Abstract
Apatinib, a small-molecule multi-targeted tyrosine kinase inhibitor, is in phase III clinical trial for treatment of patients with non-small cell lung cancer and gastric cancer in China. In this study, we determined the effect of apatinib on the interaction of specific antineoplastic compounds with P-glycoprotein (P-gp, ABCB1), multidrug resistance protein 1 (MRP1, ABCC1) and breast cancer resistance protein (BCRP, ABCG2). Our results showed that apatinib significantly enhanced the cytotoxicity of ABCB1 or ABCG2 substrate drugs in KBv200, MCF-7/adr and HEK293/ABCB1 cells overexpressing ABCB1 and S1-M1-80, MCF-7/FLV1000 and HEK293/ABCG2-R2 cells overexpressing ABCG2 (wild-type). In contrast, apatinib did not alter the cytotoxicity of specific substrates in the parental cells and cells overexpressing ABCC1. Apatinib significantly increased the intracellular accumulation of rhodamine 123 and doxorubicin in the multidrug resistance (MDR) cells. Furthermore, apatinib significantly inhibited the photolabeling of both ABCB1 and ABCG2 with [125I]-iodoarylazidoprazosin in a concentration-dependent fashion. The ATPase activity of both ABCB1 and ABCG2 was significantly increased by apatinib. However, apatinib, at a concentration the produced a reversal of MDRl, did not significantly alter the expression of the ABCB1 or ABCG2 protein or mRNA levels or the phosphorylation of AKT and ERK1/2. Importantly, apatinib significantly enhanced the effect of paclitaxel against the ABCB1 resistant KBv200 cancer cell xenografts in nude mice. In conclusion, apatinib reverses ABCB1- and ABCG2-mediated MDR by inhibiting their transport function, but not by blocking AKT or ERK1/2 pathway or downregulating ABCB1 or ABCG2 expression. Apatinib may be useful in circumventing MDR to other conventional antineoplastic drugs.
Keywords: Apatinib, Multidrug resistance, ATP-binding cassette transporters, P-glycoprotein, ABCG2, Xenograft
Introduction
Multi-drug resistance (MDR) in cancer cells produces resistantance to the cytotoxic effects of numerous antioneoplastic drugs that are structurally and mechanistically unrelated and this significantly decreases the efficacy of cancer chemotherapy (1). The most common cause of MDR results from the overexpression of cell membrane-bound ATP binding cassette (ABC) transporters, which actively extrude a variety of chemotherapeutic drugs out of the cancer cells, thereby attenuating their cytotoxic actions (2). Forty-eight ABC proteins have been identified in the human genome and are divided into seven subfamilies (A-G) based on sequence similarities (3). The ABC transporter-subfamily B member 1 (ABCB1/MDR1/ P-glycoprotein, P-gp), subfamily C member 1 (ABCC1/MRP1) and subfamily G member 2 (ABCG2/BCRP) play a major role in producing MDR in tumor cells (4, 5).
ABCB1 was first discovered in drug-resistant Chinese hamster ovarian cells (6). It can transport a wide range of antineoplastic drugs such as the anthracyclines, vinca alkaloids, taxanes, and epipodophyllotoxins (6). ABCG2 was identified independently from human colon cancinoma cells (S1-M1-80) (7), the placenta (8) and a drug-selected human breast cancer cell line MCF-7 (9). ABCG2 can actively efflux a wide variety of antineoplastic drugs including mitoxantrone, indolocarbazole, topoisomerase I inhbitors and anthracyclines, as well as fluorescent dyes such as Hoechst 33342 (10). The side population (SP) phenotype cells is present in diverse tumor types and they over express ABCG2, producing inherent drug resistance (11, 12). Currently, ABCG2 is considered a molecular marker for the SP cells (13). Thus, targeting ABCG2 in these tumor stem cells represents a promising and novel strategy to eradicate the entire cancer cell population.
Tyrosine kinase inhibitors (TKIs), a relatively new class of anti-neoplastic drugs, are believed to exert their mechanism of action by competing with ATP for binding to the ATP site of the catalytic domain of several oncogenic tyrosine kinase. Subsequently, the TKIs can attenuate downstream signaling pathways involved in cancer proliferation, invasion, metastasis and angiogenesis. Previously, it has been reported that the BCR-Abl TKIs imatinib (Gleevec) and nilotinib (Tasigna) interact with ABCB1 and ABCG2 transporters and significantly inhibit their transport activity (14, 15). In addition, epidermal growth factor receptor TKIs (e.g. lapatinib (16), gefitinib (17), erlotinib (18)), VEGFR (vascular endothelial growth factor receptor) TKIs (e.g. cediranib (19), vandetanib (20)); and the multi-kinase TKI sunitinib (21) have been shown to significantly attenuate or reverse ABC transporters-mediated MDR in cancer cells. Thus, it is possible that TKIs could be utilized as MDR inhibitors. Apatinib (YN968D1) is small-molecule TKI that inhibits VEGFR-2 (Flk-1/KDR), RET (rearranged during transfection), c-Kit (stem cell factor receptor) and c-Src tyrosine kinases. Apatinib has been used in a Phase III clinical trial in China to determine its efficacy in treating gastric carcinoma and non-small cell lung cancer (NSCLC). Currently, no studies have examined the effect of apatinib in cells lines or animal models that overexpress ABCB1 or ABCG2 transporters. Therefore, in this study, we conducted experiments in order to determine if apatinib can potentiate the efficacy of conventional antineoplastic drugs via interaction with ABC transporters in MDR cancer cells.
Materials and Methods
Reagents
Apatinib was obtained from Jiangsu Hengrui Medicine Co. (China), with a molecular structure as shown in Fig. S1A. Monoclonal antibodies against ABCB1 (sc-55510) and ABCC1 (sc-18835) were from Santa Cruz Biotechnology. ABCG2 antibody (MAB4146) was obtained from Chemicon International, Inc (Billerica, MA). Akt antibody (#4685) was a product of Cell Signaling Technology Inc (Danvers, MA). Monoclonal antibodies C-219 (against ABCB1) and BXP-34 (against ABCG2) were acquired from Signet laboratories Inc. (Dedham, MA). Phosphorylated Akt (KC-5A04), phosphorylated extracellular signal-regulated kinase (KC-5E04), MAPK1/2 (Erk1/2) (KC-5E01), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies were purchased from Kangchen Co. (Shanghai, China). [125I]-Iodoarylazidoprazosin (IAAP; 2,200 Ci/mmol) was obtained from Perkin-Elmer Life Sciences. Dulbecco’s modified Eagle’s medium (DMEM) and RPMI-1640 were products of Gibco BRL (Gaithersburg, MD). Rhodamine 123 (Rho 123), 1-(4, 5-dimethylthiazol-2-yl)-3, 5-diphenylformazan (MTT), paclitaxel, doxorubicin (DOX), vincristine (VCR), verapamil (VRP), topotecan, and other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO).
Cell lines
The following cell lines were cultured in DMEM or RPMI 1640 supplemented with 10% FBS at 37°C in a humidified atmosphere of 5% CO2: the human breast carcinoma cell lines MCF-7, its DOX-selected ABCB1-overexpressing derivative MCF-7/adr (22) andflavopiridol-resistant ABCG2 overexpressing MCF-7/FLV1000 sublines(23) were kindly provided by Dr. S.E. Bates (National Cancer Institute, NIH). The human oral epidermoid carcinoma cell line KB and its VCR-selected ABCB1-overexpressing derivative KBv200 was obtained as a gift from Dr. Xu-Yi Liu, Cancer Hospital of Beijing (24). KB-3-1 and KB/ABCC1 transfectant cells were kindly provided by Dr. S. Akiyama (Kagoshima University, Japan) (reference, Ueda’s group, PNAS, see my reference of BBRC paper in 1997!). The following cell lines were obtained from Dr. S.E. Bates (National Cancer Institute, NIH): the human colon carcinoma cell line S1 and its mitoxantrone (MX)-selected ABCG2-overexpressing derivative S1-M1-80 (Honjo, Y., Hrycyna, C. A., Yan, Q. W., Medina-Perez, W. Y., Robey, R. W., van de Laar, A., Litman, T., Dean, M., and Bates, S. E. (2001) Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells, Cancer Res. 61, 6635–6639), the human primary embryonic kidney cell line HEK293 and its pcDNA3.1, ABCB1, ABCG2 and ABCC1 stable gene transfected cell lines HEK293/pcDNA3.1, HEK293/ABCB1(Robey, R.W., Shukla, S., Finely, E.M., Oldham, R.K., Barnett, D., Ambudkar, S.V., Fojo, T., and Bates, S.E.: Inhibition of P-glycoprotein (ABCB1)- and multidrug resistance-associated protein 1 (ABCC1)-mediated transport by the orally administered inhibitor, CBT-1®. Biochem. Pharmacol., 75: 1302–1312, 2008, HEK293/ABCG2-R2 (Robey et al, British J. Cancer, 2003, which is reference #25) and HEK293/ABCC1 (Müller, M., Yong, M., Peng, X.H., Petre, B., Arora, S., and Ambudkar, S.V.: Evidence for the role of glycosylation in accessibility of the external domains of human MRP1 (ABCC1). Biochemistry 41: 10123–10132, 2002). All of the transfected cells were cultured in a medium with 2 mg/mL of G418 (except HEK293/ABCC1 cell line was cultured with 800 µg/ml G418) (25). All resistant cells were authenticated by comparing the fold-resistance with parental drug sensitive cells and examining the expression levels of ABC transporters. All cells were grown in drug-free culture medium for > 2 weeks before assay.
Animals
Athymic nude mice (BALB/c-nu-nu), 5–6 weeks old and weighing 18–24 g, were obtained from the Center of Experimental Animals, Sun Yat-Sen University (China) and used for the KBv200 cell xenografts. All animals received sterilized food and water. All experiments were carried out in accordance with the guidelines on animal care and experiments of laboratory animals (Center of Experimental Animals, Sun Yat-Sen University, China), which was approved by the ethics committee for animal experiments.
Cytotoxicity test
The MTT assay was performed as described previously to assess the sensitivity of cells to drugs (26). The concentration required to inhibit cell growth by 50% (IC50) was calculated from survival curves using the Bliss method (27). The degree of resistance was estimated by dividing the IC50 for the MDR cells by that of the parental sensitive cells; and the fold-reversal factor of MDR was calculated by dividing the IC50 of the anticancer drug in the absence of apatinib by that obtained in the presence of apatinib
Nude mouse xenograft model
The KBv200-inoculated nude xenograft model previously established by Chen and colleagues was employed in this study (28). The xenograft was found to maintain the MDR phenotype in vivo and was extremely resistant to paclitaxel treatment. Briefly, KBv200 cells grown in vitro were harvested and implanted subcutaneously under the shoulder in the nude mice. When the tumors reached a mean diameter of 0.5 cm, the mice were randomized into 4 groups and treated with various regimens: 1) saline (q3d×4), 2) paclitaxel (18 mg/kg, i.p., q3d×4); 3) apatinib (70 mg/kg, p.o., q3d×4), and 4) paclitaxel (18 mg/kg, i.p., q3d×4) + apatinib (70 mg/kg, p.o., q3d×4 given 1 h before injecting paclitaxel). The body weight of the animals and the two perpendicular diameters (A and B) were recorded every 3 days and tumor volume (V) was estimated according to the following formula (28):
The curve of tumor growth was drawn according to tumor volume and time of implantation. The mice were anesthetized and sacrificed when the mean of tumor weights was over 1 g in the control group. Tumor tissues were excised from the mice and their weight was measured. The ratio of growth inhibition (IR) was calculated according to the following formula (28):
DOX and Rho 123 accumulation
The effect of apatinib on the intracellular accumulation of DOX and Rho 123 was performed as previously described (22). VRP, an ABCB1 inhibitor, was used as a positive control for KB, KBv200, MCF-7 and MCF-7/adr cells and FTC was used as a positive control for ABCG2 in S1 and S1-M1-80 cells (29, 30).
In vitro transport assays
DOX was added to the medium to obtain final concentrations of 2.5 µM to 20 µM in the absence or presence of apatinib and cells were incubated at 37°C for 3 h. The cells were collected, centrifuged and washed once with cold PBS, and resuspended in the medium with free DOX in the absence or presence of apatinib. Subsequently, cells were incubated for 5 min at 37°C, centrifuged and washed 3 times with cold PBS. In the control experiments, the apical uptake reaction was kept at 0°C. Finally, the intracellular concentration of DOX was determined by flow cytometric analysis (Beckman Coulter, Cytomics FC500, USA) (31). The quantity of DOX efflux by ABC transporter was calculated by subtracting values obtained at 37°C from that at 0°C. The inhibitory effect of apatinib was analyzed using Lineweaver–Burk plots as previously described (32).
Reverse transcription PCR
ABCB1 and ABCG2 expression were assayed as described (16). Total RNA was isolated using the Trizol Reagent RNA extraction kit (Molecular Research Center, USA) and subjected to RT-PCR (Promega Corp.). The PCR primers were shown below: 1) ABCB1 premiers 5’-ccc atc att gca ata gca gg-3’ (forward) and 5’-gtt caa act tct gct cct ga-3’ (reverse); 2) ABCG2 primers 5’-tgg ctg tca tgg ctt cag ta-3’ (forward) and 5’-gcc acg tga ttc ttc cac aa-3’ (reverse) and 3) GAPDH primers 5’-ctt tgg tat cgt gga agg a-3’ (forward) and 5’-cac cct gtt gct gta gcc-3’ (reverse). The products were resolved using gel electrophoresis (1.5% agarose gel).
Western blot analysis
Cells were lysed after washing two times with ice cold PBS. The protein concentration was quantified using the Bradford method (33). Equal amounts of protein were resolved by SDS-PAGE, transferred to nitrocellulose membranes and chemoluminescence was used to detect the protein.
ATPase assay of ABCB1 and ABCG2
The Vi-sensitive ATPase activity of ABCB1 and BeFx-sensitive ABCG2 in the membrane vesicles of High Five insect cells was measured as previously described (34). Apatinib was added and incubated at 37°C for the duration of the experiment. The ATPase reaction was initiated by the adding 5 mM Mg-ATP into the total reaction mixture of 0.1 mL. After incubation at 37°C for 20 min, the reactions were terminated by the addition of 0.1 mL of 5% SDS solution. The liberated inorganic phosphate (Pi) was measured as previously described (16, 34)
Photo-affinity labeling of ABCB1 and ABCG2 with [125I]-IAAP
The photoaffinity labeling of ABCB1 or ABCG2 with [125I]-IAAP was performed as previously described (16, 34). ABCB1 was immunoprecipitated with the C219 antibody, whereas ABCG2 was immunoprecipitated using the BXP21 antibody (35). The samples were subjected to SDS-PAGE using a 7% Tris-acetate NuPAGE gel, dried and exposed to Bio-Max MR film (Eastman Kodak Co., Rochester, New York) at −80°C for 3 to 5 h. The radioactivity incorporated into the ABCB1 or ABCG2 band was quantified using the Storm 860 PhosphorImager system and ImageQuant (Molecular Dynamics).
Statistical analysis
All experiments were repeated at least three times and the differences were determined by using the Student’s t-test. Statistical significance was set at p < 0.05.
Results
Apatinib reverses MDR in cells overexpressing ABCB1 and ABCG2
The cytotoxicity of apatinib in different cell lines was determined by the MTT assay. The IC50 values were 15.18 ± 0.63, 11.95 ± 0.69, 17.16 ± 0.25, 14.54 ± 0.26, 9.30 ± 0.72, 11.91 ± 0.32 and 19.13 ± 1.13 µM for KB, KBv200, MCF-7, MCF-7/adr, S1, S1-M1-80, MCF-7/FLV1000 cells, respectively (Fig. S1). For HEK293/pcDNA3.1, HEK/ABCB1, HEK/ABCG2-R2 and HEK293/ABCC1 cells, the IC50 values of apatinib were > 30 µM (data not shown). Based on the cytotoxicity curves, apatinib was used at a maximum concentration of 3.0 µM, a concentration where more than 90% of the cells were viable in all cell lines used in the MDR reversal study. The IC50 values of the antineoplastic drugs in sensitive and resistant cells at different concentrations of apatinib are shown in Table 1. Apatinib produced a concentration-dependent decrease in the IC50 values of 1) DOX and paclitaxel in the KBv200 cells, DOX in MCF-7/adr cells; 2) mitoxantrone and topotecan in the S1-M1-80 cells and 3) mitoxantrone in MCF-7/FLV1000 cells. In addition, 3 µM of apatinib completely reversed ABCG2-mediated resistance to mitoxantrone and SN-38 in wild type HEK293/ABCG2-R2 cells transfected with ABCG2 (Table 2). Furthermore, 3 µM of apatinib significantly decreased the IC50 values of mitoxantrone, vincristine and DOX in stably transfected HEK293/ABCB1 cells (Table 2). However, apatinib did not significantly alter the cytotoxicity of the antineoplastic drugs in the parental cells (Tables 1 and 2). Furthermore, apatinib did not significantly alter the cytotoxicity of non-ABCB1 or non-ABCG2 substrates (cisplatin) in either MDR cells or their parental sensitive cells (Tables 1 and 2). Apatinib significantly decreased the IC50 values of DOX and paclitaxel compared to the ABCB1 inhibitor VRP in KBv200 and MCF-7/adr cells. Similarly, apatinib significantly decreased the IC50 of topotecan (from 10.3 to 0.7 µM) compared to the ABCG2 inhibitor FTC (positive control) in S1-M1-80 cells. In contrast, apatinib does not significantly alter the sensitivity of the drug-sensitive parental cells to the antineoplastic drugs used in this study. In addition, apatinib had no significant reversal effect on ABCC1-mediated drug resistance in ABCC1 gene transfectant cell lines such as KB/ABCC1 and HEK293/ABCC1(data not shown). Therefore, our results suggest that apatinib significantly sensitizes cells overexpressing ABCB1- or ABCG2 to antineoplastic drugs that are substrates of ABCB1 or ABCG2.
Table 1.
Effect of apatinib on reversing ABCB1- and ABCG2-mediated MDR in drug selection cell lines
| IC50 ± SD (µM) (fold-reversal) | ||||
|---|---|---|---|---|
| Compounds | ||||
| KB | KBv200 (ABCB1) | |||
| Doxorubicin | 0.029 ± 0.002 | (1.00) | 2.277 ± 0.134 | (1.00) |
| + 0.75 µM Apatinib | 0.029 ± 0.002 | (1.00) | 1.259 ± 0.099** | (1.81) |
| + 1.5 µM Apatinib | 0.030 ± 0.001 | (0.98) | 0.964 ± 0.070** | (2.36) |
| + 3.0 µM Apatinib | 0.028 ± 0.003 | (1.04) | 0.337 ± 0.016** | (6.81) |
| + 10 µM Verapamil | 0.029 ± 0.003 | (1.00) | 0.106 ± 0.006** | (21.4) |
| Paclitaxel | 0.0018 ± 0.0002 | (1.00) | 0.296 ± 0.027 | (1.00) |
| + 0.75 µM Apatinib | 0.0018 ± 0.0003 | (1.00) | 0.142 ± 0.013** | (2.08) |
| + 1.5 µM Apatinib | 0.0019 ± 0.0002 | (0.95) | 0.045 ± 0.004** | (6.58) |
| + 3.0 µM Apatinib | 0.0018 ± 0.0002 | (1.00) | 0.009 ± 0.001** | (32.9) |
| + 10 µM Verapamil | 0.0019 ± 0.0002 | (0.95) | 0.007 ± 0.001** | (42.3) |
| Cispatin | 0.726 ± 0.055 | (1.00) | 1.284 ± 0.141 | (1.00) |
| + 3.0 µM Apatinib | 0.714 ± 0.057 | (1.01) | 1.292 ± 0.125 | (0.99) |
| MCF-7 | MCF-7/adr (ABCB1) | |||
| Doxorubicin | 0.344 ± 0.037 | (1.00 ) | 11.504 ± 1.186 | (1.00) |
| + 0.75 µM Apatinib | 0.349 ± 0.011 | (0.99) | 3.021 ± 0.196** | (3.81) |
| + 1.5 µM Apatinib | 0.331 ± 0.019 | (1.04) | 2.177 ± 0.273** | (5.28) |
| + 3.0 µM Apatinib | 0.350 ± 0.036 | (0.98 ) | 0.854 ± 0.056** | (13.5) |
| + 10 µM Verapamil | 0.340 ± 0.038 | (1.01) | 0.540 ± 0.076** | (21.3) |
| Cispatin | 5.811 ± 0.533 | (1.00) | 4.622 ± 0.371 | (1.00) |
| + 3.0 µM Apatinib | 5.624 ± 0.211 | (1.03 ) | 4.531 ± 0.352 | (1.02) |
| S1 | S1-M1-80 (ABCG2) | |||
| Mitoxantrone | 0.194 ± 0.027 | (1.00) | 13.651 ± 0.922 | (1.00) |
| + 0.75 µM Apatinib | 0.196 ± 0.041 | (0.98) | 6.434 ± 0.478** | (2.12) |
| + 1.5 µM Apatinib | 0.185 ± 0.058 | (1.05) | 2.070 ± 0.621** | (6.59) |
| + 3.0 µM Apatinib | 0.136 ± 0.067 | (1.42) | 1.188 ± 0.495** | (11.5) |
| + 2.5 µM FTC | 0.188 ± 0.011 | (1.03) | 0.892 ± 0.056** | (15.3) |
| Topotecan | 0.262 ± 0.042 | (1.00) | 10.28 ± 0.455 | (1.00) |
| + 0.75 µM Apatinib | 0.264 ± 0.022 | (0.99) | 4.089 ± 0.026** | (2.51) |
| + 1.5 µM Apatinib | 0.247 ± 0.017 | (1.05) | 2.037 ± 0.083** | (5.04) |
| + 3.0 µM Apatinib | 0.196 ± 0.055 | (1.33) | 1.188 ± 0.055** | (8.65) |
| + 2.5 µM FTC | 0.254 ± 0.016 | (1.02) | 0.745 ± 0.068** | (13.8) |
| Cisplatin | 12.811 ± 1.181 | (1.00) | 12.092 ± 1.322 | (1.00) |
| + 3.0 µM Apatinib | 12.280 ± 1.990 | (1.04) | 12.143 ± 1.452 | (1.00) |
| MCF-7 | MCF-7/FLV1000 (ABCG2) | |||
| Mitoxantrone | 0.015 ± 0.001 | (1.00) | 3.791 ± 0.420 | (1.00) |
| + 0.75 µM Apatinib | 0.014 ± 0.004 | (1.07) | 1.718 ± 0.157** | (2.21) |
| + 1.5 µM Apatinib | 0.014 ± 0.003 | (1.06) | 0.679 ± 0.089** | (5.59) |
| + 3.0 µM Apatinib | 0.011 ± 0.002 | (1.37) | 0.301 ± 0.044** | (12.6) |
| + 2.5 µM FTC | 0.015 ± 0.004 | (1.03) | 0.170 ± 0.014** | (22.3) |
Cell survival was determined by MTT assays as described in “Materials and Methods”. Data are the mean ± standard deviation (SD) of at least three independent experiments performed in triplicate. The fold-reversal of MDR (values given in parenthesis in last column) was calculated by dividing the IC50 for cells with the anticancer drugs in the absence of apatinib by that obtained in the presence of apatinib.
represent P < 0.01 for values versus that obtained in the absence of apatinib.
Table 2.
Effect of apatinib on reversing ABCB1- and ABCG2-mediated MDR in transfected cell lines.
| Compounds | IC50 ± SD (µM) (fold-reversal) | |||||
|---|---|---|---|---|---|---|
| HEK293/pcDNA3.1 | HEK293/ABCG2-R2 | HEK293/ABCB1 | ||||
| Mitoxantrone | 0.0569 ± 0.0035 | (1.00) | 1.3460 ± 0.3143 | (1.00) | 0.1381 ± 0.0274 | (1.00) |
| + 3 µM Apatinib | 0.0349 ± 0.0097 | (1.63) | 0.0655 ± 0.0199** | (20.6) | 0.0616 ± 0.0357** | (2.24) |
| + 3 µM FTC | 0.0528 ± 0.0093 | (1.08) | 0.0687 ± 0.0126** | (19.6) | - | - |
| + 3 µM PSC833 | 0.0543 ± 0.0069 | (1.04) | - | - | 0.0688 ± 0.0459** | (2.01) |
| Doxorubicin | 0.0724 ± 0.0054 | (1.00) | 1.1936 ± 0.1654 | (1.00) | 1.0821 ± 0.5424 | (1.00) |
| + 3 µM Apatinib | 0.0512 ± 0.0033 | (1.41) | 0.1412 ± 0.0033 ** | (8.45) | 0.3168 ± 0.0045** | (3.42) |
| + 10 µM Verapamil | 0.0957 ± 0.0142 | (0.77) | - | - | 0.0964 ± 0.0153** | (11.2) |
| + 3 µM FTC | 0.0528 ± 0.0093 | (1.08) | 0.1086 ± 0.0099** | (11.0) | - | |
| SN-38 | 0.0073 ± 0.0003 | (1.00) | 0.1530 ± 0.1636 | (1.00) | - | - |
| + 3 µM Apatinib | 0.0045 ± 0.0009 | (1.62) | 0.0079 ± 0.0021** | (19.4) | - | - |
| + 3 µM FTC | 0.0050 ± 0.0003 | (1.46) | 0.0085 ± 0.0016** | (18.0) | - | - |
| Vincristine | 0.0437 ± 0.0022 | (1.00) | - | - | 0.6405 ± 0.0349 | (1.00) |
| + 3 µM Apatinib | 0.0335 ± 0.0039 | (1.30) | - | - | 0.1792 ± 0.0485** | (3.57) |
| + 10 µM Verapamil | 0.0450 ± 0.0003 | (0.97) | - | - | 0.0514 ± 0.0025** | (12.5) |
| Cisplatin | 1.8240 ± 0.4728 | (1.00) | 1.6521 ± 0.3892 | (1.00) | 1.4899 ± 0.5321 | (1.00) |
| + 3 µM Apatinib | 1.5256 ± 0.3717 | (1.19) | 1.4193 ± 0.4820 | (1.16) | 1.6479 ± 0.2402 | (0.90) |
Cell survival was determined by MTT assays as described in “Materials and Methods”.Data are the mean ± standard deviation (SD) of at least three independent experiments performed in triplicate. The fold-reversal of MDR (values given in parenthesis in last column) was calculated by dividing the IC50 for cells with the anticancer drugs in the absence of apatinib by that obtained in the presence of apatinib.
represent P < 0.01 for values versus that obtained in the absence of apatinib.
Apatinib significantly increases the accumulation of DOX and Rho 123 in cells overexpressing ABCB1 and ABCG2
To ascertain the potential mechanism by which apatinib sensitizes the MDR cells to antineoplastic drugs, we examined the effect of apatinib on the accumulation of DOX and Rho 123 in cells overexpressing ABCB1 or ABCG2. In the absence of apatinib, the intracellular levels of DOX and Rho 123 were low in MDR cells, whereas apatinib significantly increased the intracellular accumulation of DOX and Rho 123 in a concentration-dependent manner (Fig. 1, Fig. S2, S3). The fluorescence index of DOX, in the presence of 0.75, 1.5 and 3 µM of apatinib, was increased by 1.21-, 1.72- and 2.19-fold, respectively, in KBv200 cells, 1.31-, 1.86- and 2.44-fold, respectively, in MCF-7/adr cells and 1.37-, 1.71-, and 2.11-fold, respectively, in S1-M1-80 cells (Fig. 1A). As shown in Fig. 1B, apatinib, at 0.75, 1.50 and 3 µM, increased the intracellular accumulation of Rho 123 by 1.91-, 3.43- and 5.17-fold, respectively, in KBv200 cells, 1.92-, 2.83- and 3.59-fold, respectively, in MCF-7/adr cells and 2.13-, 3.42-, and 4.16-fold, respectively, in S1-M1-80 cells. However, apatinib did not significantly alter the intracellular accumulation of DOX and Rho 123 in the parental sensitive KB, MCF-7 and S1 cells. It should be noted that S1-M1-80, but not the wild type ABCG2, overexpress the R482G variant, which can transport Rho 123 (36). Taken together, these results suggest that apatinib significantly inhibits ABCB1- and ABCG2-mediated transport in MDR cells.
Figure 1. Effect of apatinib on the intracellular accumulation of DOX and Rho 123.
The accumulation of DOX (A) and Rho 123 (B) was measured by flow cytometric analysis after cells were pre-incubated with or without apatinib, verapamil (VRP) or fumitremorgin C (FTC) for 3 h at 37°C and then incubated with 10 µM DOX or 5 µM Rho 123 for another 3 h or 0.5 h at 37°C, respectively, as described in “Materials and Methods”. The results are presented as fold change in fluorescence intensity relative to untreated control MDR cells. Columns, means of triplicate determinations; bars, SD. **, P < 0.01, versus the MDR control group. Independent experiments were performed at least three times, and a representative experiment is shown.
Inhibition kinetics of apatinib on intracellular DOX efflux by ABCB1 or ABCG2
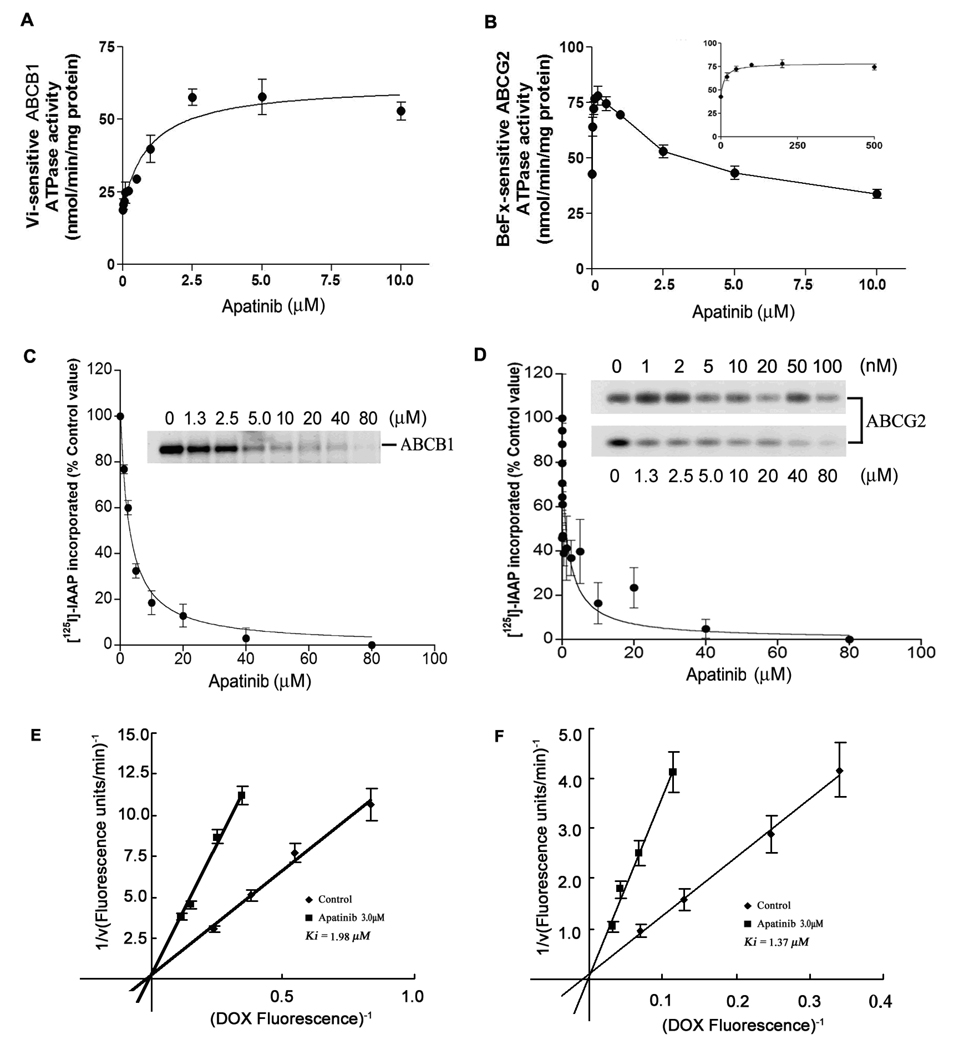
In order to obtain information about the mechanism of transport inhibition of ABCB1 and ABCG2 by apatinib, we determined the effect of apatinib on the kinetics of the intracellular DOX efflux by ABCB1 or ABCG2 transporter using KBv200 and S1-M1-80 cells, respectively. The inhibitory effect of apatinib was analyzed using Lineweaver–Burk plots in the presence or absence of apatinib. Subsequent analysis indicated that apatinib was a competitive inhibitor of DOX efflux (Fig. 2E and F). The Ki values of apatinib for DOX transport by ABCB1 and ABCG2 were 1.98 ± 0.21 and 1.37 ± 0.17 µM, respectively.
Figure 2. Effect of apatinib on the ATPase activity ABCB1 and ABCG2, the photoaffinity labeling of ABCB1 and ABCG2 with [125I]-Iodoarylazidoprazosin ([125I]-IAAP) and the transport kinetics of DOX mediated by the ABCB1 and ABCG2 transporter.
(A) Vi-sensitive ATPase activity of ABCB1. (B) beryllium fluoride-sensitive ATPase activity of ABCG2. (B, inset) Effect of lower concentrations of apatinib on ABCG2 ATPase activity. The amount of inorganic phosphate released was quantitated using a colorimetric method. The photoaffinity labeling of ABCB1 (C) and ABCG2 (D) with [125I]IAAP was performed using indicated concentrations of apatinib as described in Materials and Methods. The amount of inorganic phosphate released was quantitated using a colorimetric method. Crude membranes from High Five insect cells expressing ABCB1 (C) and from MCF7/FLV1000 cells expressing ABCG2 (D) were incubated with various concentrations of apatinib for 10 min at room temperature, and 3 to 6 nM [125I]-IAAP (2,200 Ci/mmol) were then added before illuminated with a UV lamp (365 nm) as described in Materials and Methods. In all three panels, lane 1 is control without apatinib. Effect of apatinib on the transport kinetics of intracellular DOX efflux mediated by the ABCB1 (E) and ABCG2 (F) transporter. The quantity of efflux DOX in MDR cells was measured for 5 min at 37°C at various DOX concentrations (2.5–20 µM) in the presence or absence of apatinib by flow cytometry. Data represent mean ± SD in at least three different experiments. The bars represent the SD value. The Ki values were determined from the double reciprocal Lineweaver-Burk plots in the absence (◆) or presence of 3 µM apatinib (▲).
Apatinib stimulates the ATPase activity of ABCB1 and ABCG2
To assess the effect of apatinib on the ATPase activity of ABCB1 and ABCG2, we evaluated the effect of apatinib on both ABCB1 and ABCG2 ATPase activities. Apatinib produced a 3-fold stimulation of ABCB1 ATPase activity in a concentration-dependent manner, and the concentration required for 50% stimulation was ≈ 950 nM (Fig. 2A). In contrast, apatinib had a biphasic effect on ABCG2 ATP hydrolysis as it stimulated the ATPase activity of ABCG2 at lower concentrations but produced inhibition at higher concentrations (Fig. 2B). The ATPase data suggest that apatinib has a higher affinity for ABCG2 compared to ABCB1 and that it is likely a substrate for both ABCG2 and ABCB1.
Apatinib inhibits the photo-affinity labeling of ABCB1 and ABCG2 with [125I]-IAAP
[125I]-IAAP photolabel of both ABCB1 and ABCG2, and its binding can be competitively inhibited by substrates or inhibitors of the respective transporters (35). Therefore, to further investigate the interaction of apatinib with the substrate binding sites of ABCB1 and ABCG2, the membrane vesicles of these transporters were incubated with [125I]-IAAP in the absence or presence of apatinib. Apatinib produced a concentration-dependent inhibition of [125I]-IAAP photo-affinity labeling of both ABCB1 (Fig. 2C) and ABCG2 (Fig. 2D), with IC50 values of 2.9 ± 0.4 µM and 11 ± 4 nM, respectively. These results suggest that apatinib binds with higher affinity to ABCG2 substrate-binding site(s) than to ABCB1 substrate-binding site(s).
Apatinib does not significantly alter the mRNA or protein levels of ABCB1 and ABCG2
The reversal of ABCB1- and ABCG2-mediated MDR can be achieved by either inhibiting their function or by lowering their expression. Therefore, we determined the effect of apatinib on the expression of the protein levels and mRNA content of ABCB1 and ABCG2. Apatinib (Fig. 3), at 0.75, 1.5 or 3 µM, did not significantly alter the expression of protein or mRNA for ABCB1 or ABCG2 transporters in KBv200, MCF-7/adr or S1-M1-80 cells. In addition, quantitative real-time PCR (QRT-PCR) results indicated that there was no significant difference in the expression of mRNA in the MDR cells (data not shown). These data suggest that the reversal of MDR was most likely obtained by direct inhibition of the efflux function of ABCB1 and ABCG2 as opposed to the downregulation of their mRNA or protein levels.
Figure 3. Effect of apatinib on the expression of ABCB1 and ABCG2 in MDR cells at the protein (A) and mRNA (B) levels.
KBv200, MCF-7/adr and S1-M1-80 cells were treated with apatinib at the indicated concentrations for 48 h. A representative result from at least three independent experiments is shown.
Apatinib does not block the phosphorylation of Akt and ERK1/2 at MDR reversal concentration
Previous studies have shown that the inhibition of the Akt and ERK1/2 pathways may decrease the resistance to antineoplastic drugs in cancer cells (37, 38). Consequently, we determined the effect of apatinib on the levels of total and phosphorylated forms of Akt and ERK1/2 in all cell lines. As shown in Fig. 4, the incubation of cell with apatinib (0.75 – 3 µM) for 48 h did not significantly later the total and phosphorylated forms of Akt and ERK1/2. This suggests that the MDR reversal effect of apatinib in KBv200, MCF-7/adr and S1-M1-80 cells is independent of the inhibition of Akt and ERK1/2 phosphorylation.
Figure 4. Effect of apatinib on the phosphorylation of AKT and ERK1/2.
Equal amount of protein was loaded for Western blot analysis as described in “Materials and Methods”. Independent experiments were performed at least three times and result from a representative experiment is shown. 1, untreated control; 2, 0.75 µM apatinib; 3, 1.5 µM apatinib; 4, 3.0 µM apatinib; 5, 10 µM apatinib; and 6, 15 µM apatinib.
Apatinib reverses ABCB1-mediated MDR in nude mice xenograft model
An established KBv200 cell xenografts model in nude mice was used to evaluate the efficacy of apatinib to reverse the resistance to paclitaxel in vivo. There was no significant difference in tumor size between animals treated with saline, apatinib, or paclitaxel, indicating the in vivo resistance to paclitaxel. However, the combination of apatinib and paclitaxel produced a significant inhibition of tumor growth compared with animals treated with saline, paclitaxel, or apatinib alone (P<0.05; Fig. 5). The ratio of tumor growth inhibition by the combination was 52.7%. Furthermore, at the doses tested, no mortality or apparent decrease in body weight was observed in the combination treatment groups, suggesting that the combination regimen did not increase the incidence of toxic side effects.
Figure 5. Potentiation of antitumor effects of paclitaxel by apatinib in a KBv200 xenograft model in athymic nude mice.
(A) Changes in tumor volume with time after tumor cell inoculation. Data points represent mean tumor volume for each group of 12 mice after implantation; bars, SD. (B) Tumor size. The picture was taken on the 17th day after implantation. The various treatments were as follows: control (vehicle alone), apatinib (70 mg/kg,p.o., q3d × 4); paclitaxel (18 mg/kg, i.p., q3d × 4) and paclitaxel (18 mg/kg, i.p., q3d × 4) plus apatinib (70 mg/kg, p.o., q3d × 4, given 1 h before paclitaxel administration).
Discussion
Molecular targeted therapy for various types of cancer has become an active field of basic science and clinical research ever since imatinib (Gleevec, STI-571) received an approval from FDA in 2001 as a first-line drug to treat chronic myeloid leukemia (CML). Cytokine receptor signal transduction pathways are pivotal mediators of cancer oncogenesis, proliferation, invasion, metastasis and angiogenesis. Particularly, the EGFR and VEGFR-2 pathways are vital in cancer cells and cancer-associated endothelial cells and hence are one of the most extensively studied pathways (39, 40). In recent few years, many compounds have been developed to block these two pathways including receptor tyrosine kinase inhibitors and monoclonal antibodies targeting EGFR, VEGFR and VEGF Trap (41).
Interestingly and of clinical significance, several TKIs were found to interact with the major MDR transporters such as ABCB1, ABCC1 and ABCG2. Initially, Imatinib (STI-571) was found to be an ABCB1 substrate, and EKI-785 was shown to interact with ABCC1 (14). More recently, CI1033 was reported to be substrates and inhibitors of ABCG2 (42). Other TKIs such as gefitinib (17, 43), erlotinib (18), vandetanib (20, 30) and lapatinib (16) have also been shown to inhibit ABCB1 and ABCG2 function. Apatinib is promising multi-TKs inhibitor drug and is in phase III clinical development. However, little is known to date about the interaction between apatinib and ABC transporters.
In the present study, we demonstrated that apatinib significantly potentiated the cytotoxicity of established ABCB1 and ABCG2 substrates and increased the accumulation of DOX and Rho 123 in ABCB1- or ABCG2-overexpressing cells. However, apatinib at 3.0 µM did not significantly sensitize the parental sensitive KB, MCF-7, S1 or HEK293/pcDNA3.1 cells to the anticancer agents used in this study (Table 1, Table 2). These findings suggest that the sensitization of the resistant cells by apatinib is specific to overexpression of ABCB1 or ABCG2. Furthermore, apatinib significantly enhanced the intracellular accumulation of DOX and Rho 123 in MDR cells. The results of the fluorescent drug accumulation studies were consistent with our cytotoxic results, suggesting that apatinib sensitizes the ABCB1- and ABCG2-mediated MDR cells to anticancer drugs. The downregulation of ABCB1 and ABCG2 expression on the treatment with apatinib could have potentiated the reversal effect of apatinib on ABCB1- and ABCG2-mediated MDR. However, protein expression of ABCB1 or ABCG2 in the corresponding resistant cells was not affected by a 48-h treatment with 0.75, 1.5 or 3.0 µM of apatinib (Fig. 3). We thus proposed that the MDR reversal effect of apatinib is due to the inhibition of efflux function of the ABC transporters as revealed in the drug accumulation assay (Fig. 1). To examine whether apatinib can also reverse MDR in vivo, we investigated the effect of apatinib on the anticancer activity of paclitaxel in nude mice xenograft model. We found that the combination of paclitaxel with apatinib remarkably enhanced the anticancer activity of paclitaxel in our ABCB1-overexpressing xenograft model (Fig. 5). Meanwhile, there was no substantial increase in body weight loss in mice treated with the drug combination compared with the individual drug treatment alone.
ABC transporters move substrates out of cells using ATP as the energy source. Therefore the rate of ATP hydrolysis (ATPase activity) is directly proportional to the transport activity of the transporters (44, 45). We have previously reported that some TKIs such as lapatinib, sunitinib and erlotinib, even at low concentrations can stimulate the ATPase activities of the transporters (16, 18, 46). In fact, apatinib stimulated both vanadate-sensitive ABCB1 and BeFx-sensitive ABCG2 ATPase at lower concentrations as seen previously with aforementioned TKIs (Fig. 2A–B), whereas inhibited BeFx-sensitive ABCG2 ATPase at higher concentrations (Fig. 2B). These results suggest that apatinib is likely to be a substrate of both ABCB1 and ABCG2. In addition, we speculate based on these findings that apatinib has a direct interaction with these transporters. Further experiments showed that apatinib inhibited the photolabeling of ABCB1 and ABCG2 with [125I]-IAAP, definitively illustrating the direct interaction between apatinib with these transporters. Taken together, these data suggest that apatinib reverses MDR by directly inhibiting the function of ABC drug transporters.
Receptor tyrosine kinases (RTKs) such as VEGFR, PDGFR and FLT3 play crucial role in modulating cell proliferation, differentiation and survival by activating downstream signal molecules such as signal transducers and activators of transcription (STAT), protein kinase B/AKT and extracellular signal-regulated kinase 1/2 (ERK1/2) (47, 48). Aberrant activation of different RTKs is believed to be associated with cancer growth, angiogenesis and metastasis. Moreover, it has been reported that activation of the PI3K/AKT and/or ERK pathways is related to resistance to conventional anticancer drugs (49, 50). To rule out the involvement of the AKT and ERK1/2 signaling pathways in the MDR reversal of apatinib, activation of AKT and ERK1/2 were examined. Our data shown that apatinib (up to 3.0 µM) did not block the phosphorylation of AKT and ERK1/2 in the all tested cell lines (Fig. 4). Therefore, the blockade of AKT and ERK1/2 activation is not involved in the reversal of ABCB1 or ABCG2-mediated MDR by apatinib.
In conclusion, apatinib reverses ABCB1- and ABCG2-mediated MDR by directly inhibiting ABCB1 and ABCG2 function, resulting in elevated intracellular concentrations of substrate chemotherapeutic drugs. Also, the reversal of MDR is not associated with the blockade of tyrosine kinases. Confirmation of MDR reversal by apatinib in tumor xenograft model further supports the potential usefulness of combining apatinib with other conventional anticancer drugs in overcoming clinical resistance in cancer chemotherapy.
Acknowledgements
We like to thank Drs S.E. Bates and R.W. Robey (National Cancer Institute, NIH) for the ABCG2 expressing cell lines, ABCB1-, ABCG2 and ABCC1 transfectant cell lines and FTC (NIH, MD.). We thank Shin-ichi Akiyama (Kagoshima University, Japan) for KB-3-1 and KB/ABCC1 cell lines. We thank Dr. Charles R. Ashby Jr. (St. John’s University) for the critical reading for the manuscript. This work was funded by grants from China National Natural Sciences Foundation No.30672407 (L-W. Fu) and No.30600769 (L.-W. Fu), and Key Subject Project Foundation of State Key Laboratory of Oncology in Southern China, St. John’s University Seed Grant No.582-2082-7601 (Z-S. Chen), and Drs. C-P Wu and SV Ambudkar were supported by the Intramural Research Program of the National Cancer Institute, NIH, Center for Cancer Research.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Ozben T. Mechanisms and strategies to overcome multiple drug resistance in cancer. FEBS Lett. 2006;580:2903–2909. doi: 10.1016/j.febslet.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Tomas R. Multidrug resistance: retrospect and prospects in anti-cancer drug treatment. Curr Med Chem. 2006;13:1859–1876. doi: 10.2174/092986706777585077. [DOI] [PubMed] [Google Scholar]
- 3.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 4.Litman T, Druley TE, Stein WD, Bates SE. From MDR to MXR: new understanding of multidrug resistance systems, their properties and clinical significance. Cell Mol Life Sci. 2001;58:931–959. doi: 10.1007/PL00000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi CH. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005;5:30. doi: 10.1186/1475-2867-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 7.Miyake K, Mickley L, Litman T, et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 8.Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58:5337–5339. [PubMed] [Google Scholar]
- 9.Doyle LA, Yang W, Abruzzo LV, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doyle LA, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2) Oncogene. 2003;22:7340–7358. doi: 10.1038/sj.onc.1206938. [DOI] [PubMed] [Google Scholar]
- 11.Haraguchi N, Utsunomiya T, Inoue H, et al. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506–513. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 12.Zhou S, Morris JJ, Barnes Y, Lan L, Schuetz JD, Sorrentino BP. Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 2002;99:12339–12344. doi: 10.1073/pnas.192276999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemos C, Jansen G, Peters GJ. Drug transporters: recent advances concerning BCRP and tyrosine kinase inhibitors. Br J Cancer. 2008;98:857–862. doi: 10.1038/sj.bjc.6604213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hegedus T, Orfi L, Seprodi A, Varadi A, Sarkadi B, Keri G. Interaction of tyrosine kinase inhibitors with the human multidrug transporter proteins, MDR1 and MRP1. Biochim Biophys Acta. 2002;1587:318–325. doi: 10.1016/s0925-4439(02)00095-9. [DOI] [PubMed] [Google Scholar]
- 15.Tiwari AK, Sodani K, Wang SR, et al. Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the activity of the ABCB1/Pgp and ABCG2/BCRP/MXR transporters. Biochem Pharmacol. 2009;78:153–161. doi: 10.1016/j.bcp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Dai CL, Tiwari AK, Wu CP, et al. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008;68:7905–7914. doi: 10.1158/0008-5472.CAN-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitazaki T, Oka M, Nakamura Y, et al. Gefitinib, an EGFR tyrosine kinase inhibitor, directly inhibits the function of P-glycoprotein in multidrug resistant cancer cells. Lung Cancer. 2005;49:337–343. doi: 10.1016/j.lungcan.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 18.Shi Z, Peng XX, Kim IW, et al. Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance. Cancer Res. 2007;67:11012–11020. doi: 10.1158/0008-5472.CAN-07-2686. [DOI] [PubMed] [Google Scholar]
- 19.Tao LY, Liang YJ, Wang F, et al. Cediranib (recentin, AZD2171) reverses ABCB1- and ABCC1-mediated multidrug resistance by inhibition of their transport function. Cancer Chemother Pharmacol. 2009;64:961–969. doi: 10.1007/s00280-009-0949-1. [DOI] [PubMed] [Google Scholar]
- 20.Zheng LS, Wang F, Li YH, et al. Vandetanib (Zactima, ZD6474) antagonizes ABCC1- and ABCG2-mediated multidrug resistance by inhibition of their transport function. PLoS One. 2009;4:e5172. doi: 10.1371/journal.pone.0005172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai CL, Liang YJ, Wang YS, et al. Sensitization of ABCG2-overexpressing cells to conventional chemotherapeutic agent by sunitinib was associated with inhibiting the function of ABCG2. Cancer Lett. 2009;279:74–83. doi: 10.1016/j.canlet.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 22.Fu L, Liang Y, Deng L, et al. Characterization of tetrandrine, a potent inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Chemother Pharmacol. 2004;53:349–356. doi: 10.1007/s00280-003-0742-5. [DOI] [PubMed] [Google Scholar]
- 23.Robey RW, Medina-Perez WY, Nishiyama K, et al. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin Cancer Res. 2001;7:145–152. [PubMed] [Google Scholar]
- 24.Zhang JY, Wu HY, Xia XK, et al. Anthracenedione derivative 1403P-3 induces apoptosis in KB and KBv200 cells via reactive oxygen species-independent mitochondrial pathway and death receptor pathway. Cancer Biol Ther. 2007;6:1413–1421. doi: 10.4161/cbt.6.9.4543. [DOI] [PubMed] [Google Scholar]
- 25.Robey RW, Honjo Y, Morisaki K, et al. Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer. 2003;89:1971–1978. doi: 10.1038/sj.bjc.6601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen LM, Wu XP, Ruan JW, et al. Screening novel, potent multidrug-resistant modulators from imidazole derivatives. Oncol Res. 2004;14:355–362. doi: 10.3727/0965040041292378. [DOI] [PubMed] [Google Scholar]
- 27.Shi Z, Liang YJ, Chen ZS, et al. Reversal of MDR1/P-glycoprotein-mediated multidrug resistance by vector-based RNA interference in vitro and in vivo. Cancer Biol Ther. 2006;5:39–47. doi: 10.4161/cbt.5.1.2236. [DOI] [PubMed] [Google Scholar]
- 28.Chen LM, Liang YJ, Ruan JW, et al. Reversal of P-gp mediated multidrug resistance in-vitro and in-vivo by FG020318. J Pharm Pharmacol. 2004;56:1061–1066. doi: 10.1211/0022357043879. [DOI] [PubMed] [Google Scholar]
- 29.Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000;60:47–50. [PubMed] [Google Scholar]
- 30.Mi Y, Lou L. ZD6474 reverses multidrug resistance by directly inhibiting the function of P-glycoprotein. Br J Cancer. 2007;97:934–940. doi: 10.1038/sj.bjc.6603985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dive C, Workman P, Watson JV. Improved methodology for intracellular enzyme reaction and inhibition kinetics by flow cytometry. Cytometry. 1987;8:552–561. doi: 10.1002/cyto.990080604. [DOI] [PubMed] [Google Scholar]
- 32.Tseng SJ, Hsu JP. A comparison of the parameter estimating procedures for the Michaelis-Menten model. J Theor Biol. 1990;145:457–464. doi: 10.1016/s0022-5193(05)80481-3. [DOI] [PubMed] [Google Scholar]
- 33.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 34.Ambudkar SV. Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells. Methods Enzymol. 1998;292:504–514. doi: 10.1016/s0076-6879(98)92039-0. [DOI] [PubMed] [Google Scholar]
- 35.Shukla S, Robey RW, Bates SE, Ambudkar SV. The calcium channel blockers, 1,4-dihydropyridines, are substrates of the multidrug resistance-linked ABC drug transporter, ABCG2. Biochemistry. 2006;45:8940–8951. doi: 10.1021/bi060552f. [DOI] [PubMed] [Google Scholar]
- 36.Ozvegy-Laczka C, Koblos G, Sarkadi B, Varadi A. Single amino acid (482) variants of the ABCG2 multidrug transporter: major differences in transport capacity and substrate recognition. Biochim Biophys Acta. 2005;1668:53–63. doi: 10.1016/j.bbamem.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Gagnon V, Van Themsche C, Turner S, Leblanc V, Asselin E. Akt and XIAP regulate the sensitivity of human uterine cancer cells to cisplatin, doxorubicin and taxol. Apoptosis. 2008;13:259–271. doi: 10.1007/s10495-007-0165-6. [DOI] [PubMed] [Google Scholar]
- 38.Oh SY, Song JH, Gil JE, Kim JH, Yeom YI, Moon EY. ERK activation by thymosin-beta-4 (TB4) overexpression induces paclitaxel-resistance. Exp Cell Res. 2006;312:1651–1657. doi: 10.1016/j.yexcr.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 39.Burgess AW. EGFR family: structure physiology signalling and therapeutic targets. Growth Factors. 2008;26:263–274. doi: 10.1080/08977190802312844. [DOI] [PubMed] [Google Scholar]
- 40.Shibuya M. Vascular endothelial growth factor (VEGF)-Receptor2: its biological functions, major signaling pathway, and specific ligand VEGF-E. Endothelium. 2006;13:63–69. doi: 10.1080/10623320600697955. [DOI] [PubMed] [Google Scholar]
- 41.Heymach JV, Nilsson M, Blumenschein G, Papadimitrakopoulou V, Herbst R. Epidermal growth factor receptor inhibitors in development for the treatment of non-small cell lung cancer. Clin Cancer Res. 2006;12:4441s–4445s. doi: 10.1158/1078-0432.CCR-06-0286. [DOI] [PubMed] [Google Scholar]
- 42.Erlichman C, Boerner SA, Hallgren CG, et al. The HER tyrosine kinase inhibitor CI1033 enhances cytotoxicity of 7-ethyl-10-hydroxycamptothecin and topotecan by inhibiting breast cancer resistance protein-mediated drug efflux. Cancer Res. 2001;61:739–748. [PubMed] [Google Scholar]
- 43.Elkind NB, Szentpetery Z, Apati A, et al. Multidrug transporter ABCG2 prevents tumor cell death induced by the epidermal growth factor receptor inhibitor Iressa (ZD1839, Gefitinib) Cancer Res. 2005;65:1770–1777. doi: 10.1158/0008-5472.CAN-04-3303. [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg MF, Velarde G, Ford RC, et al. Repacking of the transmembrane domains of P-glycoprotein during the transport ATPase cycle. EMBO J. 2001;20:5615–5625. doi: 10.1093/emboj/20.20.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annual review of pharmacology and toxicology. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 46.Shukla S, Robey RW, Bates SE, Ambudkar SV. Sunitinib (Sutent, SU11248), a small-molecule receptor tyrosine kinase inhibitor, blocks function of the ATP-binding cassette (ABC) transporters P-glycoprotein (ABCB1) and ABCG2. Drug Metab Dispos. 2009;37:359–365. doi: 10.1124/dmd.108.024612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roskoski R., Jr Structure and regulation of Kit protein-tyrosine kinase--the stem cell factor receptor. Biochem Biophys Res Commun. 2005;338:1307–1315. doi: 10.1016/j.bbrc.2005.09.150. [DOI] [PubMed] [Google Scholar]
- 48.Kessler T, Fehrmann F, Bieker R, Berdel WE, Mesters RM. Vascular endothelial growth factor and its receptor as drug targets in hematological malignancies. Curr Drug Targets. 2007;8:257–268. doi: 10.2174/138945007779940089. [DOI] [PubMed] [Google Scholar]
- 49.West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat. 2002;5:234–248. doi: 10.1016/s1368-7646(02)00120-6. [DOI] [PubMed] [Google Scholar]
- 50.Knuefermann C, Lu Y, Liu B, et al. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene. 2003;22:3205–3212. doi: 10.1038/sj.onc.1206394. [DOI] [PubMed] [Google Scholar]