Abstract
The molecule of the title heteroaryl chalcone, C16H16O4S, which consists of substituted thiophene and benzene rings bridged by the prop-2-en-1-one group, is slightly twisted. The dihedral angle between the thiophene and 3,4,5-trimethoxyphenyl rings is 12.18 (4)°. The three methoxy groups have two different conformations; two methoxy groups are coplanar [C—O—C—C torsion angles = −1.38 (12) and 0.47 (12)°] whereas the third is (-)-synclinal with the benzene ring. In the crystal structure, adjacent molecules are linked in a face-to-side manner into chains along the c axis by weak C—H⋯O(enone) interactions. These chains are stacked along the b axis by weak C—H⋯O(methoxy) interactions.
Related literature
For bond-length data, see: Allen et al. (1987 ▶). For hydrogen-bond motifs, see: Bernstein et al. (1995 ▶). For related structures, see: Chantrapromma et al. (2009 ▶); Patil et al. (2006 ▶; 2007 ▶); Suwunwong et al. (2009a
▶,b
▶). For background to and applications of chalcones, see: Dimmock et al. (1999 ▶); Go et al. (2005 ▶); Jung et al. (2008 ▶); Ni et al. (2004 ▶); Patil et al. (2007 ▶); Patil & Dharmaprakash (2008 ▶). For the stability of the temperature controller used in the data collection, see: Cosier & Glazer, (1986 ▶).
Experimental
Crystal data
C16H16O4S
M r = 304.36
Orthorhombic,
a = 25.3323 (8) Å
b = 3.9816 (1) Å
c = 14.0163 (4) Å
V = 1413.73 (7) Å3
Z = 4
Mo Kα radiation
μ = 0.24 mm−1
T = 100 K
0.58 × 0.31 × 0.21 mm
Data collection
Bruker APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2005 ▶) T min = 0.872, T max = 0.951
56940 measured reflections
7416 independent reflections
7177 reflections with I > 2σ(I)
R int = 0.028
Refinement
R[F 2 > 2σ(F 2)] = 0.033
wR(F 2) = 0.099
S = 1.10
7416 reflections
193 parameters
1 restraint
H-atom parameters constrained
Δρmax = 0.66 e Å−3
Δρmin = −0.54 e Å−3
Absolute structure: Flack (1983 ▶), 3588 Friedel pairs
Flack parameter: 0.04 (4)
Data collection: APEX2 (Bruker, 2005 ▶); cell refinement: SAINT (Bruker, 2005 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809021850/sj2627sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809021850/sj2627Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C1—H1A⋯O1i | 0.93 | 2.52 | 3.1827 (14) | 129 |
| C15—H15C⋯O3ii | 0.96 | 2.39 | 3.3340 (11) | 169 |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Acknowledgments
Financial support from the Prince of Songkla University through the Crystal Materials Research Unit is gratefully acknowledged. TS thanks the Graduate School, Prince of Songkla University, for partial financial support. The authors also thank Universiti Sains Malaysia for the Research University Golden Goose grant No. 1001/PFIZIK/811012.
supplementary crystallographic information
Comment
Chalcone or 1,3-diaryl-2-propen-1-one, originally isolated from natural sources, and its derivatives are known to display a variety of biological activities, demonstrating analgesic, anti-inflammatory, antibacterial and antimycotic properties (Dimmock et al., 1999; Go et al., 2005; Ni et al., 2004). Moreover synthetic chalcones have also been found to be non-linear optical (NLO) (Patil & Dharmaprakash, 2008) and electro-active fluorescent materials (Jung et al., 2008). We have previously reported the synthesis and crystal structures of chalcone derivatives (Chantrapromma et al., 2009; Suwunwong et al., 2009a, b). Our research into the NLO and biological properties of chalcone derivatives led us to synthesize the title heteroaryl chalcone (I). (I) crystallizes in the non-centrosymmetric orthorhombic space group Pna21 and should therefore exhibit second-order nonlinear optical properties.
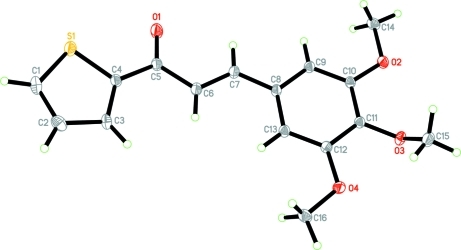
The molecule of the title heteroaryl chalcone (Fig. 1) exists in an E configuration with respect to the C6═C7 double bond [1.3437 (11) Å] with a C5–C6–C7–C8 torsion angle 176.81 (8)°. The whole molecule is twisted as shown by the interplanar angle between thiophene and 3,4,5-trimethoxyphenyl rings being 12.18 (4)°. The propenone unit (C5—C7/O1) is also twisted with the O1–C5–C6–C7 torsion angle 10.94 (15)°. The three substituted methoxy groups of 3,4,5-trimethoxyphenyl unit have two different orientations: two methoxy groups (at the C10 and C12 positions) are co-planar with the phenyl ring with torsion angles C14–O2–C10–C9 = -1.38 (12)° and C16–O4–C12–C13 = 0.47 (12)° whereas the one at C11 is (-)-syn-clinally attached with the C15–O3–C11–C12 torsion angle -76.76 (10)°. In the structure, weak intramolecular C7—H7A···O1 and C15—H15B···O4 interactions generate S(5) and S(6) ring motifs, respectively (Bernstein et al., 1995). The bond distances have normal values (Allen et al., 1987) and bond lengths and angles are comparable with closely related structures (Chantrapromma et al., 2009; Patil et al., 2006; 2007; Suwunwong et al., 2009a, b).
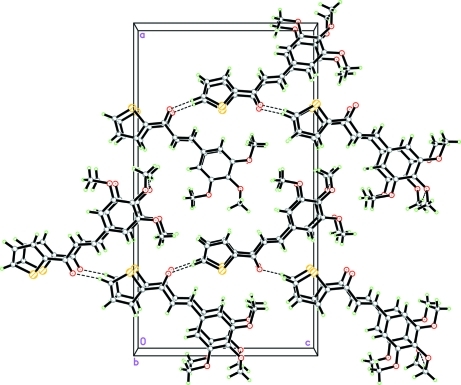
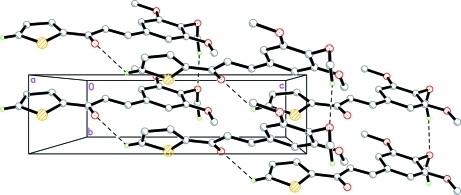
In the crystal packing, the adjacent molecules are linked in a face-to-side manner into chains along the c axis through the enone unit by weak C1—H1A···O1 interactions (Fig. 2, Table 1). Weak C15—H15C···O3 interactions involving one of methoxy groups further stack these chains along the b axis (Fig. 3, Table 1).
Experimental
The title compound was synthesized by the condensation of 3,4,5-trimethoxybenzaldehyde (0.40 g, 2 mmol) with 2-acetylthiophene (0.35 ml, 2 mmol) in ethanol (30 ml) in the presence of 30% NaOH (aq) (5 ml). After stirring for 3 h in ice bath at 278 K, the resulting pale yellow solid was collected by filtration, washed with distilled water, dried in air and purified by repeated recrystallization from acetone (72% yield). Pale yellow block-shaped single crystals of the title compound suitable for x-ray structure determination were recrystalized from acetone/ethanol (1:1 v/v) by the slow evaporation of the solvent at room temperature after several days, Mp. 420–421 K.
Refinement
All H atoms were placed in calculated positions, with C—H = 0.93 Å, Uiso = 1.2Ueq(C) for aromatic and CH and C—H = 0.96 Å, Uiso = 1.5Ueq(C) for CH3 atoms. A rotating group model was used for the methyl groups. The highest residual electron density peak is located at 0.22 Å from C3 and the deepest hole is located at 0.20 Å from S1.
Figures
Fig. 1.
The molecular structure of the title compound, showing 50% probability displacement ellipsoids and the atom-numbering scheme.
Fig. 2.
The crystal packing of the title compound viewed along the b axis, showing chains running along the c axis. Weak C—H···O interactions are shown as dashed lines.
Fig. 3.
The crystal packing of the title compound viewed along the a axis, showing chains stacking along the b axis. Weak C—H···O interactions are shown as dashed lines and hydrogen atoms not involved in C—H···O interactions were omitted for clarity.
Crystal data
| C16H16O4S | Dx = 1.430 Mg m−3 |
| Mr = 304.36 | Melting point = 420–421 K |
| Orthorhombic, Pna21 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2c -2n | Cell parameters from 7416 reflections |
| a = 25.3323 (8) Å | θ = 2.2–37.5° |
| b = 3.9816 (1) Å | µ = 0.24 mm−1 |
| c = 14.0163 (4) Å | T = 100 K |
| V = 1413.73 (7) Å3 | Block, pale yellow |
| Z = 4 | 0.58 × 0.31 × 0.21 mm |
| F(000) = 640 |
Data collection
| Bruker APEXII CCD area-detector diffractometer | 7416 independent reflections |
| Radiation source: sealed tube | 7177 reflections with I > 2σ(I) |
| graphite | Rint = 0.028 |
| φ and ω scans | θmax = 37.5°, θmin = 2.2° |
| Absorption correction: multi-scan (SADABS; Bruker, 2005) | h = −43→43 |
| Tmin = 0.872, Tmax = 0.951 | k = −6→6 |
| 56940 measured reflections | l = −23→24 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.033 | H-atom parameters constrained |
| wR(F2) = 0.099 | w = 1/[σ2(Fo2) + (0.0672P)2 + 0.1429P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.10 | (Δ/σ)max = 0.001 |
| 7416 reflections | Δρmax = 0.66 e Å−3 |
| 193 parameters | Δρmin = −0.54 e Å−3 |
| 1 restraint | Absolute structure: Flack (1983), 3588 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: 0.04 (4) |
Special details
| Experimental. The crystal was placed in the cold stream of an Oxford Cryosystems Cobra open-flow nitrogen cryostat (Cosier & Glazer, 1986) operating at 120.0 (1) K. |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.249987 (8) | 0.46794 (6) | 0.47989 (2) | 0.01892 (5) | |
| O1 | 0.25370 (3) | 0.4617 (3) | 0.68801 (7) | 0.02618 (18) | |
| O2 | 0.40965 (3) | 0.51095 (18) | 1.13056 (5) | 0.01662 (11) | |
| O3 | 0.49824 (2) | 0.83176 (19) | 1.08244 (5) | 0.01615 (10) | |
| O4 | 0.51691 (3) | 1.02802 (19) | 0.90201 (5) | 0.01753 (11) | |
| C1 | 0.28105 (4) | 0.5872 (3) | 0.37853 (6) | 0.02121 (16) | |
| H1A | 0.2679 | 0.5465 | 0.3177 | 0.025* | |
| C2 | 0.32771 (4) | 0.7503 (3) | 0.39595 (7) | 0.02174 (16) | |
| H2A | 0.3493 | 0.8331 | 0.3477 | 0.026* | |
| C3 | 0.34021 (3) | 0.7815 (2) | 0.49530 (5) | 0.01517 (12) | |
| H3A | 0.3702 | 0.8844 | 0.5200 | 0.018* | |
| C4 | 0.29890 (3) | 0.6283 (2) | 0.55061 (6) | 0.01440 (12) | |
| C5 | 0.29401 (3) | 0.5880 (2) | 0.65390 (6) | 0.01617 (13) | |
| C6 | 0.33914 (3) | 0.6919 (2) | 0.71401 (6) | 0.01613 (13) | |
| H6A | 0.3662 | 0.8202 | 0.6878 | 0.019* | |
| C7 | 0.34122 (3) | 0.6018 (2) | 0.80632 (6) | 0.01549 (13) | |
| H7A | 0.3122 | 0.4836 | 0.8294 | 0.019* | |
| C8 | 0.38341 (3) | 0.6666 (2) | 0.87483 (5) | 0.01329 (11) | |
| C9 | 0.37539 (3) | 0.5559 (2) | 0.96864 (6) | 0.01372 (11) | |
| H9A | 0.3441 | 0.4479 | 0.9849 | 0.016* | |
| C10 | 0.41415 (3) | 0.6073 (2) | 1.03751 (5) | 0.01261 (11) | |
| C11 | 0.46146 (3) | 0.7683 (2) | 1.01284 (5) | 0.01296 (11) | |
| C12 | 0.46951 (3) | 0.8767 (2) | 0.91840 (5) | 0.01333 (12) | |
| C13 | 0.43078 (3) | 0.8266 (2) | 0.84955 (6) | 0.01399 (12) | |
| H13A | 0.4362 | 0.8985 | 0.7872 | 0.017* | |
| C14 | 0.36101 (4) | 0.3538 (2) | 1.15750 (6) | 0.01816 (14) | |
| H14A | 0.3619 | 0.3001 | 1.2243 | 0.027* | |
| H14B | 0.3563 | 0.1516 | 1.1212 | 0.027* | |
| H14C | 0.3322 | 0.5043 | 1.1452 | 0.027* | |
| C15 | 0.54355 (3) | 0.6178 (2) | 1.07845 (8) | 0.02027 (15) | |
| H15A | 0.5681 | 0.6817 | 1.1274 | 0.030* | |
| H15B | 0.5601 | 0.6391 | 1.0172 | 0.030* | |
| H15C | 0.5329 | 0.3890 | 1.0881 | 0.030* | |
| C16 | 0.52661 (4) | 1.1439 (3) | 0.80695 (6) | 0.01969 (15) | |
| H16A | 0.5598 | 1.2597 | 0.8049 | 0.030* | |
| H16B | 0.4989 | 1.2941 | 0.7880 | 0.030* | |
| H16C | 0.5276 | 0.9555 | 0.7642 | 0.030* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.01673 (9) | 0.02390 (10) | 0.01613 (9) | −0.00032 (6) | −0.00311 (6) | 0.00034 (9) |
| O1 | 0.0181 (3) | 0.0441 (5) | 0.0164 (3) | −0.0106 (3) | −0.0019 (2) | 0.0083 (3) |
| O2 | 0.0169 (2) | 0.0225 (3) | 0.0104 (2) | −0.0028 (2) | 0.00034 (19) | 0.00240 (19) |
| O3 | 0.0152 (2) | 0.0203 (3) | 0.0130 (2) | −0.00066 (19) | −0.00317 (18) | −0.0026 (2) |
| O4 | 0.0148 (2) | 0.0241 (3) | 0.0136 (2) | −0.0053 (2) | 0.0005 (2) | 0.0021 (2) |
| C1 | 0.0243 (4) | 0.0266 (4) | 0.0127 (3) | 0.0053 (3) | −0.0033 (3) | −0.0005 (3) |
| C2 | 0.0220 (4) | 0.0247 (4) | 0.0185 (3) | 0.0027 (3) | 0.0059 (3) | 0.0038 (3) |
| C3 | 0.0144 (3) | 0.0203 (3) | 0.0108 (3) | 0.0051 (2) | −0.0017 (2) | −0.0003 (2) |
| C4 | 0.0135 (3) | 0.0168 (3) | 0.0129 (3) | 0.0002 (2) | −0.0012 (2) | 0.0020 (2) |
| C5 | 0.0142 (3) | 0.0215 (3) | 0.0128 (3) | −0.0013 (3) | −0.0015 (2) | 0.0025 (2) |
| C6 | 0.0147 (3) | 0.0199 (3) | 0.0138 (3) | −0.0014 (2) | −0.0017 (2) | 0.0012 (2) |
| C7 | 0.0134 (3) | 0.0206 (3) | 0.0125 (3) | −0.0006 (2) | −0.0014 (2) | 0.0006 (2) |
| C8 | 0.0123 (3) | 0.0168 (3) | 0.0108 (2) | 0.0004 (2) | −0.0006 (2) | −0.0002 (2) |
| C9 | 0.0125 (3) | 0.0169 (3) | 0.0117 (3) | −0.0006 (2) | −0.0001 (2) | 0.0002 (2) |
| C10 | 0.0128 (3) | 0.0149 (3) | 0.0101 (3) | 0.0006 (2) | 0.0003 (2) | 0.0001 (2) |
| C11 | 0.0130 (3) | 0.0153 (3) | 0.0105 (2) | −0.0002 (2) | −0.0003 (2) | −0.0003 (2) |
| C12 | 0.0127 (3) | 0.0157 (3) | 0.0116 (3) | −0.0003 (2) | 0.0010 (2) | −0.0007 (2) |
| C13 | 0.0129 (3) | 0.0178 (3) | 0.0113 (3) | 0.0000 (2) | 0.0002 (2) | 0.0001 (2) |
| C14 | 0.0186 (3) | 0.0206 (4) | 0.0152 (3) | −0.0016 (3) | 0.0039 (2) | 0.0030 (3) |
| C15 | 0.0174 (3) | 0.0188 (3) | 0.0246 (4) | 0.0001 (3) | −0.0066 (3) | −0.0001 (3) |
| C16 | 0.0171 (3) | 0.0252 (4) | 0.0168 (3) | −0.0013 (3) | 0.0032 (3) | 0.0051 (3) |
Geometric parameters (Å, °)
| S1—C1 | 1.6921 (11) | C7—C8 | 1.4598 (11) |
| S1—C4 | 1.7104 (8) | C7—H7A | 0.9300 |
| O1—C5 | 1.2347 (11) | C8—C9 | 1.4015 (11) |
| O2—C10 | 1.3642 (10) | C8—C13 | 1.4040 (11) |
| O2—C14 | 1.4325 (11) | C9—C10 | 1.3920 (11) |
| O3—C11 | 1.3725 (10) | C9—H9A | 0.9300 |
| O3—C15 | 1.4304 (12) | C10—C11 | 1.4023 (11) |
| O4—C12 | 1.3630 (10) | C11—C12 | 1.4073 (11) |
| O4—C16 | 1.4312 (11) | C12—C13 | 1.3905 (11) |
| C1—C2 | 1.3704 (15) | C13—H13A | 0.9300 |
| C1—H1A | 0.9300 | C14—H14A | 0.9600 |
| C2—C3 | 1.4335 (13) | C14—H14B | 0.9600 |
| C2—H2A | 0.9300 | C14—H14C | 0.9600 |
| C3—C4 | 1.4382 (12) | C15—H15A | 0.9600 |
| C3—H3A | 0.9300 | C15—H15B | 0.9600 |
| C4—C5 | 1.4619 (12) | C15—H15C | 0.9600 |
| C5—C6 | 1.4792 (12) | C16—H16A | 0.9600 |
| C6—C7 | 1.3437 (11) | C16—H16B | 0.9600 |
| C6—H6A | 0.9300 | C16—H16C | 0.9600 |
| C1—S1—C4 | 92.57 (5) | O2—C10—C9 | 124.24 (7) |
| C10—O2—C14 | 116.54 (7) | O2—C10—C11 | 115.83 (7) |
| C11—O3—C15 | 114.04 (7) | C9—C10—C11 | 119.93 (7) |
| C12—O4—C16 | 116.78 (7) | O3—C11—C10 | 119.29 (7) |
| C2—C1—S1 | 112.61 (7) | O3—C11—C12 | 120.91 (7) |
| C2—C1—H1A | 123.7 | C10—C11—C12 | 119.73 (7) |
| S1—C1—H1A | 123.7 | O4—C12—C13 | 124.62 (7) |
| C1—C2—C3 | 113.88 (8) | O4—C12—C11 | 114.94 (7) |
| C1—C2—H2A | 123.1 | C13—C12—C11 | 120.43 (7) |
| C3—C2—H2A | 123.1 | C12—C13—C8 | 119.54 (7) |
| C2—C3—C4 | 109.02 (8) | C12—C13—H13A | 120.2 |
| C2—C3—H3A | 125.5 | C8—C13—H13A | 120.2 |
| C4—C3—H3A | 125.5 | O2—C14—H14A | 109.5 |
| C3—C4—C5 | 129.94 (7) | O2—C14—H14B | 109.5 |
| C3—C4—S1 | 111.91 (6) | H14A—C14—H14B | 109.5 |
| C5—C4—S1 | 118.14 (6) | O2—C14—H14C | 109.5 |
| O1—C5—C4 | 119.89 (8) | H14A—C14—H14C | 109.5 |
| O1—C5—C6 | 122.19 (8) | H14B—C14—H14C | 109.5 |
| C4—C5—C6 | 117.90 (7) | O3—C15—H15A | 109.5 |
| C7—C6—C5 | 120.26 (8) | O3—C15—H15B | 109.5 |
| C7—C6—H6A | 119.9 | H15A—C15—H15B | 109.5 |
| C5—C6—H6A | 119.9 | O3—C15—H15C | 109.5 |
| C6—C7—C8 | 127.94 (8) | H15A—C15—H15C | 109.5 |
| C6—C7—H7A | 116.0 | H15B—C15—H15C | 109.5 |
| C8—C7—H7A | 116.0 | O4—C16—H16A | 109.5 |
| C9—C8—C13 | 120.22 (7) | O4—C16—H16B | 109.5 |
| C9—C8—C7 | 117.10 (7) | H16A—C16—H16B | 109.5 |
| C13—C8—C7 | 122.68 (7) | O4—C16—H16C | 109.5 |
| C10—C9—C8 | 120.15 (7) | H16A—C16—H16C | 109.5 |
| C10—C9—H9A | 119.9 | H16B—C16—H16C | 109.5 |
| C8—C9—H9A | 119.9 | ||
| C4—S1—C1—C2 | −0.66 (8) | C14—O2—C10—C11 | 178.47 (7) |
| S1—C1—C2—C3 | 0.53 (11) | C8—C9—C10—O2 | 179.44 (8) |
| C1—C2—C3—C4 | −0.06 (11) | C8—C9—C10—C11 | −0.40 (12) |
| C2—C3—C4—C5 | 178.30 (9) | C15—O3—C11—C10 | 106.33 (9) |
| C2—C3—C4—S1 | −0.43 (9) | C15—O3—C11—C12 | −76.76 (10) |
| C1—S1—C4—C3 | 0.62 (7) | O2—C10—C11—O3 | −2.98 (11) |
| C1—S1—C4—C5 | −178.27 (7) | C9—C10—C11—O3 | 176.88 (8) |
| C3—C4—C5—O1 | 176.49 (10) | O2—C10—C11—C12 | −179.93 (7) |
| S1—C4—C5—O1 | −4.85 (13) | C9—C10—C11—C12 | −0.07 (12) |
| C3—C4—C5—C6 | −5.52 (14) | C16—O4—C12—C13 | 0.47 (12) |
| S1—C4—C5—C6 | 173.14 (7) | C16—O4—C12—C11 | −179.67 (8) |
| O1—C5—C6—C7 | 10.94 (15) | O3—C11—C12—O4 | 3.51 (11) |
| C4—C5—C6—C7 | −166.99 (8) | C10—C11—C12—O4 | −179.59 (7) |
| C5—C6—C7—C8 | 176.81 (8) | O3—C11—C12—C13 | −176.63 (8) |
| C6—C7—C8—C9 | 177.83 (9) | C10—C11—C12—C13 | 0.27 (12) |
| C6—C7—C8—C13 | −3.34 (14) | O4—C12—C13—C8 | 179.86 (8) |
| C13—C8—C9—C10 | 0.69 (12) | C11—C12—C13—C8 | 0.01 (12) |
| C7—C8—C9—C10 | 179.55 (8) | C9—C8—C13—C12 | −0.49 (12) |
| C14—O2—C10—C9 | −1.38 (12) | C7—C8—C13—C12 | −179.29 (8) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C1—H1A···O1i | 0.93 | 2.52 | 3.1827 (14) | 129 |
| C7—H7A···O1 | 0.93 | 2.48 | 2.8243 (12) | 102 |
| C15—H15B···O4 | 0.96 | 2.49 | 3.0396 (13) | 116 |
| C15—H15C···O3ii | 0.96 | 2.39 | 3.3340 (11) | 169 |
| C7—H7A···O1 | 0.93 | 2.48 | 2.8243 (12) | 102 |
| C15—H15B···O4 | 0.96 | 2.49 | 3.0396 (13) | 116 |
Symmetry codes: (i) −x+1/2, y+1/2, z−1/2; (ii) x, y−1, z.
Footnotes
This paper is dedicated to the late Her Royal Highness Princess Galyani Vadhana Krom Luang Naradhiwas Rajanagarindra for her patronage of Science in Thailand.
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SJ2627).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl 34, 1555–1573.
- Bruker (2005). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Chantrapromma, S., Suwunwong, T., Karalai, C. & Fun, H.-K. (2009). Acta Cryst. E65, o893–o894. [DOI] [PMC free article] [PubMed]
- Cosier, J. & Glazer, A. M. (1986). J. Appl. Cryst.19, 105–107.
- Dimmock, J. R., Elias, D. W., Beazely, M. A. & Kandepu, N. M. (1999). Curr. Med. Chem 6, 1125–1149. [PubMed]
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Go, M.-L., Wu, X. & Liu, X.-L. (2005). Curr. Med. Chem 12, 483–499.
- Jung, Y. J., Son, K. I., Oh, Y. E. & Noh, D. Y. (2008). Polyhedron, 27, 861–867.
- Ni, L., Meng, C. Q. & Sikorski, J. A. (2004). Expert Opin. Ther. Patents, 14, 1669–1691.
- Patil, P. S., Chantrapromma, S., Fun, H.-K., Dharmaprakash, S. M. & Babu, H. B. R. (2007). Acta Cryst E63, o2612.
- Patil, P. S. & Dharmaprakash, S. M. (2008). Mater. Lett 62, 451–453.
- Patil, P. S., Rosli, M. M., Fun, H.-K., Razak, I. A., Puranik, V. G. & Dharmaprakash, S. M. (2006). Acta Cryst. E62, o4798–o4799.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Suwunwong, T., Chantrapromma, S. & Fun, H.-K. (2009a). Acta Cryst. E65, o120. [DOI] [PMC free article] [PubMed]
- Suwunwong, T., Chantrapromma, S., Karalai, C., Pakdeevanich, P. & Fun, H.-K. (2009b). Acta Cryst. E65, o420–o421. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809021850/sj2627sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809021850/sj2627Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report