Abstract
Purpose
The causes of male infertility are heterogeneous but more than 50% of cases have a genetic basis. Specific genetic defects have been identified in less than 20% of infertile males and, thus, most causes remain to be elucidated. The most common cytogenetic defects associated with nonobstructive azoospermia are numerical and structural chromosome abnormalities, including Klinefelter syndrome (47,XXY) and Y chromosome microdeletions. To refine the incidence and nature of chromosomal aberrations in males with infertility we reviewed cytogenetic results in 668 infertile men with oligozoospermia and azoospermia.
Materials and Methods
High resolution Giemsa banding chromosome analysis and/or fluorescence in situ hybridization were done in 668 infertile males referred for routine cytogenetic analysis between January 2004 and March 2009.
Results
The overall incidence of chromosomal abnormalities was about 8.2%. Of the 55 patients with abnormal cytogenetic findings sex chromosome aneuploidies were observed in 29 (53%), including Klinefelter syndrome in 27 (49%). Structural chromosome abnormalities involving autosomes (29%) and sex chromosomes (18%) were detected in 26 infertile men. Abnormal cytogenetic findings were observed in 35 of 264 patients (13.3%) with azoospermia and 19 of 365 (5.2%) with oligozoospermia.
Conclusions
Structural chromosomal defects and low level sex chromosome mosaicism are common in oligozoospermia cases. Extensive cytogenetic assessment and fluorescence in situ hybridization may improve the detection rate in males with oligozoospermia. These findings highlight the need for efficient genetic testing in infertile men so that couples may make informed decisions on assisted reproductive technologies to achieve parenthood.
Keywords: infertility, male, aneuploidy, azoospermia, oligospermia, sex chromosome aberrations
Infertility is the inability of a couple to conceive in 1 year of regular unprotected intercourse. Infertility is a major health problem of multifactorial etiology that involves males and females, and affects almost 6 million couples in the United States.1–3 According to the American Urological Association and American Society for Reproductive Medicine in almost 50% of infertile couples a male factor is a primary or contributory cause of infertility.4 Male infertility factor is typically defined as abnormal semen analysis, although an infertility diagnosis may be made in patients with normal semen parameters.5 Abnormal semen parameters are not definitive indicators of male infertility but they correlate with lower probability of achieving pregnancy. The 2 most common semen abnormalities are OS and AS.4,6
Spermatogenesis is one of the most complex cell differentiation processes known, involving about 2,300 genes in the regulation of testicular development, germ cell development and maturation.7 Investigators estimate that almost 50% of patients with idiopathic male infertility have a genetic contribution but most of these genetic factors remain to be elucidated. To date specific genetic defects, including chromosomal aberrations and gene defects, ie sex chromosome aneuploidy and cystic fibrosis mutation, have been identified in fewer than 20% of male patients with infertility.8 Recent reports of genetic defects associated with abnormal semen parameters, such as SYCP3, PRM1, KLHL10, SPATA16 and AURKC, lack epidemiological data.9–14 Thus, future studies may collectively increase the incidence of known genetic defects in infertile men.
Almost 6% to 8% of nonobstructive AS cases and a smaller percent of severe OS cases are associated with microdeletions on the AZF regions of the Y chromosome according to Online Mendelian Inheritance in Men No. 415000 of the National Center for Biotechnology Information.15,16 Of male infertility cases aneuploidy of sex chromosomes such as 47,XXY, ie Klinefelter syndrome, accounts for up to 3% and structural rearrangements involving X and/or Y chromosomes account for an estimated 1% to 3%.8,17 Less commonly OS and AS are associated with numerical or structural autosomal abnormalities.6,18 Some patients with abnormal semen parameters have balanced Robertsonian translocations.19 Balanced reciprocal autosomal translocations, inversions and duplications are less often associated with impaired fertility.3 However, only a few male infertility associated genes have been elucidated from these cytogenetic reports. We present the results of cytogenetic investigation in 668 infertile males to define the nature of chromosomal abnormalities and determine the importance of these findings to advance our understanding of the causes of severe male factor infertility.
Materials and Methods
Patients
In a 5-year period 5,325 patients were evaluated for male factor infertility at the division of male reproductive medicine and surgery at our institution. Blood samples from 668 patients 21 to 50 years old were submitted for routine cytogenetic analysis. As part of the evaluation each patient provided a history and obstetrical history of the wife, and underwent physical examination. Two semen analyses were obtained per patient, and centrifugation and pellet analysis were done as indicated. Serum follicle-stimulating hormone and testosterone were measured as well as other serum hormones as indicated. Patient whole blood was examined for Y chromosome microdeletions and karyotype abnormalities when sperm density was less than 5 × 106/ml or karyotype abnormality was suspected. Based on semen parameters cases were classified into 5 categories, including 1—AS for no sperm on semen analysis or centrifugation and pellet analysis, 2—sOS for sperm density less than 0.5 × 106/ml, 3—OS for sperm density between 0.5 × 106/ml and 5 × 106/ml, 4—mOS for sperm density between 5 × 106/ml and 20 × 106/ml, and 5—NS for sperm density greater than 20 × 106/ml. The Baylor College of Medicine institutional review board for human subject research approved this study.
Cytogenetic Analysis and FISH
Cytogenetic studies were done as part of routine evaluation in males with severe male factor infertility according to previous guidelines and best practice statements.4,5 Cases of sperm density less than 5 × 106/ml and those suspicious for a genetic cause of infertility were routinely screened for Y chromosome microdeletions and karyotype abnormalities. Metaphase chromosome preparations for Giemsa banding and FISH were obtained from phytohemagglutinin stimulated lymphocyte cultures of peripheral blood. Cytogenetic chromosome analysis was done at 550 to 700 band resolution according to standard techniques. FISH was done on metaphase chromosomes and/or interphase nuclei using commercially available centromere, subtelomere or SRY gene specific probes (Abbott/Vysis, Downers Grove, Illinois) according to the manufacturer. FISH with locus specific probes was done with bacterial artificial chromosome clones from the RPCI-11 human library. DNA from bacterial artificial chromosomes clones was directly labeled with SpectrumOrange™ deoxyuridine triphosphate or SpectrumGreen™ deoxyuridine triphosphate using a commercially available kit, as previously described.20 At least 100 cells per case of suspected mosaicism were examined by Giemsa banding or FISH. Analysis of 100 cells ruled out 3% mosaicism at the 95% confidence level.
Results
Increased Chromosomal Abnormality Rate in Infertile Men
A total of 5,325 infertile males were classified into 5 major categories based on semen parameter results (table 1). In the screened cohort AS, sOS, OS, mOS and NS were identified in 678 (12.7%), 246 (4.6%), 492 (9.2%), 1,280 (24%) and 2,629 patients (49.4%), respectively. Conventional cytogenetic analysis was done in 668 infertile males, and various numerical and structural chromosome abnormalities were identified in 55 (8.2%) (table 1). Of 55 patients with an abnormal karyotype 29 (53%) had sex chromosome aneuploidies (table 1 and fig. 1, A). The most common aneuploidy was 47,XXY (Klinefelter syndrome), accounting for 27 of the 55 cytogenetic defects (49%). In this group 7 patients had 5% to 80% mosaicism for a cell line, ie 47,XXY/46,XY (table 1 and fig. 1, B). A less common finding was Y chromosome aneuploidy. Two patients were identified with Y chromosome numerical aberrations only, including 1 with mosaicism for a 45,X (Turner syndrome) cell line (ie 45,X/46,XY) and 1 with disomy for chromosome Y, ie 47,XYY (table 1).
Table 1. Cytogenetic findings in infertile males.
| Total No. | No. Azoospermia | No. Severe Oligozoospermia | No. Oligozoospermia | No. Mild Oligozoospermia | No. Normozoospermia | |
|---|---|---|---|---|---|---|
| Pts: | ||||||
| Evaluated in clinic (%) | 5,325 | 678 (12.7) | 246 (4.6) | 492 (9.2) | 1,280 (24) | 2,629 (49.4) |
| Studied by cytogenetic analysis (%) | 668 (13) | 264 (39) | 91 (37) | 192 (39) | 82 (6.4) | 39 (1.5) |
| Abnormal cytogenetic analysis (%) | 55 (8.2) | 35 (13.3) | 10 (10.9) | 8 (4.2) | 1 (1.2) | 1 (2.6) |
| Numerical sex chromosome abnormalities: | 29 | 24 | 1 | 3 | 1 | 0 |
| Klinefelter syndrome 47,XXY | 20 | 19 | 1 | — | — | — |
| Mosaic 47,XXY/46,XY | 7 | 5 | — | 1 | 1 | — |
| Mosaic 45,X/46,XY | 1 | — | — | 1 | — | — |
| 47,XYY | 1 | — | — | 1 | — | — |
| Structural aberrations involving sex chromosomes: | 10 | 8 | 1 | 0 | 0 | 1 |
| Yq deletions | 5 | 5 | — | — | — | — |
| X;Y translocations | 1 | 1 | — | — | — | — |
| X chromosome rearrangements | 2 | — | 1 | — | — | 1 |
| X;autosome translocations | 1 | 1 | — | — | — | — |
| Y;autosome translocations | 1 | 1 | — | — | — | — |
| Structural autosome abnormalities | 16 | 3 | 8 | 5 | 0 | 0 |
Total number of cytogenetic aberrations in each category is shown as underlined number.
Figure 1.
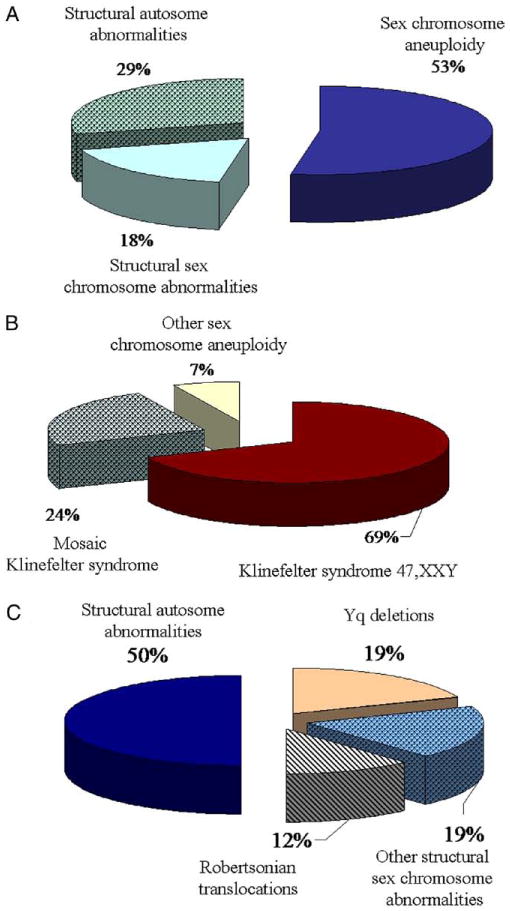
Different rearrangements in male infertile cohort. A, proportion of each category detected in 55 men with chromosomal defects. B, X and Y chromosome aneuploidy in 29 men with numerical chromosome defects. C, sex and autosomal chromosome aberrations in 26 men with structural rearrangements.
Structural chromosome rearrangements were identified in 26 patients (47%) (table 2 and fig. 1, C), of whom 10 had structural sex chromosome abnormalities. Patients 1 to 4 had X chromosome aberrations (table 2). Patient 5 to 10 had Y chromosome rearrangements resulting in nullisomy for the distal part of the Yq region, including AZF genes associated with AS (table 2 and fig. 2, B). AS was observed in 2 patients with X chromosome abnormalities and in all with Y chromosome rearrangements. Male patient 3 with AS had the female chromosome constitution 46,XX (table 2). Further FISH analysis with the Yp11.2 locus specific probe that detects the male sex determination SRY gene showed positive hybridization on the long arm of 1 chromosome X (fig. 2, C). Thus, this XX male patient had a derivative X chromosome due to cryptic translocation between the X and Y chromosomes, and nullisomy for the Yq chromosome material, including AZF genes.
Table 2. Identified structural chromosome abnormalities in infertile males.
| Pt No. | Results | Semen Analysis |
|---|---|---|
| 1 | 46,inv(X)(p22.3q21.2),Y | sOS |
| 2 | 46,Xqs,Y* | NS |
| 3 | 46,XX.ish der(X)t(X;Y)(q28;p11.3) | AS |
| 4 | 46,Y,t(X;17)(q27.3;q21.1) | AS |
| 5 | 45,X[16]/46,X,i(Y)(p10)[14] | AS |
| 6 | 46,X,del(Y)(q11.2) | AS |
| 7 | 46,X,i(Y)(p10)[18]/45,X[2] | AS |
| 8 | 46,X,idic(Y)(q11.2) | AS |
| 9 | 46,X,t(Y;6)(q12;p12.3) | AS |
| 10 | 46,X,Yqs | AS |
| 11 | 46,XY,inv(1)(p36.3q24) | sOS |
| 12 | 46,XY,inv(1)(q23q42.1) | OS |
| 13 | 46,XY,t(1;20)(p32.1;p13) | sOS |
| 14 | 46,XY,inv(2)(p11.2q13) | sOS |
| 15 | 46,XY,t(2;13)(p13;p12) | sOS |
| 16 | 46,XY,t(3;8)(q25.1;q24.11) | OS |
| 17 | 46,XY,inv(4)(p15.32q21.3) | sOS |
| 18 | 46,XY,t(6;10)(p21.3;q26.1) | OS |
| 19 | 46,XY,inv(7)(p13q32) | AS |
| 20 | 46,XY,t(8;15)(q13.3;p13) | AS |
| 21 | 46,XY,inv(9)(pterq21.2) | sOS |
| 22 | 45,XY,der(13;14)(q10;q10) | sOS |
| 23 | 45,XY,der(13;14)(q10;q10) | OS |
| 24 | 45,XY,der(14;21)(q10;q10) | OS |
| 25 | 46,XY,t(14;16;20)(q22;p13;q13) | AS |
| 26 | 45,XY,der(12)t(12;22)(p13.3;q11.1),–22[77]/ | |
| 26 | 45,XY,der(2)t(2;22)(q37.3;q11.1),–22[13]/ | sOS |
| 26 | 45,XY,der(20)t(20;22) (q13.3;q11.1),–22[7]/ | |
| 26 | 45,XY,der(10)t(10;22)(q26.3;q11.1),–22[6] |
Satellite positive by C-banding.
Figure 2.
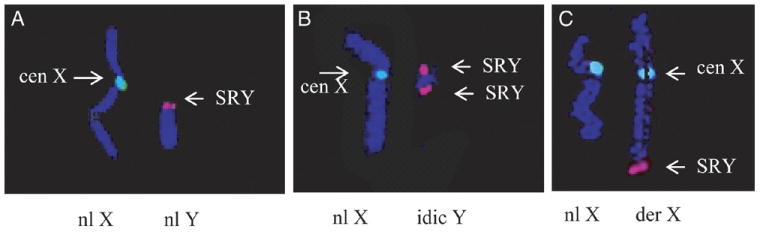
FISH using X (green dye) and Y (red dye) specific probes. A, normal hybridization pattern using X chromosome centromere and SRY specific probes in male patient. B, isodicentric Y chromosome in patient 8. C, infertile patient 3 with 46,XX karyotype and derivative chromosome X resulting from translocation between X and Y chromosomes. Yp11.2 region containing SRY gene was detected at long arm of chromosome X.
In the remaining 16 infertile patients different structural autosomal rearrangements were identified (table 2). We noted apparently balanced translocations in patients 13, 15, 16, 18, 20 and 25, pericentric inversions of chromosomes 1, 2, 4, 7 and 9 in patients 11, 14, 17, 19 and 21, and paracentric inversion of chromosome 1 in patient 12. Patients 22 to 24 had translocations involving acrocentric chromosomes (Robertsonian translocations) (table 2). Patient 26 had an unbalanced rearrangement known as jumping translocation with mosaicism for 4 abnormal cell lines (table 2). Structural autosomal rearrangements were commonly associated with OS but only 3 patients had AS.
More Common Chromosomal Aberrations Associated With OS and AS
To define the relationship between identified chromosomal aberrations and clinical phenotypes we analyzed the proportion of aberrations for each of the 4 abnormal categories (fig. 3). The chromosomal abnormality rate was increased in the AS and sOS categories (13.3% and 10.9%, respectively, table 1 and fig. 3, A). In the OS and mOS categories fewer patients had chromosomal defects but the rate remained high at 4.2% (8 of 192) and 1.2% (1 of 82), respectively. In the NS category 1 chromosomal aberration was noted that most likely represented a polymorphic variant.
Figure 3.
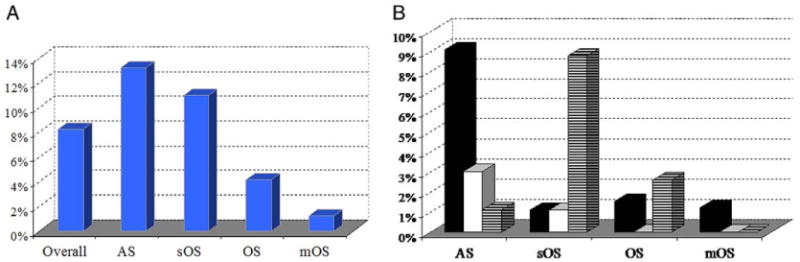
Cytogenetic findings in semen categories of tested infertile men. A, chromosome abnormalities. B, cytogenetic abnormalities. Black bars represent numerical sex chromosome abnormalities. Open bars represent structural sex chromosome abnormalities. Hatched bars represent structural autosomal aberrations.
To further examine genotype-phenotype correlations we calculated the incidence of numerical and structural chromosomal rearrangements in each infertility category (fig. 3, B). Sex chromosome aneuploidies were more common for AS than for the 3 OS categories. Klinefelter syndrome, including 47,XXY/46XY mosaicism cases, was the most common abnormality associated with OS and AS (table 1). Two patients with Klinefelter syndrome and mosaicism with an additional X chromosome in 5% to 10% of cells had OS and another 5 with 40% to 80% mosaicism for a 47,XXY/46,XY cell line had AS.
We compared structural rearrangement rates in patients with AS and OS (fig. 3). Structural rearrangements involving autosomes were more common than those involving sex chromosomes. The combined incidence of structural aberrations in sex and autosomal chromosomes was higher for AS than for OS (about 5% vs 3%). We also noted that OS was the only category with a higher proportion of structural rearrangements, involving sex chromosome and autosomes, than aneuploidies.
Discussion
Retrospective analysis of cytogenetic results in 668 infertile patients diagnosed with various nonobstructive spermatogenic defects revealed constitutional chromosomal abnormalities in 55 (8.2%). The observed incidence was almost 20-fold greater than reported in healthy fertile men (0.37%).21 We correlated cytogenetic aberration types with male reproductive phenotypes and noted that sex chromosome aneuploidy was the most common finding in AS cases, accounting for about 9% (fig. 3, B). Remarkably Klinefelter syndrome (47,XXY karyotype and variants) accounted for about 4% of all infertile males. The 47,XXY karyotype was detected at a considerably higher rate in men with AS vs OS (27 of 668 or 9.1% vs 3 of 365 or 0.8%).
Mosaicism for an additional X chromosome may occur in up to 22% of Klinefelter syndrome cases and is associated with a milder phenotype.19,22 In our cohort 7 of 27 patients (26%) were diagnosed with mosaicism for a 47,XXY/46,XY cell line, including 2 with low level mosaicism for the 47,XXY/46,XY cell line and OS, and 5 with a higher level of 47,XXY/46,XY mosaicism and AS (table 1). Because the extent of mosaicism may fluctuate among different tissues, analysis of a cultured peripheral blood sample does not always reflect the 46,XY/47,XXY ratio in gonadal and other tissues.23 Thus, patients with OS and Klinefelter syndrome may have a lower level of XXY cell line mosaicism in gonads than in the peripheral white blood cells used for karyotype analysis. Conversely some patients with a normal karyotype may have chromosome abnormalities in testicular or other tissues. In 1 study 20% of patients with a normal karyotype had XXY/XY mosaicism detected by FISH analysis.19 In such cases low level mosaicism may be undetectable by conventional cytogenetic analysis. Accordingly hidden sex chromosome aneuploidy may be detected by interphase FISH in uncultured blood cells or buccal epithelial cells. Analysis of cells derived from different germ layers (mesoderm and ectoderm) may improve the detection rate of cytogenetic defects.
In addition to Klinefelter syndrome, we noted 2 infertile males with Y chromosome aneuploidies with gain or loss of a Y chromosome (47,XYY and mosaic 45,X/46,XY karyotypes, respectively). Their infertility may be associated with a Y chromosome gene dose effect (gain or loss) but the clinical significance of 47,XYY syndrome currently is controversial. Recent studies show that the 47,XYY karyotype may be associated with altered meiotic segregation, resulting in sperm apoptosis and necrosis, leading to male infertility.24,25 However, most 47,XYY males are fertile.26
The 8.2% overall incidence of cytogenetic abnormalities is comparable to that in previously reported studies. This finding likely reflects selection bias of the patients analyzed, of whom many were tertiary referrals, due to the highly specialized expertise of the physician (LIL) ordering the tests. In our cohort various structural chromosomal aberrations were identified, including inversions, balanced and unbalanced translocations, and deletions (table 1). Structural rearrangements accounted for about 47% of all chromosome aberrations (fig. 1, A), considerably greater than previously reported.6,8 Another potential explanation for this finding is the use of high resolution chromosome analysis, which may increase the detection rate of subtle chromosomal defects.
Our study suggests that sex and autosomal chromosome structural rearrangements may result in AS or OS (table 1). Previous reports show that structural chromosomal aberrations are relatively common in infertile males.19,27 Our series shows that structural defects are more common in patients with OS, especially sOS. These rearrangements may involve genes that are critical for spermatogenesis.
Previous cytogenetic studies indicate a high incidence of Robertsonian translocations and chromosome 1 rearrangements in men with sOS and AS.18,28 However, we observed no high incidence of such rearrangements. We identified 3 of 26 infertile men (11% of those with structural aberrations) who were carriers of Robertsonian translocations and 3 of 26 (11% of those with structural aberrations) with OS and AS who had different rearrangements involving chromosome 1.
To date the most common clinical test to resolve uncertainty about subtle structural rearrangements and low level mosaicism is FISH in peripheral blood lymphocytes and spermatozoa.29 Recently new molecular technologies were developed, including single nucleotide polymorphism microarray and array CGH, that efficiently detect small genomic alterations and low level mosaicism in various tissues.30 Widespread application of array CGH technology for clinical diagnostics may significantly improve the detection rate of subtle aberrations in infertile males.
Conclusions
Despite recent significant progress in identifying novel molecular mechanisms responsible for spermatogenesis and improved assisted reproductive techniques the etiology of male infertility is often descriptive or unknown.3 Furthermore, in vitro fertilization and intracytoplasmic sperm injection are widely perceived as therapeutic applications but in reality these assisted reproductive technologies do not treat the infertility defect. Intracytoplasmic sperm injection and in vitro fertilization bypass the natural reproductive defect(s) causing infertility, leaving the disease unidentified and ultimately untreated.
Although it is estimated that genetic factors may contribute up to 75% of male infertility, karyotype analysis, cystic fibrosis mutation detection and Y chromosome microdeletion analysis are the only clinically available genetic tests for male infertility. Thus, structural submicroscopic chromosomal defects and/or low level mosaicism may remain undetected genetic causes of OS and AS. As a result, molecular technologies such as spermatozoal FISH and microarray CGH are likely to improve the diagnosis of and consequently future treatments for male infertility. These molecular techniques may be beneficial to identify new genes/regions that have a vital role in the human reproductive system and are associated with male infertility.
Acknowledgments
Supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development P01HD36289 (DJL and MMM) and K08HD058073 (ANY), and National Institute of Diabetes Kidney and Digestive Diseases 1R01DK078121 (DJL)
Abbreviations and Acronyms
- AS
azoospermia
- CGH
comparative genome hybridization
- FISH
fluorescence in situ hybridization
- mOS
mild OS
- NS
normozoospermia
- OS
oligozoospermia
- sOS
severe OS
Footnotes
Study received approval from Baylor College of Medicine institutional review board for human subject research
References
- 1.Report OTA: Infertility: Medical and Social Choices OTA-BA-358. Washington, D.C.: United States Congress, Office of Technology Assessment, United States Government Printing Office; 1988. [Google Scholar]
- 2.Abma JC, Chandra A, Mosher WD, et al. Fertility, family planning, and women's health: new data from the 1995 National Survey of Family Growth. Vital Health Stat. 1997;23:1. [PubMed] [Google Scholar]
- 3.Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14:1197. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Report on the Optimal Evaluation of the Infertile Male. American Urological Association Best Practice Statement and American Society of Reproductive Medicine Practice Committee Report. Linthicum Maryland: American Urological Association; 2001. pp. 1–14. [Google Scholar]
- 5.Evaluation of the azoospermic male. Practice Committee of American Society for Reproductive Medicine in Collaboration With Society for Male Reproduction and Urology. Fertil Steril. 2008;90:S74. doi: 10.1016/j.fertnstert.2008.08.092. [DOI] [PubMed] [Google Scholar]
- 6.Brugh VM, 3rd, Lipshultz LI. Male factor infertility: evaluation and management. Med Clin North Am. 2004;88:367. doi: 10.1016/S0025-7125(03)00150-0. [DOI] [PubMed] [Google Scholar]
- 7.Schultz N, Hamra FK, Garbers DL. A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. Proc Natl Acad Sci U S A. 2003;100:12201. doi: 10.1073/pnas.1635054100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipshultz LI, Lamb DJ. Risk of transmission of genetic diseases by assisted reproduction. Nat Clin Pract Urol. 2007;4:460. doi: 10.1038/ncpuro0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyamoto T, Hasuike S, Yogev L, et al. Azoospermia in patients heterozygous for a mutation in SYCP3. Lancet. 2003;362:1714. doi: 10.1016/S0140-6736(03)14845-3. [DOI] [PubMed] [Google Scholar]
- 10.Chillón M, Casals T, Mercier B, et al. Mutations in the cystic fibrosis gene in patients with congenital absence of the vas deferens. N Engl J Med. 1995;332:1475. doi: 10.1056/NEJM199506013322204. [DOI] [PubMed] [Google Scholar]
- 11.Iguchi N, Yang S, Lamb DJ, et al. A protamine SNP: one genetic cause of male infertility. J Med Genet. 2005;43:382. doi: 10.1136/jmg.2005.037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dam AH, Koscinski I, Kremer JA, et al. Homozygous mutation in SPATA16 is associated with male infertility in human globozoospermia. Am J Hum Genet. 2007;81:813. doi: 10.1086/521314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yatsenko AN, Roy A, Chen R, et al. Non-invasive genetic diagnosis of male infertility using spermatozoal RNA: KLHL10 mutations in oligozoospermic patients impair homodimerization. Hum Mol Genet. 2006;15:3411. doi: 10.1093/hmg/ddl417. [DOI] [PubMed] [Google Scholar]
- 14.Dieterich K, Soto Rifo R, Faure AK, et al. Homozygous mutation of AURKC yields large-headed polyploid spermatozoa and causes male infertility. Nat Genet. 2007;39:661. doi: 10.1038/ng2027. [DOI] [PubMed] [Google Scholar]
- 15.Reijo R, Alagappan RK, Patrizio P, et al. Severe oligozoospermia resulting from deletions of azoospermia factor gene on Y chromosome. Lancet. 1996;347:1290. doi: 10.1016/s0140-6736(96)90938-1. [DOI] [PubMed] [Google Scholar]
- 16.Reijo R, Lee TY, Salo P, et al. Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nat Genet. 1995;10:383. doi: 10.1038/ng0895-383. [DOI] [PubMed] [Google Scholar]
- 17.Bojesen A, Gravholt CH. Klinefelter syndrome in clinical practice. Nat Clin Pract Urol. 2007;4:192. doi: 10.1038/ncpuro0775. [DOI] [PubMed] [Google Scholar]
- 18.Bache I, Van Assche E, Cingoz S, et al. An excess of chromosome 1 breakpoints in male infertility. Eur J Hum Genet. 2004;12:993. doi: 10.1038/sj.ejhg.5201263. [DOI] [PubMed] [Google Scholar]
- 19.Elghezal H, Hidar S, Braham R, et al. Chromosome abnormalities in one thousand infertile males with nonobstructive sperm disorders. Fertil Steril. 2006;86:1792. doi: 10.1016/j.fertnstert.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 20.Yatsenko SA, Cheung SW, Scott DA, et al. Deletion 9q34.3 syndrome: genotype-phenotype correlations and an extended deletion in a patient with features of Opitz C trigonocephaly. J Med Genet. 2005;42:328. doi: 10.1136/jmg.2004.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravel C, Berthaut I, Bresson JL, et al. Prevalence of chromosomal abnormalities in phenotypically normal and fertile adult males: large-scale survey of over 10,000 sperm donor karyotypes. Hum Reprod. 2006;21:1484. doi: 10.1093/humrep/del024. [DOI] [PubMed] [Google Scholar]
- 22.Rives N, Joly G, Machy A, et al. Assessment of sex chromosome aneuploidy in sperm nuclei from 47,XXY and 46,XY/47,XXY males: comparison with fertile and infertile males with normal karyotype. Mol Hum Reprod. 2000;6:107. doi: 10.1093/molehr/6.2.107. [DOI] [PubMed] [Google Scholar]
- 23.Stipoljev F, Vujisić S, Parazajder J, et al. Cytogenetic analysis of azoospermic patients: karyotype comparison of peripheral blood lymphocytes and testicular tissue. Eur J Obstet Gynecol Reprod Biol. 2006;124:197. doi: 10.1016/j.ejogrb.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Moretti E, Anichini C, Sartini B, et al. Sperm ultrastructure and meiotic segregation in an infertile 47, XYY man. Andrologia. 2007;39:229. doi: 10.1111/j.1439-0272.2007.00791.x. [DOI] [PubMed] [Google Scholar]
- 25.Wong EC, Ferguson KA, Chow V, et al. Sperm aneuploidy and meiotic sex chromosome configurations in an infertile XYY male. Hum Reprod. 2008;23:374. doi: 10.1093/humrep/dem377. [DOI] [PubMed] [Google Scholar]
- 26.Chantot-Bastaraud S, Ravel C, Siffroi JP. Underlying karyotype abnormalities in IVF/ICSI patients. Reprod Biomed Online. 2008;16:514. doi: 10.1016/s1472-6483(10)60458-0. [DOI] [PubMed] [Google Scholar]
- 27.Gekas J, Thepot F, Turleau C, et al. Chromosomal factors of infertility in candidate couples for ICSI: an equal risk of constitutional aberrations in women and men. Hum Reprod. 2001;16:82. doi: 10.1093/humrep/16.1.82. [DOI] [PubMed] [Google Scholar]
- 28.Kumar R, Tanwar M, Ammini AC, et al. Robertsonian translocation and their role in pathogenesis of recurrent in vitro fertilization failure. Med Sci Monit. 2008;14:CR617. [PubMed] [Google Scholar]
- 29.Foresta C, Ferlin A, Gianaroli L, et al. Guidelines for the appropriate use of genetic tests in infertile couples. Eur J Hum Genet. 2002;10:303. doi: 10.1038/sj.ejhg.5200805. [DOI] [PubMed] [Google Scholar]
- 30.Cheung SW, Shaw CA, Scott DA, et al. Microarray-based CGH detects chromosomal mosaicism not revealed by conventional cytogenetics. Am J Med Genet A. 2007;143A:1679. doi: 10.1002/ajmg.a.31740. [DOI] [PubMed] [Google Scholar]


