Abstract
The title compound, C24H32O5·C2H4O2, is a derivative of abietic acid. The two fused and unbridged cyclohexane rings have chair conformations and the anhydride ring is planar. Of the other three six-membered rings, two have boat conformations and one has a twist-boat conformation. The crystal structure is stabilized by intermolecular O—H⋯O and C—H⋯O hydrogen bonds.
Related literature
For general background, see: McCoy (2000 ▶); Schweizer et al. (2003 ▶); Savluchinske-Feio et al. (2007 ▶). For the crystal structure of a similar compound, see: Pan et al. (2006 ▶). For standard bond-length data, see: Allen et al. (1987 ▶).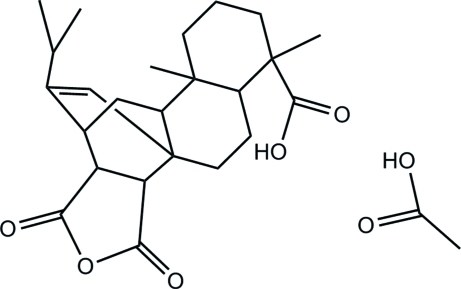
Experimental
Crystal data
C24H32O5·C2H4O2
M r = 460.55
Orthorhombic,

a = 7.9469 (10) Å
b = 12.7755 (16) Å
c = 24.884 (3) Å
V = 2526.3 (5) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 291 K
0.30 × 0.26 × 0.24 mm
Data collection
Bruker SMART APEX CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2000 ▶) T min = 0.97, T max = 0.98
13853 measured reflections
2837 independent reflections
2432 reflections with I > 2σ(I)
R int = 0.059
Refinement
R[F 2 > 2σ(F 2)] = 0.047
wR(F 2) = 0.110
S = 1.04
2837 reflections
304 parameters
H-atom parameters constrained
Δρmax = 0.19 e Å−3
Δρmin = −0.17 e Å−3
Data collection: SMART (Bruker, 2000 ▶); cell refinement: SAINT (Bruker, 2000 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809023745/wn2334sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809023745/wn2334Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O4—H4C⋯O6 | 0.93 | 1.70 | 2.617 (3) | 169 |
| O7—H7A⋯O5 | 0.93 | 1.76 | 2.681 (3) | 171 |
| C13—H13C⋯O5i | 0.96 | 2.59 | 3.137 (5) | 117 |
| C26—H26B⋯O1ii | 0.96 | 2.56 | 3.369 (4) | 142 |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Acknowledgments
This work was supported by the President of the Chinese Academy of Forestry Foundation (CAFYBB2008009).
supplementary crystallographic information
Comment
Rosin, a versatile natural resin, possesses a rare combination of many desirable properties and has consequently found innumerable industrial uses in a modified form or in conjunction with other natural or synthetic resins (McCoy, 2000). Abietic type resin acid is the major component of gum rosin, including abietic acid, neoabietic acid, levo-pimaric acid, palustric acid and is a high quality biomass resource in developing chiral new drugs (Schweizer et al., 2003). Abietic acid and its derivatives are readily available hydrophenanthrene compounds which form useful starting materials for the design and synthesis of industrially and physiologically important products (Savluchinske-Feio et al.,2007).
The crystal structure of a similar compound, also a derivative of maleopimaric acid, has already been published (Pan et al., 2006). The molecular structure of the title compound, is shown in Fig. 1. The asymmetric unit consists of two molecules, viz. maleopimaric anhydride and acetic acid. The cyclohexane rings C5, C6, C14–C16, C21 and C16—C21 have typical chair forms. The cyclohexane ring C2–C7 has a slightly distorted twist-boat conformation; the other two six-membered rings adopt boat conformations. The configuration about the C9═C10 bond is Z (Fig. 1), with the H atom and the isopropyl group cis with respect to each other. The bond lengths (Allen et al., 1987) and bond angles exhibit normal values. In the crystal structure, the molecules are linked (Fig.2) by O—H···O and C—H···O intermolecular hydrogen bonds, also by van der Waals forces.
Experimental
Rosin (10.0 g), acetic acid (7 ml), and maleic anhydride (3.0 g) were put into a 50-ml three-necked flask and magnetically stirred; the mixture was stirred for 20 min with power 450w. The solution was put into 5 ml glacial acetic acid and cooled, washed with hot water (10 ml), dried (MgSO4), and concentrated to dryness. Recrystallization from ethanol afforded the adduct (7.5 g, 50%).
Refinement
All H atoms were initially located in a difference Fourier map. The methyl H atoms were then constrained to an ideal geometry, with C—H distances of 0.96 Å and Uiso(H) = 1.5Ueq, but each group was allowed to rotate freely about its C—C bond. All other H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms,with C—H distances in the range 0.93–0.98 Å and Uiso(H) = 1.2Ueq(C); O—H = 0.93 Å and Uiso(H) = 1.2Ueq(O). In the absence of significant anomalous scattering effects, Friedel pairs were merged.
Figures
Fig. 1.
A view of the molecular structure of the title compound, showing displacement ellipsoids at the 30% probability level. Hydrogen atoms are drawn as spheres of arbitrary radius.
Fig. 2.
The packing of the title compound, viewed along the a axis. Dashed lines indicate hydrogen bonds.
Crystal data
| C24H32O5·C2H4O2 | Dx = 1.211 Mg m−3 |
| Mr = 460.55 | Melting point: 498 K |
| Orthorhombic, P212121 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 3636 reflections |
| a = 7.9469 (10) Å | θ = 2.3–23.2° |
| b = 12.7755 (16) Å | µ = 0.09 mm−1 |
| c = 24.884 (3) Å | T = 291 K |
| V = 2526.3 (5) Å3 | Block, colorless |
| Z = 4 | 0.30 × 0.26 × 0.24 mm |
| F(000) = 992 |
Data collection
| Bruker SMART APEX CCD diffractometer | 2837 independent reflections |
| Radiation source: sealed tube | 2432 reflections with I > 2σ(I) |
| graphite | Rint = 0.059 |
| φ and ω scans | θmax = 26.0°, θmin = 1.8° |
| Absorption correction: multi-scan (SADABS; Bruker, 2000) | h = −9→8 |
| Tmin = 0.97, Tmax = 0.98 | k = −15→15 |
| 13853 measured reflections | l = −27→30 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.047 | H-atom parameters constrained |
| wR(F2) = 0.110 | w = 1/[σ2(Fo2) + (0.05P)2 + 0.55P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.04 | (Δ/σ)max < 0.001 |
| 2837 reflections | Δρmax = 0.19 e Å−3 |
| 304 parameters | Δρmin = −0.17 e Å−3 |
| 0 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0076 (9) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. The Friedel pairs have been merged in the absence of anomalous scattering. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.5002 (4) | −0.1408 (3) | 0.21013 (13) | 0.0419 (7) | |
| C2 | 0.5980 (4) | −0.0411 (2) | 0.20070 (12) | 0.0398 (7) | |
| H2 | 0.6794 | −0.0301 | 0.2297 | 0.048* | |
| C3 | 0.6887 (4) | −0.0420 (2) | 0.14559 (13) | 0.0417 (7) | |
| H3 | 0.7696 | −0.0998 | 0.1434 | 0.050* | |
| C4 | 0.7802 (4) | 0.0670 (2) | 0.14272 (13) | 0.0426 (7) | |
| H4A | 0.8616 | 0.0723 | 0.1716 | 0.051* | |
| H4B | 0.8400 | 0.0730 | 0.1089 | 0.051* | |
| C5 | 0.6514 (4) | 0.1559 (2) | 0.14754 (14) | 0.0411 (7) | |
| H5 | 0.6683 | 0.1867 | 0.1832 | 0.049* | |
| C6 | 0.4713 (4) | 0.1086 (2) | 0.14794 (13) | 0.0414 (7) | |
| C7 | 0.4657 (4) | 0.0448 (2) | 0.20125 (12) | 0.0409 (7) | |
| H7 | 0.4851 | 0.0916 | 0.2318 | 0.049* | |
| C8 | 0.3020 (4) | −0.0147 (3) | 0.20917 (13) | 0.0442 (8) | |
| C9 | 0.4523 (4) | 0.0321 (3) | 0.10233 (12) | 0.0429 (7) | |
| H9 | 0.3687 | 0.0390 | 0.0764 | 0.052* | |
| C10 | 0.5648 (4) | −0.0472 (3) | 0.10145 (12) | 0.0453 (8) | |
| C11 | 0.5732 (5) | −0.1285 (3) | 0.05890 (15) | 0.0552 (10) | |
| H11 | 0.6773 | −0.1203 | 0.0383 | 0.066* | |
| C12 | 0.4230 (5) | −0.1334 (3) | 0.02054 (15) | 0.0553 (10) | |
| H12A | 0.3219 | −0.1455 | 0.0407 | 0.083* | |
| H12B | 0.4391 | −0.1895 | −0.0046 | 0.083* | |
| H12C | 0.4137 | −0.0683 | 0.0015 | 0.083* | |
| C13 | 0.5604 (5) | −0.2392 (3) | 0.07953 (13) | 0.0503 (9) | |
| H13A | 0.6437 | −0.2503 | 0.1069 | 0.075* | |
| H13B | 0.5788 | −0.2873 | 0.0505 | 0.075* | |
| H13C | 0.4504 | −0.2505 | 0.0944 | 0.075* | |
| C14 | 0.3351 (4) | 0.1953 (2) | 0.14874 (13) | 0.0434 (7) | |
| H14A | 0.2267 | 0.1642 | 0.1406 | 0.052* | |
| H14B | 0.3287 | 0.2247 | 0.1846 | 0.052* | |
| C15 | 0.3695 (4) | 0.2827 (2) | 0.10897 (13) | 0.0427 (8) | |
| H15A | 0.3630 | 0.2555 | 0.0726 | 0.051* | |
| H15B | 0.2849 | 0.3369 | 0.1128 | 0.051* | |
| C16 | 0.5419 (4) | 0.3288 (2) | 0.11854 (13) | 0.0409 (7) | |
| H16 | 0.5485 | 0.3405 | 0.1574 | 0.049* | |
| C17 | 0.5691 (4) | 0.4405 (3) | 0.09213 (12) | 0.0408 (7) | |
| C18 | 0.7484 (4) | 0.4797 (3) | 0.10755 (14) | 0.0442 (8) | |
| H18A | 0.7707 | 0.5453 | 0.0893 | 0.053* | |
| H18B | 0.7528 | 0.4925 | 0.1459 | 0.053* | |
| C19 | 0.8818 (4) | 0.4017 (3) | 0.09266 (15) | 0.0491 (9) | |
| H19A | 0.9912 | 0.4293 | 0.1026 | 0.059* | |
| H19B | 0.8809 | 0.3914 | 0.0540 | 0.059* | |
| C20 | 0.8550 (4) | 0.2952 (3) | 0.12078 (14) | 0.0450 (8) | |
| H20A | 0.8616 | 0.3047 | 0.1594 | 0.054* | |
| H20B | 0.9437 | 0.2474 | 0.1102 | 0.054* | |
| C21 | 0.6810 (4) | 0.2471 (2) | 0.10598 (12) | 0.0405 (7) | |
| C22 | 0.6830 (4) | 0.2061 (3) | 0.04687 (12) | 0.0429 (7) | |
| H22A | 0.5701 | 0.1906 | 0.0357 | 0.064* | |
| H22B | 0.7501 | 0.1437 | 0.0449 | 0.064* | |
| H22C | 0.7300 | 0.2587 | 0.0238 | 0.064* | |
| C23 | 0.5378 (5) | 0.4443 (3) | 0.03186 (12) | 0.0445 (7) | |
| H23A | 0.4192 | 0.4508 | 0.0252 | 0.067* | |
| H23B | 0.5789 | 0.3812 | 0.0156 | 0.067* | |
| H23C | 0.5955 | 0.5034 | 0.0168 | 0.067* | |
| C24 | 0.4419 (4) | 0.5116 (3) | 0.11930 (13) | 0.0426 (7) | |
| C25 | 0.1024 (4) | 0.6778 (3) | 0.18987 (14) | 0.0460 (8) | |
| C26 | −0.0272 (4) | 0.7410 (3) | 0.21888 (13) | 0.0484 (8) | |
| H26A | −0.0330 | 0.7187 | 0.2557 | 0.073* | |
| H26B | −0.1348 | 0.7313 | 0.2021 | 0.073* | |
| H26C | 0.0031 | 0.8137 | 0.2175 | 0.073* | |
| O1 | 0.5518 (3) | −0.22935 (16) | 0.21417 (9) | 0.0463 (6) | |
| O2 | 0.3332 (3) | −0.12062 (18) | 0.21447 (10) | 0.0472 (6) | |
| O3 | 0.1594 (3) | 0.01506 (17) | 0.21128 (9) | 0.0453 (5) | |
| O4 | 0.4493 (3) | 0.51827 (17) | 0.17031 (8) | 0.0455 (6) | |
| H4C | 0.3728 | 0.5602 | 0.1887 | 0.055* | |
| O5 | 0.3379 (3) | 0.55953 (19) | 0.09249 (8) | 0.0472 (6) | |
| O6 | 0.2043 (3) | 0.62457 (17) | 0.21453 (9) | 0.0465 (6) | |
| O7 | 0.1034 (3) | 0.68154 (18) | 0.13804 (9) | 0.0462 (6) | |
| H7A | 0.1831 | 0.6430 | 0.1191 | 0.055* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0453 (18) | 0.0411 (16) | 0.0395 (17) | 0.0089 (14) | −0.0018 (14) | 0.0098 (14) |
| C2 | 0.0398 (17) | 0.0409 (17) | 0.0389 (16) | 0.0087 (14) | 0.0007 (13) | −0.0023 (13) |
| C3 | 0.0443 (17) | 0.0377 (15) | 0.0431 (17) | 0.0077 (14) | 0.0041 (15) | 0.0005 (13) |
| C4 | 0.0427 (17) | 0.0423 (17) | 0.0428 (17) | 0.0049 (14) | 0.0031 (14) | 0.0012 (14) |
| C5 | 0.0417 (17) | 0.0386 (16) | 0.0430 (17) | 0.0036 (13) | 0.0036 (15) | −0.0003 (13) |
| C6 | 0.0400 (16) | 0.0405 (16) | 0.0437 (17) | 0.0004 (13) | −0.0006 (15) | −0.0001 (14) |
| C7 | 0.0403 (17) | 0.0403 (16) | 0.0420 (17) | 0.0049 (14) | −0.0029 (14) | −0.0019 (13) |
| C8 | 0.0434 (18) | 0.0452 (18) | 0.0441 (18) | 0.0096 (15) | 0.0095 (15) | 0.0081 (14) |
| C9 | 0.0470 (17) | 0.0430 (17) | 0.0388 (16) | 0.0055 (15) | −0.0113 (15) | −0.0024 (13) |
| C10 | 0.0439 (18) | 0.0488 (19) | 0.0433 (17) | 0.0118 (15) | 0.0043 (15) | −0.0102 (14) |
| C11 | 0.067 (2) | 0.0469 (19) | 0.051 (2) | 0.0168 (19) | −0.0152 (19) | −0.0133 (16) |
| C12 | 0.059 (2) | 0.050 (2) | 0.057 (2) | 0.0163 (18) | −0.0149 (19) | −0.0216 (17) |
| C13 | 0.0488 (19) | 0.056 (2) | 0.0461 (19) | −0.0147 (17) | 0.0107 (16) | −0.0149 (15) |
| C14 | 0.0414 (17) | 0.0470 (17) | 0.0418 (17) | 0.0044 (15) | −0.0055 (15) | 0.0019 (14) |
| C15 | 0.0506 (19) | 0.0365 (16) | 0.0411 (17) | 0.0070 (15) | −0.0127 (15) | −0.0028 (13) |
| C16 | 0.0417 (17) | 0.0392 (16) | 0.0418 (17) | 0.0102 (14) | 0.0007 (14) | 0.0027 (13) |
| C17 | 0.0387 (16) | 0.0441 (17) | 0.0397 (15) | 0.0031 (14) | 0.0028 (14) | 0.0037 (14) |
| C18 | 0.0451 (17) | 0.0406 (18) | 0.0469 (18) | −0.0079 (15) | 0.0056 (15) | 0.0080 (14) |
| C19 | 0.0444 (18) | 0.051 (2) | 0.0522 (19) | −0.0018 (15) | 0.0170 (16) | 0.0135 (16) |
| C20 | 0.0445 (18) | 0.0445 (18) | 0.0459 (18) | −0.0015 (15) | 0.0029 (15) | 0.0114 (15) |
| C21 | 0.0416 (17) | 0.0394 (16) | 0.0406 (16) | 0.0058 (14) | 0.0125 (14) | 0.0013 (13) |
| C22 | 0.0426 (17) | 0.0441 (17) | 0.0420 (17) | 0.0118 (15) | 0.0126 (14) | −0.0080 (14) |
| C23 | 0.0463 (18) | 0.0417 (17) | 0.0456 (17) | 0.0112 (15) | 0.0012 (15) | 0.0056 (14) |
| C24 | 0.0422 (17) | 0.0411 (17) | 0.0443 (17) | 0.0118 (15) | −0.0131 (14) | −0.0023 (13) |
| C25 | 0.0419 (18) | 0.0466 (18) | 0.0496 (19) | 0.0143 (15) | −0.0064 (15) | 0.0150 (15) |
| C26 | 0.0450 (18) | 0.0536 (19) | 0.0468 (18) | 0.0114 (16) | 0.0139 (16) | 0.0172 (16) |
| O1 | 0.0498 (13) | 0.0413 (12) | 0.0480 (12) | 0.0178 (11) | 0.0177 (12) | 0.0161 (10) |
| O2 | 0.0437 (13) | 0.0468 (12) | 0.0511 (13) | 0.0058 (11) | 0.0054 (12) | 0.0177 (10) |
| O3 | 0.0417 (13) | 0.0465 (12) | 0.0477 (12) | 0.0104 (11) | 0.0100 (11) | 0.0107 (10) |
| O4 | 0.0480 (13) | 0.0462 (12) | 0.0422 (12) | 0.0174 (11) | −0.0129 (11) | −0.0074 (9) |
| O5 | 0.0502 (13) | 0.0539 (14) | 0.0376 (11) | 0.0186 (11) | −0.0092 (11) | 0.0108 (10) |
| O6 | 0.0465 (13) | 0.0495 (13) | 0.0436 (12) | 0.0148 (11) | −0.0067 (11) | 0.0144 (10) |
| O7 | 0.0442 (13) | 0.0469 (12) | 0.0475 (13) | 0.0169 (10) | −0.0119 (10) | 0.0153 (11) |
Geometric parameters (Å, °)
| C1—O1 | 1.207 (4) | C14—H14B | 0.9700 |
| C1—O2 | 1.356 (4) | C15—C16 | 1.510 (5) |
| C1—C2 | 1.511 (5) | C15—H15A | 0.9700 |
| C2—C7 | 1.520 (4) | C15—H15B | 0.9700 |
| C2—C3 | 1.550 (4) | C16—C21 | 1.552 (4) |
| C2—H2 | 0.9800 | C16—C17 | 1.586 (4) |
| C3—C10 | 1.477 (5) | C16—H16 | 0.9800 |
| C3—C4 | 1.573 (4) | C17—C24 | 1.518 (5) |
| C3—H3 | 0.9800 | C17—C23 | 1.521 (4) |
| C4—C5 | 1.533 (4) | C17—C18 | 1.558 (5) |
| C4—H4A | 0.9700 | C18—C19 | 1.501 (5) |
| C4—H4B | 0.9700 | C18—H18A | 0.9700 |
| C5—C6 | 1.553 (4) | C18—H18B | 0.9700 |
| C5—C21 | 1.576 (4) | C19—C20 | 1.544 (4) |
| C5—H5 | 0.9800 | C19—H19A | 0.9700 |
| C6—C9 | 1.505 (4) | C19—H19B | 0.9700 |
| C6—C14 | 1.549 (4) | C20—C21 | 1.557 (5) |
| C6—C7 | 1.557 (4) | C20—H20A | 0.9700 |
| C7—C8 | 1.519 (5) | C20—H20B | 0.9700 |
| C7—H7 | 0.9800 | C21—C22 | 1.561 (4) |
| C8—O3 | 1.197 (4) | C22—H22A | 0.9600 |
| C8—O2 | 1.382 (4) | C22—H22B | 0.9600 |
| C9—C10 | 1.351 (5) | C22—H22C | 0.9600 |
| C9—H9 | 0.9300 | C23—H23A | 0.9600 |
| C10—C11 | 1.485 (4) | C23—H23B | 0.9600 |
| C11—C13 | 1.507 (5) | C23—H23C | 0.9600 |
| C11—C12 | 1.529 (5) | C24—O5 | 1.226 (4) |
| C11—H11 | 0.9800 | C24—O4 | 1.274 (4) |
| C12—H12A | 0.9600 | C25—O6 | 1.223 (4) |
| C12—H12B | 0.9600 | C25—O7 | 1.291 (4) |
| C12—H12C | 0.9600 | C25—C26 | 1.494 (5) |
| C13—H13A | 0.9600 | C26—H26A | 0.9600 |
| C13—H13B | 0.9600 | C26—H26B | 0.9600 |
| C13—H13C | 0.9600 | C26—H26C | 0.9600 |
| C14—C15 | 1.517 (4) | O4—H4C | 0.9300 |
| C14—H14A | 0.9700 | O7—H7A | 0.9300 |
| O1—C1—O2 | 120.3 (3) | H14A—C14—H14B | 107.8 |
| O1—C1—C2 | 128.9 (3) | C16—C15—C14 | 110.3 (3) |
| O2—C1—C2 | 110.8 (3) | C16—C15—H15A | 109.6 |
| C1—C2—C7 | 104.6 (3) | C14—C15—H15A | 109.6 |
| C1—C2—C3 | 111.8 (3) | C16—C15—H15B | 109.6 |
| C7—C2—C3 | 109.6 (2) | C14—C15—H15B | 109.6 |
| C1—C2—H2 | 110.3 | H15A—C15—H15B | 108.1 |
| C7—C2—H2 | 110.3 | C15—C16—C21 | 110.7 (3) |
| C3—C2—H2 | 110.3 | C15—C16—C17 | 114.1 (3) |
| C10—C3—C2 | 110.4 (3) | C21—C16—C17 | 115.1 (3) |
| C10—C3—C4 | 108.3 (3) | C15—C16—H16 | 105.3 |
| C2—C3—C4 | 104.4 (3) | C21—C16—H16 | 105.3 |
| C10—C3—H3 | 111.2 | C17—C16—H16 | 105.3 |
| C2—C3—H3 | 111.2 | C24—C17—C23 | 108.1 (3) |
| C4—C3—H3 | 111.2 | C24—C17—C18 | 107.9 (3) |
| C5—C4—C3 | 110.1 (3) | C23—C17—C18 | 112.5 (3) |
| C5—C4—H4A | 109.6 | C24—C17—C16 | 105.3 (3) |
| C3—C4—H4A | 109.6 | C23—C17—C16 | 114.5 (3) |
| C5—C4—H4B | 109.6 | C18—C17—C16 | 108.2 (3) |
| C3—C4—H4B | 109.6 | C19—C18—C17 | 111.8 (3) |
| H4A—C4—H4B | 108.2 | C19—C18—H18A | 109.2 |
| C4—C5—C6 | 109.1 (2) | C17—C18—H18A | 109.2 |
| C4—C5—C21 | 113.4 (3) | C19—C18—H18B | 109.2 |
| C6—C5—C21 | 115.4 (3) | C17—C18—H18B | 109.2 |
| C4—C5—H5 | 106.1 | H18A—C18—H18B | 107.9 |
| C6—C5—H5 | 106.1 | C18—C19—C20 | 112.0 (3) |
| C21—C5—H5 | 106.1 | C18—C19—H19A | 109.2 |
| C9—C6—C14 | 113.8 (3) | C20—C19—H19A | 109.2 |
| C9—C6—C5 | 109.9 (3) | C18—C19—H19B | 109.2 |
| C14—C6—C5 | 111.5 (2) | C20—C19—H19B | 109.2 |
| C9—C6—C7 | 107.5 (3) | H19A—C19—H19B | 107.9 |
| C14—C6—C7 | 110.1 (3) | C19—C20—C21 | 111.3 (3) |
| C5—C6—C7 | 103.6 (3) | C19—C20—H20A | 109.4 |
| C8—C7—C2 | 103.4 (2) | C21—C20—H20A | 109.4 |
| C8—C7—C6 | 113.4 (3) | C19—C20—H20B | 109.4 |
| C2—C7—C6 | 110.5 (3) | C21—C20—H20B | 109.4 |
| C8—C7—H7 | 109.8 | H20A—C20—H20B | 108.0 |
| C2—C7—H7 | 109.8 | C16—C21—C20 | 108.6 (3) |
| C6—C7—H7 | 109.8 | C16—C21—C22 | 115.0 (3) |
| O3—C8—O2 | 118.4 (3) | C20—C21—C22 | 110.3 (3) |
| O3—C8—C7 | 131.1 (3) | C16—C21—C5 | 105.0 (2) |
| O2—C8—C7 | 110.4 (3) | C20—C21—C5 | 105.6 (3) |
| C10—C9—C6 | 115.6 (3) | C22—C21—C5 | 111.8 (3) |
| C10—C9—H9 | 122.2 | C21—C22—H22A | 109.5 |
| C6—C9—H9 | 122.2 | C21—C22—H22B | 109.5 |
| C9—C10—C3 | 113.3 (3) | H22A—C22—H22B | 109.5 |
| C9—C10—C11 | 124.5 (3) | C21—C22—H22C | 109.5 |
| C3—C10—C11 | 122.1 (3) | H22A—C22—H22C | 109.5 |
| C10—C11—C13 | 114.2 (3) | H22B—C22—H22C | 109.5 |
| C10—C11—C12 | 116.0 (3) | C17—C23—H23A | 109.5 |
| C13—C11—C12 | 97.0 (3) | C17—C23—H23B | 109.5 |
| C10—C11—H11 | 109.7 | H23A—C23—H23B | 109.5 |
| C13—C11—H11 | 109.7 | C17—C23—H23C | 109.5 |
| C12—C11—H11 | 109.7 | H23A—C23—H23C | 109.5 |
| C11—C12—H12A | 109.5 | H23B—C23—H23C | 109.5 |
| C11—C12—H12B | 109.5 | O5—C24—O4 | 122.7 (3) |
| H12A—C12—H12B | 109.5 | O5—C24—C17 | 120.4 (3) |
| C11—C12—H12C | 109.5 | O4—C24—C17 | 116.9 (3) |
| H12A—C12—H12C | 109.5 | O6—C25—O7 | 121.2 (3) |
| H12B—C12—H12C | 109.5 | O6—C25—C26 | 121.0 (3) |
| C11—C13—H13A | 109.5 | O7—C25—C26 | 117.9 (3) |
| C11—C13—H13B | 109.5 | C25—C26—H26A | 109.5 |
| H13A—C13—H13B | 109.5 | C25—C26—H26B | 109.5 |
| C11—C13—H13C | 109.5 | H26A—C26—H26B | 109.5 |
| H13A—C13—H13C | 109.5 | C25—C26—H26C | 109.5 |
| H13B—C13—H13C | 109.5 | H26A—C26—H26C | 109.5 |
| C15—C14—C6 | 113.1 (3) | H26B—C26—H26C | 109.5 |
| C15—C14—H14A | 109.0 | C1—O2—C8 | 110.7 (3) |
| C6—C14—H14A | 109.0 | C24—O4—H4C | 119.9 |
| C15—C14—H14B | 109.0 | C25—O7—H7A | 119.5 |
| C6—C14—H14B | 109.0 | ||
| O1—C1—C2—C7 | −178.2 (3) | C3—C10—C11—C12 | 171.8 (3) |
| O2—C1—C2—C7 | 1.4 (4) | C9—C6—C14—C15 | −79.4 (3) |
| O1—C1—C2—C3 | 63.3 (5) | C5—C6—C14—C15 | 45.5 (4) |
| O2—C1—C2—C3 | −117.1 (3) | C7—C6—C14—C15 | 159.9 (3) |
| C1—C2—C3—C10 | 63.1 (3) | C6—C14—C15—C16 | −55.4 (4) |
| C7—C2—C3—C10 | −52.4 (3) | C14—C15—C16—C21 | 66.0 (3) |
| C1—C2—C3—C4 | 179.3 (3) | C14—C15—C16—C17 | −162.3 (3) |
| C7—C2—C3—C4 | 63.8 (3) | C15—C16—C17—C24 | 62.4 (3) |
| C10—C3—C4—C5 | 58.8 (3) | C21—C16—C17—C24 | −168.1 (3) |
| C2—C3—C4—C5 | −58.8 (3) | C15—C16—C17—C23 | −56.2 (4) |
| C3—C4—C5—C6 | −5.6 (4) | C21—C16—C17—C23 | 73.3 (4) |
| C3—C4—C5—C21 | −135.8 (3) | C15—C16—C17—C18 | 177.5 (3) |
| C4—C5—C6—C9 | −48.9 (3) | C21—C16—C17—C18 | −53.0 (3) |
| C21—C5—C6—C9 | 80.1 (3) | C24—C17—C18—C19 | 167.7 (3) |
| C4—C5—C6—C14 | −176.0 (3) | C23—C17—C18—C19 | −73.2 (4) |
| C21—C5—C6—C14 | −47.0 (4) | C16—C17—C18—C19 | 54.3 (3) |
| C4—C5—C6—C7 | 65.7 (3) | C17—C18—C19—C20 | −59.5 (4) |
| C21—C5—C6—C7 | −165.3 (3) | C18—C19—C20—C21 | 59.2 (4) |
| C1—C2—C7—C8 | −1.8 (3) | C15—C16—C21—C20 | −175.5 (3) |
| C3—C2—C7—C8 | 118.1 (3) | C17—C16—C21—C20 | 53.2 (3) |
| C1—C2—C7—C6 | −123.5 (3) | C15—C16—C21—C22 | 60.3 (4) |
| C3—C2—C7—C6 | −3.5 (4) | C17—C16—C21—C22 | −70.9 (4) |
| C9—C6—C7—C8 | −60.5 (3) | C15—C16—C21—C5 | −62.9 (3) |
| C14—C6—C7—C8 | 64.0 (3) | C17—C16—C21—C5 | 165.8 (3) |
| C5—C6—C7—C8 | −176.7 (2) | C19—C20—C21—C16 | −54.0 (3) |
| C9—C6—C7—C2 | 55.1 (3) | C19—C20—C21—C22 | 72.8 (3) |
| C14—C6—C7—C2 | 179.5 (3) | C19—C20—C21—C5 | −166.2 (3) |
| C5—C6—C7—C2 | −61.2 (3) | C4—C5—C21—C16 | −178.6 (3) |
| C2—C7—C8—O3 | −178.1 (4) | C6—C5—C21—C16 | 54.4 (3) |
| C6—C7—C8—O3 | −58.4 (5) | C4—C5—C21—C20 | −63.9 (3) |
| C2—C7—C8—O2 | 1.8 (3) | C6—C5—C21—C20 | 169.2 (3) |
| C6—C7—C8—O2 | 121.5 (3) | C4—C5—C21—C22 | 56.1 (4) |
| C14—C6—C9—C10 | −177.5 (3) | C6—C5—C21—C22 | −70.9 (3) |
| C5—C6—C9—C10 | 56.7 (4) | C23—C17—C24—O5 | 0.2 (4) |
| C7—C6—C9—C10 | −55.3 (4) | C18—C17—C24—O5 | 122.0 (3) |
| C6—C9—C10—C3 | −1.2 (4) | C16—C17—C24—O5 | −122.7 (3) |
| C6—C9—C10—C11 | −177.3 (3) | C23—C17—C24—O4 | −180.0 (3) |
| C2—C3—C10—C9 | 57.3 (4) | C18—C17—C24—O4 | −58.1 (4) |
| C4—C3—C10—C9 | −56.4 (4) | C16—C17—C24—O4 | 57.2 (4) |
| C2—C3—C10—C11 | −126.5 (3) | O1—C1—O2—C8 | 179.4 (3) |
| C4—C3—C10—C11 | 119.8 (4) | C2—C1—O2—C8 | −0.2 (4) |
| C9—C10—C11—C13 | −124.0 (4) | O3—C8—O2—C1 | 178.9 (3) |
| C3—C10—C11—C13 | 60.1 (5) | C7—C8—O2—C1 | −1.1 (4) |
| C9—C10—C11—C12 | −12.4 (6) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O4—H4C···O6 | 0.93 | 1.70 | 2.617 (3) | 169 |
| O7—H7A···O5 | 0.93 | 1.76 | 2.681 (3) | 171 |
| C13—H13C···O5i | 0.96 | 2.59 | 3.137 (5) | 117 |
| C26—H26B···O1ii | 0.96 | 2.56 | 3.369 (4) | 142 |
Symmetry codes: (i) x, y−1, z; (ii) x−1, y+1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: WN2334).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Bruker (2000). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- McCoy, M. (2000). Chem. Eng. News, 78, 13–15.
- Pan, Y.-M., Yang, L., Wang, H.-S., Zhao, Z.-C. & Zhang, Y. (2006). Acta Cryst. E62, o5701–o5703.
- Savluchinske-Feio, S., Nunes, L., Pereira, P. T., Silva, A. M., Roseiro, J. C., Gigante, B. & Curto, M. J. M. (2007). J. Microbiol. Methods, 70, 465–470. [DOI] [PubMed]
- Schweizer, R. A. S., Atanasoc, A. G., Frey, B. M. & Odermatt, A. (2003). Mol. Cell. Endocrinol.212, 41–49. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809023745/wn2334sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809023745/wn2334Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




