT20), we first verified the efficiency of SD by analyzing whole brain c-fos expression, a well-established marker of neuronal activity and wakefulness (Basheer et al. 1997). As expected, a stronger activation of c-fos was observed in all sleep-deprived fish (n=12) when compared to controls (n=12, Figure 3D, 3E) in both 3 and 6 hour SD experiments. Also confirming SD, we found that recovering sleep deprived animals (at CT21.5 and CT22.5) displayed reduced activity levels compared to controls (Figure S3C). Taken together, these data suggest that diurnal fluctuations of synapse numbers in HCRT axons projecting to the pineal gland are primarily driven by the circadian clock; however, the reduced rhythm under DD and the increased punctum number after 6 hours of SD also demonstrates a role for homeostatic control.
nptx2b is expressed in the lateral hypothalamus and the pineal gland
To investigate the mechanisms that could regulate rhythmic synaptic plasticity in this circuit, we studied the neuronal pentraxin nptx2 gene. In zebrafish, we cloned two nptx2 paralogues, nptx2a and nptx2b, with high homology to mammalian nptx2 (Figure S4). The two zebrafish predicted protein sequences contain the characteristic neuronal pentraxin structure with a signal peptide followed by two coiled-coil domains and a pentraxin domain in the C-terminal (Figure S4). Nptx2a is widely expressed, resembling the mammalian pattern of expression of nptx2 (Figure S4). Remarkably, nptx2b is initially discretely expressed in the hypothalamus and the pineal gland (Figure 4A, 4B), a pattern of expression later expanded to include the habenula (Figure 4C), retina and cranial ganglia (data not shown). Double in situ hybridization (ISH) studies in adults and larvae indicated that nptx2b positive cells in the lateral hypothalamus (LH) are glutamatergic (Figure 4H). Some nptx2b cells also colocalize with dynorphin (pdyn, Figure 4F) and hcrt (Figure 4E) in the LH, and with aanat2, a marker of melatonin-producing photoreceptor cells, in the pineal gland (Figure 4G). These data largely recapitulate the expression pattern of nptx2 in the mammalian LH (Reti et al., 2002; Crocker et al., 2005), where pdyn and nptx2 also colocalize in HCRT cells.
Figure 4. Nptx2b cell identity and rhythmic nptx2b expression in the lateral hypothalamus (LH).
(A, B) Lateral and dorsal views of 2dpf larvae expressing nptx2b in the LH and the pineal gland (P).
(C, D) Similar pattern of nptx2b expression is shown in the LH (D), habenula (Hb, C) and pineal gland (C) in adult fish.
(E-G) Double ISH in 2dpf larvae showing colocalization of nptx2b with hcrt (E) and pdyn (F) in the LH and with aanat2 in the pineal gland (G).
(H) Double ISH in adult shows that nptx2b neurons express vglut2b, a marker of glutamatergic neurons.
(I-L) Time course analysis under light:dark (LD) and constant darkness (DD, gray bar represents subjective day) conditions demonstrate that endogenous nptx2b expression increases during the day and decreases during the night (K, L, n=7 adult brains per time point, p<0.001). Representative adult brain sections for day (I) and night (J) are shown. Statistical comparisons were performed using ANOVA. Each value represents mean ± SEM.
Rhythmic expression of nptx2b is independent of the light regime
The localization of nptx2b (a gene involved in synaptic plasticity of glutamatergic neurons) in sleep- and circadian-related tissues (i.e. hypothalamus and pineal gland) suggested a possible involvement of this protein in the regulation of rhythmic synaptic plasticity in HCRT-pineal circuitry. To explore this hypothesis, we studied whether the expression of nptx2b is rhythmic, and possibly regulated by the circadian clock. The expression pattern was analyzed in adults because nuclei-specific expression can be more easily quantified on adult brain sections. Fish were sampled every 4 hours throughout the first cycle under LD and DD conditions. Under both conditions, nptx2b mRNA levels in the LH were highest during the day and lowest during the night (p<0.001, Figure 4I–4L), with minimal differences between LD and DD, suggesting a circadian control. Interestingly, nptx2b mRNA levels in the LH peak toward the evening (CT11, Figure 4K, 4L), 3 hours before the number of SYP-EGFP puncta reach their maximum level (Figure 2L, 2M). Rhythmic nptx2b expression was not restricted solely to the LH. In the habenula, nptx2b mRNA levels were high during the night (CT11-CT19) and low during the day (CT23-CT7) under DD (p<0.05, Figure S5). In contrast, no apparent differences were observed in the levels of nptx2b mRNA after 6 hours of SD (Figure S5). Taken together, these results suggest that nptx2b expression is primarily regulated by the circadian clock.
Overexpression of nptx2b increases synaptic density in HCRT axons
The coincidence of nptx2b rhythmicity with synapse number fluctuations in HCRT cells prompted us to investigate the existence of a functional relationship. To test the hypothesis that nptx2b is involved in the circadian regulation of synaptic plasticity in these neurons, we first analyzed the effect of nptx2b on synaptic density. We were unable to pursue a loss-of-function strategy, as no nptx2b−/− mutants are available and antisense morpholino approaches are inefficient at the developmental stage of fish in our experiments (7dpf). Further, NPTX2b loss could be compensated by the effects of other pentraxins such as NPTX2a and NPTX1 (see discussion). Therefore, we pursued a gain-of-function strategy and generated a stable transgenic line overexpressing nptx2b under the control of the hcrt promoter (Figure 5D). This promoter drives a strong, arrhythmic expression specifically in HCRT neurons (Faraco et al., 2006; Appelbaum et al., 2009). To further validate that the HCRT promoter is arrhythmic, we show that, in adult zebrafish, hcrt mRNA levels are arrhythmic with a slight increase toward the end of the night (CT23, Figure S5). These data match well with mammalian studies that show that hcrt mRNA expression is arrhythmic (Stütz et al., 2007). Both pre- and post-synaptic density structures were monitored using SYP-EGFP (Figure 5) and PSD95-EGFP (Figure S6), respectively, as NPTX2 has been shown to act both pre- and post-synaptically in vitro (O’Brien et al., 1999). PSD95 is a validated post-synaptic marker of excitatory synapses in mammals and zebrafish (Ebihara et al., 2003; Niell et al., 2004; Livneh et al., 2009). To study the function of NPTX2b in a single HCRT neuron, we used transient expression (Figure 5A), taking advantage of mosaic effects obtained after injections of the hcrt:SYP-EGFP or hcrt:PSD95-EGFP construct at the one-cell stage of stable hcrt:NPTX2b transgenic or wild-type sibling embryos. The number of synaptic protein clusters was then quantified in all dendrites or axons in both wild-type sibling and NPTX2b overexpression fish. We found a significant increase (43%) of SYP-EGFP puncta in fish overexpressing NPTX2b versus control wild-type siblings (264.8 ± 32.4 SEM in hcrt:NPTX2b, n=7 vs 184.2 ± 11.4 SEM in WT sibling, n=8, p<0.05, Figure 5E). In contrast, PSD95-EGFP punctum distribution in the dendrites remained unchanged (68.6 ± 7.9 SEM in hcrt:NPTX2b, n=9 vs 60.8 ± 10.5 SEM in WT sibling, n=11, Figure S6). These results indicate that in vivo overexpression of NPTX2b increases synaptic density in HCRT axons without a similar effect in the dendrites, suggesting that NPTX2b functions in axon terminals of HCRT neurons.
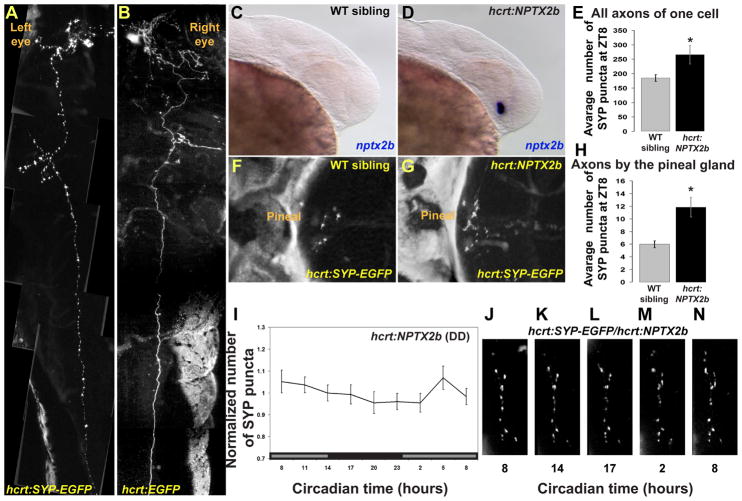
Figure 5. Arrhythmic overexpression of nptx2b increases synaptic density and abolishes synaptic rhythms in HCRT axons.
(A, B) Dorsal views, head to the left, of 4 dpf larvae transiently expressing either hcrt:SYP-EGFP (A) or hcrt:EGFP (B) transgenes. Pictures were cropped in the animal midline and juxtaposed to mirror each other for comparison. Transient expression of the SYP-EGFP presynaptic fusion protein is used to tag and quantify punctum number in all axons of single HCRT neurons (compare SYP-EGFP in A versus cytosolic EGFP in B).
(C, D) Lateral views, head to the right, of 30 hpf wildtype (C) and transgenic embryos overexpressing nptx2b (hcrt:NPTX2b, D). hcrt:NPTX2b larvae (D) display strong nptx2b ISH staining, prior to the appearance of endogenous nptx2b expression in their wildtype siblings (C).
(E) hcrt:SYP-EGFP transgene was injected into hcrt-NPTX2b and WT sibling embryos. 4dpf larvae that express EGFP in a single HCRT neuron (as shown in A) were selected. Overexpression of nptx2b in HCRT neurons increases total synapse numbers in all axons of a single HCRT neuron.
(F-H) The number of puncta present on HCRT axons projecting to the pineal gland were quantified in control hcrt:SYP-EGFP (F) and hcrt:NPTX2b/hcrt:SYP-EGFP double transgenic (G) 7dpf fish at ZT8 (H).
(I) Quantification of SYP-EGFP punctum number under DD (n=11, gray bars represent subjective day) across 24 hours in hcrt:NPTX2b larvae. (J-N) Time-lapse imaging of HCRT axons located near the pineal gland in representative hcrt:SYP-EGFP/hcrt:NPTX2b double transgenic individual. Note the absence of rhythmic variation. Statistical comparisons were performed using ANOVA and student t-tests. Each value represents mean ± SEM.
Arrhythmic expression of nptx2b abolishes synapse rhythmicity in HCRT axons
We next studied the effects of NPTX2b arrhythmic overexpression in HCRT axons projecting to the pineal gland (Figure 5F–5N), a region where we found rhythmic structural synapse changes in HCRT axons (Figure 2). For this experiment, we used progeny of crosses between stable homozygous hcrt:SYP-EGFP and homozygous hcrt:NPTX2b lines. We observed a two-fold increase in SYP-EGFP punctum density (at zeitgeber time, ZT8) in nptx2b overexpressing fish (11.8± 1.5 SEM in hcrt:NPTX2b, n=14 vs 6± 0.5 SEM in WT sibling, n=25, p<0.001, Figure 5F–5H). Importantly, to test whether the overexpression of NPTX2b could affect synapse rhythmicity, the numbers of SYP-EGFP puncta were also monitored in hcrt:NPTX2b 7dpf larvae across the entire 24-hour cycle (Figure 5I–5N) as described above (Figure 2) for wild type fish. Remarkably, rhythmic fluctuation of synapse density was almost entirely abolished in hcrt-NPTX2b fish (Compare Figure 5I and Figure 2M), although SYP-EGFP puncta were slightly increased (p<0.05) above baseline at CT5 (Figure 5I). These results indicate that NPTX2b overexpression not only increases presynaptic structure density, but also suppresses synapse rhythmicity. These data suggest that the rhythmically expressed nptx2b gene is involved in the mechanism regulating circadian variation of synaptic density in this circuit.
Fish overexpressing NPTX2b are resistant to hypnotic effect of melatonin
Since the increase of presynaptic structures in HCRT axons may lead to an increase in excitatory output of the glutamatergic HCRT neurons, the effects of NPTX2b overexpression on behavior were next studied in both larvae and adults. In larvae, we monitored activity across the day under dim light conditions. In adults, we monitored sleep and wake under the LD cycle using the Adult Fish Sleep Recording System (Yokogawa et al. 2007). At both larval and adult stages, hcrt:NPTX2b fish behaved the same way as their wild type siblings (Figure 6A for larvae; Table S1 for adults), suggesting that, in normal conditions, the increase of synapse number in HCRT axons has undetectable effects on activity and sleep.
Figure 6. nptx2b overexpression in HCRT cells reduces sensitivity to the sleep-promoting effect of melatonin.
(A-C) Larvae kept under 14h light:10h dark conditions (LD) were recorded under constant dim (10 lux) light (LL, gray bar represent the subjective dark period). All larvae (n=48–52 per genotype for each treatment) demonstrate rhythmic activity under control conditions (A, p<0.0001), but after melatonin treatment (1μM), activity was arrhythmic and reduced while genotype effect was significant (p<0.005), with hcrt:NPTX2b fish being more resistant than wild type siblings (B). (C) Notably, hcrt:NPTX2b larvae are resistant while hcrtr−/− larvae are sensitive (Appelbaum et al., 2009) to melatonin.
(D-H) A similar phenotype was observed in adults under LD (n=11 for each genotype). Sleep time of representative individuals did not increase in hcrt:NPTX2b transgenic fish (E) after melatonin (100μM) as compared to wild type siblings (D). Percentage increase of sleep time (F), number of wake/sleep transitions (G) and sleep bout length (H) per hour during the day and after melatonin administration are shown. Statistical comparisons were performed using t-tests in adults, and repeated measure ANOVA with grouping factor in larvae. Each value represents mean ± SEM.
We recently identified a specific interaction between the HCRT and melatonin systems illustrated by the hypersensitivity to melatonin of HCRT receptor mutant fish (hcrtr−/−, Appelbaum et al., 2009). Melatonin has a strong sleep-promoting effect in zebrafish (Zhdanova et al., 2001; Appelbaum et al., 2009). Thus, we next tested the effect of melatonin on hcrt:NPTX2b fish. Interestingly, in contrast to hcrtr−/− fish, we found that hcrt:NPTX2b larvae are resistant to melatonin (p<0.005, Figure 6B, 6C). Likewise, in adult fish, when melatonin was added at the end of the second day, it increased sleep time 3-fold (p<0.01) and the number of sleep/wake transitions (2-fold, p<0.01) during the day in wild type but not transgenic hcrt:NPTX2b siblings (Figure 6D–6H). These data are opposite to the results obtained with fish lacking a functional HCRT system (Appelbaum et al., 2009), suggesting an increased activation of the HCRT system by NPTX2b, in agreement with our finding of increased synaptic density in HCRT axons. Thus, NPTX2b may modulate and strengthen synaptic connectivity between HCRT and downstream post-synaptic targets, and this modification may be reflected in behaviors regulated by the HCRT and melatonin systems.
Discussion
Visualization of synaptic markers across the day in individual living animals
Visualizing synapses in vivo is increasingly important in neurobiology. Much of what is currently known about the circadian and sleep-dependent dynamics of synapse formation and elimination comes from measurements of fixed anatomical material in the fly (Fernández et al., 2008; Pyza and Górska-Andrzejak 2008; Donlea et al. 2009; Gilestro at al., 2009). Here, we used time-lapse imaging of fluorescently tagged SYP to visualize and quantify synapses in HCRT axons of living zebrafish larvae across the day. With this novel approach, we were able to observe longitudinal changes in the same individual fish under various physiological conditions. Using SV2 and PSD95 staining with SYP-EGFP and array tomography, we found that our procedure labeled synaptic structures. Although SYP-EGFP does not differentiate active versus silent synapses, the synapses measured here are likely to be functional considering the associated behavioral effects. Indeed, in HCRT neurons of hcrt:NPTX2b fish, the number of PSD95-EGFP clusters in dendrites (input) is stable while the number of SYP-EGFP clusters (output) increase. Further, hcrt:NPTX2b fish have a behavioral phenotype opposite to hcrtr−/− fish. Taken together, we hypothesize that the output of complex neuronal circuits involved in behavior, such as HCRT, can be modulated at the axonal level independently of changes in dendritic input, providing changes in circuitry “gain”. Additional studies using novel calcium indicators fused to SYP that can identify locations and activity of synapses simultaneously would be helpful to further document changes in active synapses (Dreosti et al., 2009).
Circadian regulation of synaptic plasticity
Studies in Drosophila have shown clock-controlled remodeling of axonal terminals in the pigment dispersing factor (PDF) circuit (Fernández et al., 2008), suggesting circadian modification of synapses connecting PDF axons to their post synaptic targets. A similar mechanism was shown in the visual system of the fly, where the circadian clock and external stimuli drive rhythmic synaptic plasticity (Pyza and Górska-Andrzejak 2008). Using our novel technique, we were able to show for the first time circadian regulation of synaptic plasticity in single axons of living vertebrates under LD or DD conditions. Further, we explored different areas of projections of the same neuron, in particular the hindbrain and pineal gland areas. Interestingly, although the amplitude of the rhythm in these target regions was similar, the phase was opposite, suggesting differential post-synaptic regulation of circadian synaptic plasticity even in the presence of a similar input. We hypothesize that HCRT output in these two regions has different functions that require differential circadian regulation. The mechanism leading to these differences may involve presynaptic regulation of axonal transport or circadian regulation of HCRT synapses by postsynaptic targeted cells. This difference illustrates the importance of looking at individual projections within circuits, rather than global changes.
Homeostatic regulation of synaptic plasticity
Both circadian and homeostatic influences regulate behaviors such as sleep. The lower fluctuation in synapse number observed during DD versus LD suggested homeostatic influences on synaptic plasticity. To test this hypothesis, we explored the effects of SD and found homeostatic influences on the number of synapses in HCRT axons. These results are in line with experiments linking synaptic homeostasis, memory and sleep. In mammals, the number of GluR1-containing AMPA receptors is high during wake and low during sleep in the cortex and hippocampus. Further, induction of long-term potentiation (LTP) is diminished after wakefulness and restored after sleep (Vyazovskiy et al., 2008). In flies, synaptic protein levels are high after waking and low after sleep and SD increases the number of synaptic terminals in the ventral lateral neurons’ projections (Donlea et al., 2009; Gilestro at al., 2009). These synaptic changes were independent of circadian effects, which were negligible in these circuits. It has been hypothesized that, by modifying synapses, sleep consolidates memories, and thus SD disrupts memory acquisition (Cirelli and Tononi 2008). Learning and associated neuronal activity during wakefulness lead to a use-dependent strengthening of synapses, while sleep downscales these changes (Tononi and Cirelli, 2006).
Circuit-specific regulation of synaptic plasticity by circadian and homeostatic influences
Whereas evidence for homeostatic regulation of synapse plasticity is strong in flies and the mammalian forebrain, it remains unclear whether all circuits are similarly regulated. In our study, homeostatic effects were modest (17%) and only observed after 6 hours of SD. Further, even under condition of SD, circadian regulation of synapses was dominant, with decreases in synapse number from baseline (CT14) to the end of SD period (CT20) as observed in non-sleep deprived controls. These results suggest a strong circadian effect and more minor homeostatic influences on this circuit.
Several studies have provided important insights into the underlying mechanisms through which sleep and synaptic homeostasis may benefit memory consolidation (Diekelmann and Born, 2010). It is possible that homeostatic regulation primarily affects synaptic plasticity in brain areas important for learning and memory such as the forebrain. In other circuits where short-term reactive adaptation is less important, long-term circadian modulation predicting light/dark transitions may be a more important regulatory mechanism of synaptic plasticity. An example of such a circuit may be the zebrafish HCRT-pineal system, a circuit we do not expect to be involved in memory. Indeed, the zebrafish HCRT system has been shown to regulate sleep/wake and melatonin secretion (Prober et al., 2006; Yokogawa et al., 2007; Appelbaum et al., 2009; Panula, 2010). It is possible that synaptic plasticity in some postsynaptic HCRT targets will be affected by the circadian clock, while other HCRT targets that regulate sleep and memory will be more sensitive to SD. In this model (Figure 7A), both circadian and homeostatic regulation of synaptic density occurs in various brain areas, with homeostatic regulation reflecting circuit needs for sleep and learning-dependent plasticity.
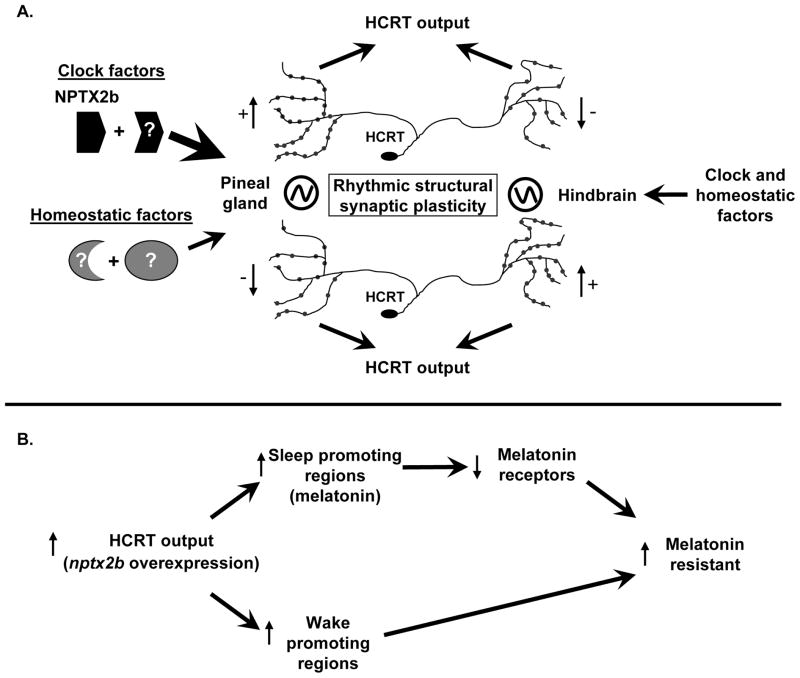
Figure 7. A model for circadian and homeostatic regulation of rhythmic structural synaptic plasticity in HCRT neurons.
(A) Circuit specific rhythmicity in synaptic density: circadian and homeostatic effects. Rhythmicity of synaptic plasticity of HCRT axons is observed in several brain regions. In HCRT axons projecting to the pineal gland, control of rhythmicity is mostly circadian (wide arrow), with minor homeostatic effects (narrow arrow). We found differences in rhythmic regulation of synapse density between axons within the same neuron projecting to the pineal gland and hindbrain. This could result in time-dependent differential output effects in selected projection targets, providing one more layer of regulation. NPTX2b, a rhythmically expressed protein, can mediate circadian fluctuations of synaptic density in HCRT axons. Other unknown clock- and homeostatic-controlled synaptic proteins are also likely involved in this process. (B) Overexpression of NPTX2b in HCRT neurons induces melatonin resistance. Overexpression of NPTX2b increases axonal synaptic density and results in a phenotype opposite to hcrtr−/− mutants (melatonin hypersensitivity), suggesting increased HCRT output. This may mediate melatonin resistance in two different ways. First, increased HCRT output can increase pineal gland melatonin release, an effect that could downregulate melatonin receptors and reduce sensitivity to melatonin application. Alternatively or in addition, other projections of HCRT with wake/activity promoting effects could be involved. In this scenario, HCRT projections to areas other than the pineal gland, such as the hindbrain, induce wake or activity. Increased activity at these sites could counterbalance sleep induced by melatonin.
nptx2b and circadian regulation of synaptic plasticity
NPTX2b is an attractive candidate protein for the circadian regulation of synaptic plasticity in HCRT neurons. This gene is rhythmically expressed in the hypothalamus under LD and DD. Further, the putative nptx2b promoter contains 16 E-boxes (CANNTG, two with CACGTG, the perfect E-box sequence, data not shown), a key DNA motif for the binding of clock proteins (Muñoz and Baler, 2003). In cultured neurons, overexpression of nptx2 in presynaptic neurons causes enhanced postsynaptic AMPA receptor clustering (O`Brien et al., 1999; O`Brien et al., 2002). In this study, we found that overexpression of nptx2b in HCRT neurons increases the number of presynaptic puncta, abolishes rhythmic synaptic plasticity in HCRT axons projecting to the pineal gland, and reduces sensitivity to melatonin, a phenotype opposite to hcrtr−/− fish. These results suggest that increased nptx2b expression in transgenic fish increases NPTX2b secretion in HCRT axon terminals, promoting clustering of AMPA receptors in postsynaptic neurons, an effect leading to increased HCRT output.
Rhythmic fluctuation of nptx2b expression in HCRT neurons is unlikely to be the sole factor regulating circadian synaptic plasticity. Indeed, nptx2b expression in the hypothalamus and synapse numbers of HCRT axons projecting to the pineal gland and hindbrain peak at different phases, suggesting multiple layers of regulation. Neural Pentraxin (NP) proteins enriched in the extracellular matrix include NPTX1, NPTX2 and NPR (the neuronal pentraxin receptor, Kirkpatrick et al., 2000). NPs have opposing effects on AMPA receptor aggregation at synapses. NPTX2 and NPTX1 heterodimerize, synergistically promoting AMPA receptor clustering while NPR regulates AMPAR removal (O’Brien et al., 1999; O’Brien et al., 2002; Xu et al.,2003, Sia et al., 2007; Cho et al., 2008). In this study, we elected to conduct gain of function experiments, as an nptx2b mutant is not available, and morpholino antisense oligonucleotides are not efficient at 7dpf. Further, loss of NPTX2b function would likely be compensated through the effects of other Neuronal Pentraxins (NPs). In knock-out mice, loss of both NPTX1 and NPTX2 is required to reduce AMPA-receptor-mediated transmission and alter synaptic refinement of the developing visual system (Bjartmar et al. 2006; Koach and Ullian, 2010).
Together, these data suggest that the rhythmically expressed nptx2b gene is involved in circadian regulation of synapse number of HCRT axons (Figure 7). As NPTX2 expression in mammals is upregulated by wake and sleep deprivation, and is not primarily clock dependant (Cirelli et al., 2004; Maret et al., 2007), it is tempting to speculate that other neuronal pentraxin members in zebrafish, such as NPTX2a, NPTX1 and NPR might regulate homeostatically controlled synaptic plasticity. For example, the role of nptx2 in zebrafish may vary between two paralogous genes. Nptx2b may regulate circadian synaptic plasticity only in HCRT and other hypothalamic neurons while nptx2a, a gene more widely expressed, may regulate homeostatic synaptic plasticity in wake-active memory circuits, for example in the forebrain and other brain area.
HCRT-melatonin interaction in zebrafish
These results extend on our recent finding indicating important interactions between HCRT and melatonin in the regulation of zebrafish sleep/wake cycles (Appelbaum et al., 2009). Melatonin has a strong hypnotic effect in zebrafish (Zhdanova et al., 2001). Although we were unable to detect baseline differences in sleep and activity in hcrt:NPTX2b transgenic fish, these animals were resistant to the hypnotic effects of melatonin, in symmetric opposition to the hypersensitivity observed in hcrtr−/− fish. Like in mammals, studies in zebrafish have shown activation of HCRT neurons in correlation with increased locomotor activity (Naumann et al., 2010), suggesting that melatonin resistance could be merely a consequence of HCRT excitatory output. However, we previously identified an interaction between HCRT and melatonin pathways suggesting alternative mechanism of regulation. In zebrafish, we found that HCRT enhances melatonin production in the pineal gland (Appelbaum et al., 2009). When the HCRT-melatonin signaling is altered, expression or activity of melatonin receptors in downstream circuits may increase or decrease to compensate for the variation in melatonin production. These may explain the hypersensitivity or resistance to melatonin of hcrtr−/− and hcrt:NPTX2b fish, respectively. Such a regulatory loop has been previously reported in birds where melatonin receptor numbers increased after pinealectomy (Lu and Cassone 1993). Interestingly, our results indicate that during the day when animals are more active, synapse number may be higher (in the pineal) or lower (hindbrain), offering the possibility of a differential modulation of HCRT cell output in various brain regions. Whether resistance to melatonin in hcrt:NPTX2b fish is secondary to disruptions of melatonin release via HCRT-pineal projections or indirect via co-modulation of other HCRT downstream targets (Figure 7B) remains to be investigated.
Conclusion and perspective
Our study demonstrates the feasibility of monitoring synapses in vivo longitudinally in individual animals, with application to the study of synaptic plasticity across the 24hr cycle. We found that diurnal regulation of synaptic plasticity is complex and circuit specific, with differential effects even within different axonal projection sites of the same neurons. In the zebrafish HCRT system, synapse number is primarily regulated by the circadian clock, although weaker homeostatic influences are also evident. A possible actor in this regulation is NPTX2b, a rhythmic gene that is expressed in the hypothalamus, although other synaptic proteins are likely to be involved.
The function of sleep is a highly debated topic (Mignot, 2008), but synaptic plasticity is believed to play a critical role (Diekelmann and Born, 2010). The longitudinal study of synaptic markers in other neuronal systems and in other HCRT projections will be necessary to expand on our findings, and will likely demonstrate various degrees of homeostatic versus circadian regulation of synaptic plasticity. Our approach offers the opportunity of studying synaptic plasticity in response to behavioral challenges or after genetic manipulation of key synaptic proteins, with complementary monitoring of the resulting behavior in a living vertebrate.
Experimental procedures
Cloning of nptx2 paralogous genes and in-situ hybridization (ISH)
Partial open reading frame sequences of nptx2a and nptx2b were identified using the online Ensembl database (http://www.ensembl.org) and the mouse nptx2 gene sequence (ENSMUSG00000059991). 5′ untranslated regions and location of start codons were determined with 5′ RACE (Invitrogen). The NCBI (http://www.ncbi.nlm.nih.gov/) accession numbers for these genes are: nptx2a, FJ232033; nptx2b, FJ232032. The full codon sequences of nptx2a and nptx2b were subcloned into pCRII-TOPO (Invitrogen) and served as a template for ISH in larvae and adults as previously described (Yokogawa et al., 2007; Appelbaum et al., 2009). Additional probes included: pdyn (AY309090), aanat2 (NM_131411), vglut2b (AB183387), c-fos (BC065466) and hcrt (DQ831346). Nptx2b expression in adult hypothalamus was quantified by setting a threshold in 8-bit pictures and calculation of pixel intensity and area using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Immunohistochemistry
Adult brains or 32hpf embryos were fixed in 4% PFA overnight at 4°C and then sliced as described (Appelbaum et al., 2009; Berman et al., 2009). After blocking, adult brain slices or embryos were incubated in rabbit polyclonal anti-GFP primary antibody (Torrey Pines Biolabs, TP401, 1:100 dilution) in block buffer overnight at 4°C. For double immunohistochemistry, embryos were also labeled with a primary monoclonal antibody to SV2 (Hybridoma Bank, Iowa City, IA, 1:1000 dilutions). Anti-GFP antibodies were detected using a secondary goat-anti-rabbit Alexa Fluor® 488 IgG (H+L) antibody (2 mg/mL, Invitrogen, A-11034). Anti-SV2 antibodies were detected using a secondary Alexa Fluor® 594 goat-anti-mouse IgG (2 mg/mL, Invitrogen, A-11005).
Array tomography was performed as described previously (Micheva and Smith 2007). In short, 4% PFA fixed hcrt:SYP-EGFP fish were embedded in LR White Resin. 100μm sections were acquired as an array from the fish hypothalamus. The array was then stained with an antibody for PSD95 (Gift of Jim Trimmer, UC Davis), visualized with alexa dyes, and the acquired images were deconvolved using the Richardson-Lucy algorithm implemented in Matlab. The images were then reconstructed in 3D to reveal the positioning of the SYP-EGFP puncta and PSD95 puncta.
Transient expression and generation of stable transgenic fish
All experiments with zebrafish are in accordance with Stanford University animal protocol. The CDS of nptx2b, the presynaptic (SYP-EGFP; Meyer and Smith, 2006) and postsynaptic (PSD95-EGFP; Niell et al., 2004) markers were subcloned into pT2-HCRT vector containing the HCRT promoter and the Tol2 (Kawakami et al., 2004) transposon elements (pT2-hcrt:NPTX2b, pT2-hcrt:SYP-EGFP, pT2-hcrt:PSD95-EGFP). Microinjections of the constructs into embryos for transient expression and generation of transgenic fish using the Tol2 system were carried out as described (Supplementary Data and Appelbaum et al., 2009).
In vivo imaging and quantification
4dpf and 7dpf larvae expressing synaptic markers were mounted in agarose and imaged with two-photon microscopy as described previously (Niell et al., 2004; Meyer and Smith, 2006; Appelbaum et al., 2009). We used two-photon imaging because the excitation wavelength is near infrared, a wavelength with undetectable effects on fish physiology and behavior, an important requirement for this study. This technique also minimizes photobleaching and toxicity because two-photon excitation is localized only to the focal plane of the objective (Carvalho and Heisenberg, 2009). Punctum counting in circadian and NPTX2b overexpression experiments was performed on image stacks processed with ImageJ software (Supplementary Data). In immunohistochemistry experiments, confocal imaging was performed using a Zeiss LSM 510 META laser scanning microscope.
Behavioral assay
To monitor larvae response to SD and melatonin application, locomotor activity of 5–7 dpf larvae was recorded using an automated video-tracking system (Videotrack; ViewPoint Life Sciences, Montreal, Canada, see Supplementary Methods) as previously described (Appelbaum et al., 2009). Progeny from out-crosses of heterozygous hcrt-NPTX2b transgenic and wild-type siblings were monitored in drug-free and melatonin (1μM) conditions by an experimental procedure blind to the genotype. A total of 4 independent experiments were performed for each treatment. Adult sleep was monitored with and without melatonin (100μM) as described (Yokogawa et al., 2007; Appelbaum et al., 2009). Sleep deprivation (SD) experiments were performed under DD as described (Zhdanova et al., 2001), with minor modifications. Specifically, larvae were placed in a Petri dish on a 3-dimentional moving shaker tray that produces variable levels of vibration with intermittent gentle tapping. Larvae were subjected to SD for 3 or 6 hours, and sibling control larvae were maintained under similar conditions without vibration.
Supplementary Material
Acknowledgments
We thank Laura Alexander and Wilfredo Marin for assistance in fish maintenance and experiments. We thank Dr. Géraldine Maro for her expertise and assistance in confocal microscopy. We also thank Dr. Jamie Zeitzer and Dr. Oren levy for helping in statistical analysis and Dr. Matthew E. Carter, Dr. Brian Grone, Dr. Juliette Faraco, Dr. Simon Warby, Dr. Yoav Gothilf and Dr. Luis de Lecea for helpful comments on the manuscript. Funding: This research was supported by the McKnight Foundation, National Institutes of Health (NS23724, NS062798), the Howard Hughes Medical Research Institute (EM) and the Vincent Coates Foundation (SJS).
Footnotes
Supplementary data includes Supplementary Experimental Procedures, six figures and one table.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achermann P, Borbély AA. Mathematical models of sleep regulation. Front Biosci. 2003;8:s683–693. doi: 10.2741/1064. [DOI] [PubMed] [Google Scholar]
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum L, Wang G, Maro G, Mori R, Tovin A, Marin W, Yokogawa T, Kawakami K, Smith SJ, Gothilf Y, Mignot E, Mourrain P. Sleep/wake regulation and hypocretin-melatonin interaction in zebrafish. Proc Natl Acad Sci U S A. 2009;106:21942–21947. doi: 10.1073/pnas.906637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamji SX, Shimazu K, Kimes N, Huelsken J, Birchmeier W, Lu B, Reichardt LF. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 2003;40:719–731. doi: 10.1016/s0896-6273(03)00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basheer R, Sherin JE, Saper CB, Morgan JI, McCarley RW, Shiromani PJ. Effects of sleep on wake-induced c-fos expression. J Neurosci. 1997;17:9746–9750. doi: 10.1523/JNEUROSCI.17-24-09746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JR, Skariah G, Maro GS, Mignot E, Mourrain P. Characterization of two melanin-concentrating hormone genes in zebrafish reveals evolutionary and physiological links with the mammalian MCH system. J Comp Neurol. 2009;517:695–710. doi: 10.1002/cne.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjartmar L, Huberman AD, Ullian EM, Rentería RC, Liu X, Xu W, Prezioso J, Susman MW, Stellwagen D, Stokes CC, et al. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J Neurosci. 2006;26:6269–6281. doi: 10.1523/JNEUROSCI.4212-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho L, Heisenberg CP. Imaging zebrafish embryos by two-photon excitation time-lapse microscopy. Methods Mol Biol. 2009;546:273–287. doi: 10.1007/978-1-60327-977-2_17. [DOI] [PubMed] [Google Scholar]
- Cho RW, Park JM, Wolff SB, Xu D, Hopf C, Kim JA, Reddy RC, Petralia RS, Perin MS, Linden DJ, Worley PF. mGluR1/5-dependent long-term depression requires the regulated ectodomain cleavage of neuronal pentraxin NPR by TACE. Neuron. 2008;57:858–871. doi: 10.1016/j.neuron.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Is Sleep Essential? PLoS Biol. 2008;6:e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker A, España RA, Papadopoulou M, Saper CB, Faraco J, Sakurai T, Honda M, Mignot E, Scammell TE. Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology. 2005;65:1184–1188. doi: 10.1212/01.wnl.0000168173.71940.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- Donlea JM, Ramanan N, Shaw PJ. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324:105–108. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreosti E, Odermatt B, Dorostkar MM, Lagnado L. A genetically encoded reporter of synaptic activity in vivo. Nat Methods. 2009;6:883–889. doi: 10.1038/nmeth.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara T, Kawabata I, Usui S, Sobue K, Okabe S. Synchronized formation and remodeling of postsynaptic densities: long-term visualization of hippocampal neurons expressing postsynaptic density proteins tagged with green fluorescent protein. J Neurosci. 2003;23:2170–2181. doi: 10.1523/JNEUROSCI.23-06-02170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco JH, Appelbaum L, Marin W, Gaus SE, Mourrain P, Mignot E. Regulation of hypocretin (orexin) expression in embryonic zebrafish. J Biol Chem. 2006;281:29753–29761. doi: 10.1074/jbc.M605811200. [DOI] [PubMed] [Google Scholar]
- Fernández MP, Berni J, Ceriani MF. Circadian remodeling of neuronal circuits involved in rhythmic behavior. Plos Biol. 2008;6:518–524. doi: 10.1371/journal.pbio.0060069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science. 2009;324:109–112. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Javaherian A, Cline HT. Coordinated motor neuron axon growth and neuromuscular synaptogenesis are promoted by CPG15 in vivo. Neuron. 2005;45:505–512. doi: 10.1016/j.neuron.2004.12.051. [DOI] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Johnson AW, Crombag HS, Takamiya K, Baraban JM, Holland PC, Huganir RL, Reti IM. A selective role for neuronal activity regulated pentraxin in the processing of sensory-specific incentive value. J Neurosci. 2007;27:13430–13435. doi: 10.1523/JNEUROSCI.4320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslin J, Nystedt JM, Ostergård M, Peitsaro N, Panula P. The orexin/hypocretin system in zebrafish is connected to the aminergic and cholinergic systems. J Neurosci. 2004;24:2678–2689. doi: 10.1523/JNEUROSCI.4908-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Takeda H, Kawakami N, Kobayashi M, Matsuda N, Mishina M. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev Cell. 2004;7:133–144. doi: 10.1016/j.devcel.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick LL, Matzuk MM, Dodds DC, Perin MS. Biochemical interactions of the neuronal pentraxins. Neuronal pentraxin (NP) receptor binds to taipoxin and taipoxin-associated calcium-binding protein 49 via NP1 and NP2. J Biol Chem. 2000;275:17786–17792. doi: 10.1074/jbc.M002254200. [DOI] [PubMed] [Google Scholar]
- Koch SM, Ullian EM. Neuronal pentraxins mediate silent synapse conversion in the developing visual system. J Neurosci. 2010;30:5404–5414. doi: 10.1523/JNEUROSCI.4893-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Livneh Y, Feinstein N, Klein M, Mizrahi A. Sensory input enhances synaptogenesis of adult-born neurons. J Neurosci. 2009;29:86–97. doi: 10.1523/JNEUROSCI.4105-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Cassone VM. Pineal regulation of circadian rhythms of 2-deoxy[14C]glucose uptake and 2[125I]iodomelatonin binding in the visual system of the house sparrow, Passer domesticus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1993;173:765–774. [Google Scholar]
- Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- Maret S, Dorsaz S, Gurcel L, Pradervand S, Petit B, Pfister C, Hagenbuchle O, O’Hara BF, Franken P, Tafti M. Homer1a is a core brain molecular correlate of sleep loss. Proc Natl Acad Sci U S A. 2007;104:20090–20095. doi: 10.1073/pnas.0710131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MP, Smith SJ. Evidence from in vivo imaging that synaptogenesis guides the growth and branching of axonal arbors by two distinct mechanisms. J Neurosci. 2006;26:3604–3614. doi: 10.1523/JNEUROSCI.0223-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva KD, Smith SJ. Array tomography: a new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron. 2007;55:25–36. doi: 10.1016/j.neuron.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot E. Why we sleep: the temporal organization of recovery. PLoS Biol. 2008;6:661–669. doi: 10.1371/journal.pbio.0060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz E, Baler R. The circadian E-box: when perfect is not good enough. Chronobiol Int. 2003;20:371–388. doi: 10.1081/cbi-120022525. [DOI] [PubMed] [Google Scholar]
- Niell CM, Meyer MP, Smith SJ. In vivo imaging of synapse formation on a growing dendritic arbor. Nat Neurosci. 2004;7:254–260. doi: 10.1038/nn1191. [DOI] [PubMed] [Google Scholar]
- Naumann EA, Kampff AR, Prober DA, Schier AF, Engert F. Monitoring neural activity with bioluminescence during natural behavior. Nat Neurosci. 2010;13:513–520. doi: 10.1038/nn.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino S, Sakurai T. The Orexin/Hypocretin System Physiology and Pathophysiology. Humana Press Inc; Totowa, New Jersey: 2006. [Google Scholar]
- O’Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron. 1999;23:309–323. doi: 10.1016/s0896-6273(00)80782-5. [DOI] [PubMed] [Google Scholar]
- O’Brien R, Xu D, Mi R, Tang X, Hopf C, Worley P. Synaptically targeted narp plays an essential role in the aggregation of AMPA receptors at excitatory synapses in cultured spinal neurons. J Neurosci. 2002;22:4487–4498. doi: 10.1523/JNEUROSCI.22-11-04487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Pando MP, Sassone-Corsi P. Unraveling the mechanisms of the vertebrate circadian clock: zebrafish may light the way. BioEssays. 2002;24:419–426. doi: 10.1002/bies.10091. [DOI] [PubMed] [Google Scholar]
- Panula P. Hypocretin/orexin in fish physiology with emphasis on zebrafish. Acta Physiol (Oxf) 2010;198:381–386. doi: 10.1111/j.1748-1716.2009.02038.x. [DOI] [PubMed] [Google Scholar]
- Prober DA, Rihel J, Onah AA, Sung RJ, Schier AF. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J Neurosci. 2006;26:13400–13410. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyza E, Górska-Andrzejak J. External and internal inputs affecting plasticity of dendrites and axons of the fly’s neurons. Acta Neurobiol Exp (Wars) 2008;68:322–333. doi: 10.55782/ane-2008-1698. [DOI] [PubMed] [Google Scholar]
- Rao Y, Liu ZW, Borok E, Rabenstein RL, Shanabrough M, Lu M, Picciotto MR, Horvath TL, Gao XB. Prolonged wakefulness induces experience-dependent synaptic plasticity in mouse hypocretin/orexin neurons. J Clin Invest. 2007;117:4022–4033. doi: 10.1172/JCI32829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reti IM, Reddy R, Worley PF, Baraban JM. Selective expression of Narp, a secreted neuronal pentraxin, in orexin neurons. J Neurochem. 2002;82:1561–1565. doi: 10.1046/j.1471-4159.2002.01141.x. [DOI] [PubMed] [Google Scholar]
- Reti IM, Miskimon M, Dickson M, Petralia RS, Takamiya K, Bland R, Saini J, During MJ, Huganir RL, Baraban JM. Activity-dependent secretion of neuronal activity regulated pentraxin from vasopressin neurons into the systemic circulation. Neuroscience. 2008a;151:352–360. doi: 10.1016/j.neuroscience.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reti IM, Crombag HS, Takamiya K, Sutton JM, Guo N, Dinenna ML, Huganir RL, Holland PC, Baraban JM. Narp regulates long-term aversive effects of morphine withdrawal. Behav Neurosci. 2008b;122:760–768. doi: 10.1037/a0012514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Cano G, Scammell TE. Homeostatic, circadian, and emotional regulation of sleep. J Comp Neurol. 2005;493:92–98. doi: 10.1002/cne.20770. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- Sia GM, Béïque JC, Rumbaugh G, Cho R, Worley PF, Huganir RL. Interaction of the N-terminal domain of the AMPA receptor GluR4 subunit with the neuronal pentraxin NP1 mediates GluR4 synaptic recruitment. Neuron. 2007;55:87–102. doi: 10.1016/j.neuron.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Stütz AM, Staszkiewicz J, Ptitsyn A, Argyropoulos G. Circadian expression of genes regulating food intake. Obesity. 2007;15:607–615. doi: 10.1038/oby.2007.564. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao A, Wisor JP, Peyron C, Apte-Deshpande A, Wurts SW, Edgar DM, Kilduff TS. Gene expression in the rat brain during sleep deprivation and recovery sleep: an Affymetrix GeneChip study. 2006;137:593–605. doi: 10.1016/j.neuroscience.2005.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Tsui CC, Copeland NG, Gilbert DJ, Jenkins NA, Barnes C, Worley PF. Narp, a novel member of the pentraxin family, promotes neurite outgrowth and is dynamically regulated by neuronal activity. J Neurosci. 1996;16:2463–2478. doi: 10.1523/JNEUROSCI.16-08-02463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtorta F, Pennuto M, Bonanomi D, Benfenati F. Synaptophysin: leading actor or walk-on role in synaptic vesicle exocytosis? BioEssays. 2004;26:445–453. doi: 10.1002/bies.20012. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Xu D, Hopf C, Reddy R, Cho RW, Guo L, Lanahan A, Petralia RS, Wenthold RJ, O’Brien RJ, Worley P. Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron. 2003;39:513–528. doi: 10.1016/s0896-6273(03)00463-x. [DOI] [PubMed] [Google Scholar]
- Yokogawa T, Marin W, Faraco J, Pézeron G, Appelbaum L, Zhang J, Rosa F, Mourrain P, Mignot E. Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. PLoS Biol. 2007;5:2379–2397. doi: 10.1371/journal.pbio.0050277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhdanova IV, Wang SY, Leclair OU, Danilova NP. Melatonin promotes sleep-like state in zebrafish. Brain Res. 2001;903:263–268. doi: 10.1016/s0006-8993(01)02444-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.