Abstract
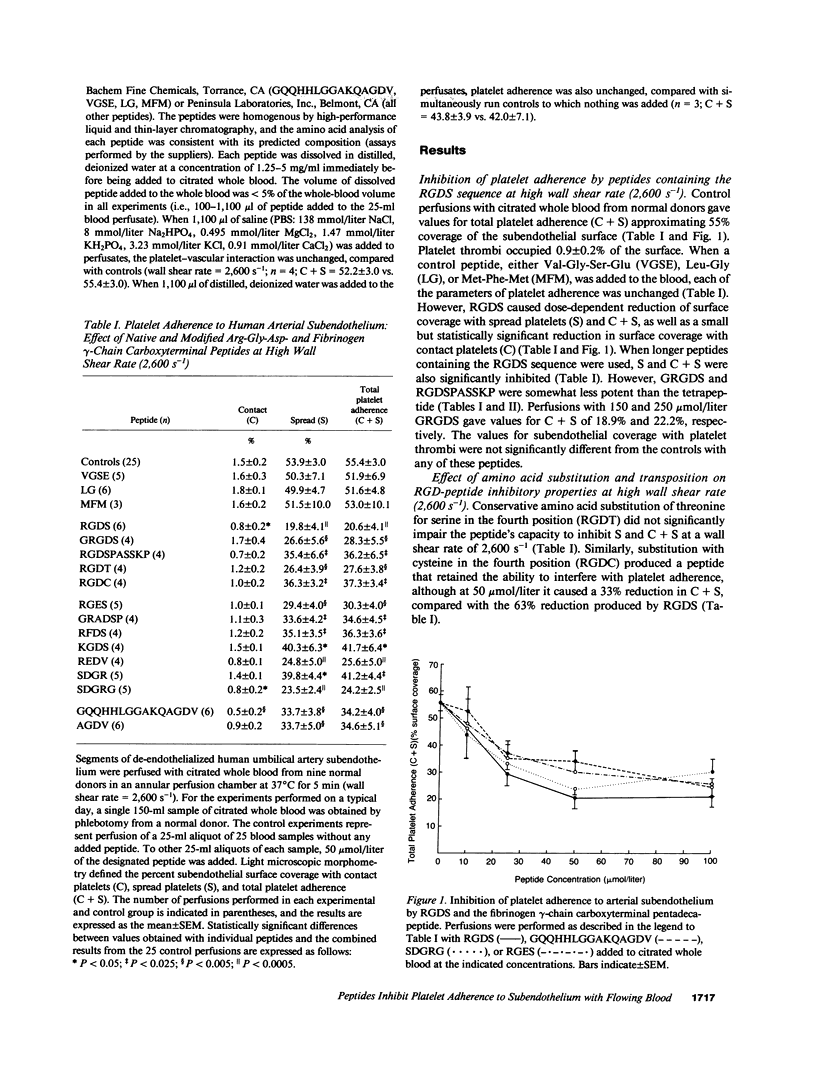
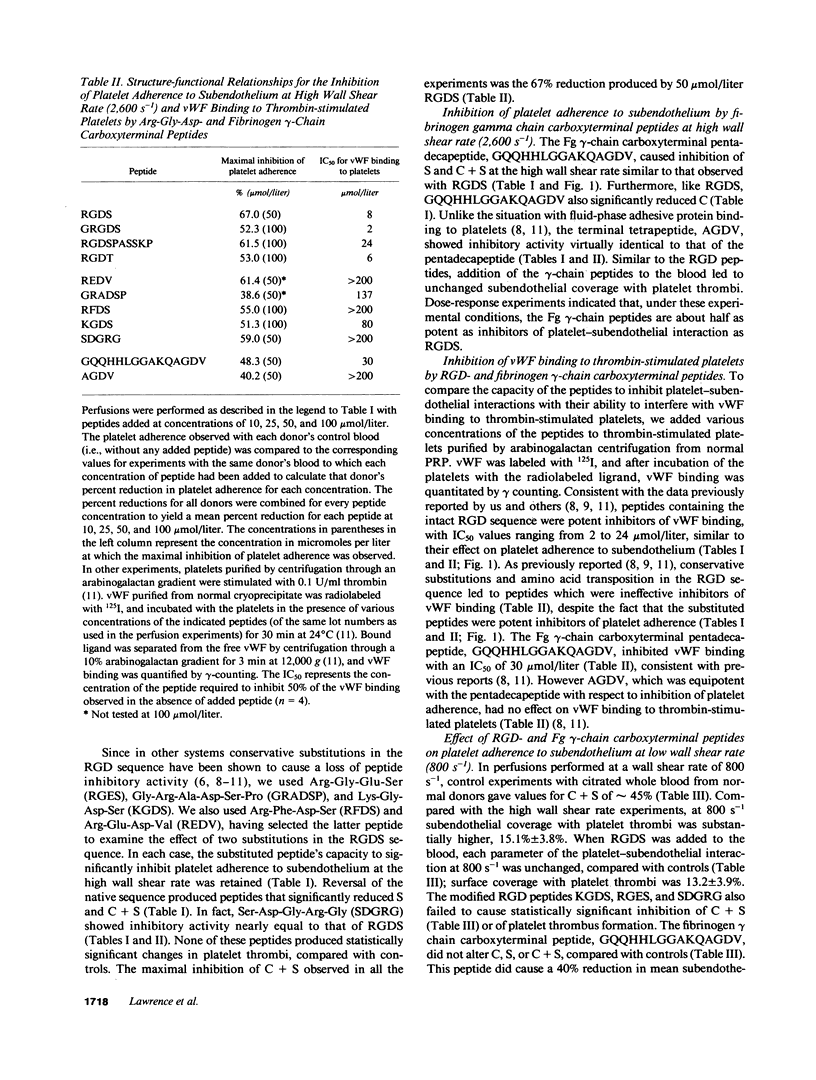
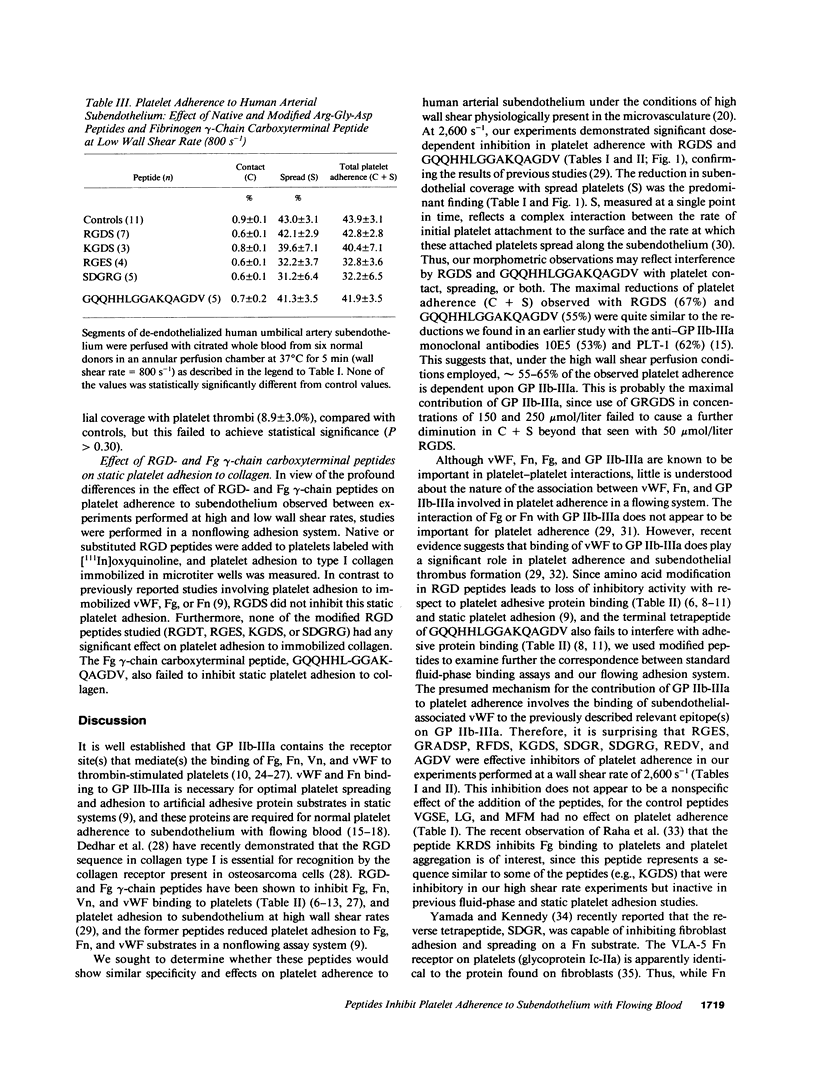
Arg-Gly-Asp (RGD)- and fibrinogen gamma-chain carboxyterminal (GQQHHLGGAKQAGDV) peptides inhibit fibrinogen, fibronectin (Fn), vitronectin, and von Willebrand factor (vWF) binding to the platelet glycoprotein IIb-IIIa complex (GP IIb-IIIa). GP IIb-IIIa, vWF, and Fn are essential for normal platelet adherence to subendothelium. We added peptides to normal citrated whole blood before perfusion over human umbilical artery subendothelium and evaluated platelet adherence morphometrically at high (2,600 s-1) and low (800 s-1) wall shear rates. We also examined the effects of the peptides on platelet adhesion to collagen in a static system. At the high wall shear rate, RGDS and GQQHHLGGAKQAGDV caused dose-dependent reduction in the surface coverage with spread and adherent platelets. Amino acid transposition and conservative substitutions of RGD peptides and the AGDV peptide significantly inhibited platelet adherence at 2,600 s-1. By contrast, the modified RGD peptides and AGDV do not affect adhesive protein binding to platelets. None of the native or modified RGD- or fibrinogen gamma-chain peptides significantly inhibited either platelet adherence to subendothelium at 800 s-1 or platelet adhesion to collagen. Our findings demonstrate that peptides that interfere with adhesive protein binding to GP IIb-IIIa inhibit platelet adherence to vascular subendothelium with flowing blood only at high wall shear rates. Platelet adherence to subendothelium at high wall shear rates appears to be mediated by different recognition specificities from those required for fluid-phase adhesive protein binding or static platelet adhesion.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedhar S., Ruoslahti E., Pierschbacher M. D. A cell surface receptor complex for collagen type I recognizes the Arg-Gly-Asp sequence. J Cell Biol. 1987 Mar;104(3):585–593. doi: 10.1083/jcb.104.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Watt K. W., Cottrell B. A., Strong D. D., Riley M. The amino acid sequence of the alpha-chain of human fibrinogen. Nature. 1979 Aug 9;280(5722):464–468. doi: 10.1038/280464a0. [DOI] [PubMed] [Google Scholar]
- Fressinaud E., Baruch D., Girma J. P., Sakariassen K. S., Baumgartner H. R., Meyer D. von Willebrand factor-mediated platelet adhesion to collagen involves platelet membrane glycoprotein IIb-IIIa as well as glycoprotein Ib. J Lab Clin Med. 1988 Jul;112(1):58–67. [PubMed] [Google Scholar]
- Gartner T. K., Bennett J. S. The tetrapeptide analogue of the cell attachment site of fibronectin inhibits platelet aggregation and fibrinogen binding to activated platelets. J Biol Chem. 1985 Oct 5;260(22):11891–11894. [PubMed] [Google Scholar]
- Ginsberg M. H., Forsyth J., Lightsey A., Chediak J., Plow E. F. Reduced surface expression and binding of fibronectin by thrombin-stimulated thrombasthenic platelets. J Clin Invest. 1983 Mar;71(3):619–624. doi: 10.1172/JCI110808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M., Pierschbacher M. D., Ruoslahti E., Marguerie G., Plow E. Inhibition of fibronectin binding to platelets by proteolytic fragments and synthetic peptides which support fibroblast adhesion. J Biol Chem. 1985 Apr 10;260(7):3931–3936. [PubMed] [Google Scholar]
- Haverstick D. M., Cowan J. F., Yamada K. M., Santoro S. A. Inhibition of platelet adhesion to fibronectin, fibrinogen, and von Willebrand factor substrates by a synthetic tetrapeptide derived from the cell-binding domain of fibronectin. Blood. 1985 Oct;66(4):946–952. [PubMed] [Google Scholar]
- Hayman E. G., Pierschbacher M. D., Ruoslahti E. Detachment of cells from culture substrate by soluble fibronectin peptides. J Cell Biol. 1985 Jun;100(6):1948–1954. doi: 10.1083/jcb.100.6.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E., Crouse C., Takada Y., Sonnenberg A. Multiple very late antigen (VLA) heterodimers on platelets. Evidence for distinct VLA-2, VLA-5 (fibronectin receptor), and VLA-6 structures. J Biol Chem. 1988 Jun 5;263(16):7660–7665. [PubMed] [Google Scholar]
- Houdijk W. P., Sixma J. J. Fibronectin in artery subendothelium is important for platelet adhesion. Blood. 1985 Mar;65(3):598–604. [PubMed] [Google Scholar]
- Kessler C. M., Floyd C. M., Rick M. E., Krizek D. M., Lee S. L., Gralnick H. R. Collagen-factor VIII/von Willebrand factor protein interaction. Blood. 1984 Jun;63(6):1291–1298. [PubMed] [Google Scholar]
- Kloczewiak M., Timmons S., Lukas T. J., Hawiger J. Platelet receptor recognition site on human fibrinogen. Synthesis and structure-function relationship of peptides corresponding to the carboxy-terminal segment of the gamma chain. Biochemistry. 1984 Apr 10;23(8):1767–1774. doi: 10.1021/bi00303a028. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Gralnick H. R. Monoclonal antibodies to the glycoprotein IIb-IIIa epitopes involved in adhesive protein binding: effects on platelet spreading and ultrastructure on human arterial subendothelium. J Lab Clin Med. 1987 Apr;109(4):495–503. [PubMed] [Google Scholar]
- Nieuwenhuis H. K., Akkerman J. W., Houdijk W. P., Sixma J. J. Human blood platelets showing no response to collagen fail to express surface glycoprotein Ia. Nature. 1985 Dec 5;318(6045):470–472. doi: 10.1038/318470a0. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis H. K., Sakariassen K. S., Houdijk W. P., Nievelstein P. F., Sixma J. J. Deficiency of platelet membrane glycoprotein Ia associated with a decreased platelet adhesion to subendothelium: a defect in platelet spreading. Blood. 1986 Sep;68(3):692–695. [PubMed] [Google Scholar]
- Nievelstein P. F., Sixma J. J. Glycoprotein IIb-IIIa and RGD(S) are not important for fibronectin-dependent platelet adhesion under flow conditions. Blood. 1988 Jul;72(1):82–88. [PubMed] [Google Scholar]
- Parise L. V., Helgerson S. L., Steiner B., Nannizzi L., Phillips D. R. Synthetic peptides derived from fibrinogen and fibronectin change the conformation of purified platelet glycoprotein IIb-IIIa. J Biol Chem. 1987 Sep 15;262(26):12597–12602. [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Influence of stereochemistry of the sequence Arg-Gly-Asp-Xaa on binding specificity in cell adhesion. J Biol Chem. 1987 Dec 25;262(36):17294–17298. [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5985–5988. doi: 10.1073/pnas.81.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowicz R. S., Orchekowski R. P., Nugent D. J., Yamada K. Y., Kunicki T. J. Glycoprotein Ic-IIa functions as an activation-independent fibronectin receptor on human platelets. J Cell Biol. 1988 Apr;106(4):1359–1364. doi: 10.1083/jcb.106.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischel K. D., Bluestein H. G., Woods V. L., Jr Platelet glycoproteins Ia, Ic, and IIa are physicochemically indistinguishable from the very late activation antigens adhesion-related proteins of lymphocytes and other cell types. J Clin Invest. 1988 Feb;81(2):505–513. doi: 10.1172/JCI113348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischel K. D., Hemler M. E., Huang C., Bluestein H. G., Woods V. L., Jr Use of the monoclonal antibody 12F1 to characterize the differentiation antigen VLA-2. J Immunol. 1987 Jan 1;138(1):226–233. [PubMed] [Google Scholar]
- Plow E. F., Pierschbacher M. D., Ruoslahti E., Marguerie G. A., Ginsberg M. H. The effect of Arg-Gly-Asp-containing peptides on fibrinogen and von Willebrand factor binding to platelets. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8057–8061. doi: 10.1073/pnas.82.23.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., Srouji A. H., Meyer D., Marguerie G., Ginsberg M. H. Evidence that three adhesive proteins interact with a common recognition site on activated platelets. J Biol Chem. 1984 May 10;259(9):5388–5391. [PubMed] [Google Scholar]
- Raha S., Dosquet C., Abgrall J. F., Jolles P., Fiat A. M., Caen J. P. KRDS--a tetrapeptide derived from lactotransferrin--inhibits binding of monoclonal antibody against glycoprotein IIb-IIIa on ADP-stimulated platelets and megakaryocytes. Blood. 1988 Jul;72(1):172–178. [PubMed] [Google Scholar]
- Ruggeri Z. M., Bader R., de Marco L. Glanzmann thrombasthenia: deficient binding of von Willebrand factor to thrombin-stimulated platelets. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6038–6041. doi: 10.1073/pnas.79.19.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Sadler J. E., Shelton-Inloes B. B., Sorace J. M., Harlan J. M., Titani K., Davie E. W. Cloning and characterization of two cDNAs coding for human von Willebrand factor. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6394–6398. doi: 10.1073/pnas.82.19.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakariassen K. S., Bolhuis P. A., Sixma J. J. Human blood platelet adhesion to artery subendothelium is mediated by factor VIII-Von Willebrand factor bound to the subendothelium. Nature. 1979 Jun 14;279(5714):636–638. doi: 10.1038/279636a0. [DOI] [PubMed] [Google Scholar]
- Staatz W. D., Rajpara S. M., Wayner E. A., Carter W. G., Santoro S. A. The membrane glycoprotein Ia-IIa (VLA-2) complex mediates the Mg++-dependent adhesion of platelets to collagen. J Cell Biol. 1989 May;108(5):1917–1924. doi: 10.1083/jcb.108.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Oldberg A., Hayman E. G., Pierschbacher M. D., Ruoslahti E. Complete amino acid sequence of human vitronectin deduced from cDNA. Similarity of cell attachment sites in vitronectin and fibronectin. EMBO J. 1985 Oct;4(10):2519–2524. doi: 10.1002/j.1460-2075.1985.tb03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Huang C., Hemler M. E. Fibronectin receptor structures in the VLA family of heterodimers. Nature. 1987 Apr 9;326(6113):607–609. doi: 10.1038/326607a0. [DOI] [PubMed] [Google Scholar]
- Thiagarajan P., Kelly K. L. Exposure of binding sites for vitronectin on platelets following stimulation. J Biol Chem. 1988 Feb 25;263(6):3035–3038. [PubMed] [Google Scholar]
- Turitto V. T., Baumgartner H. R. Platelet interaction with subendothelium in flowing rabbit blood: effect of blood shear rate. Microvasc Res. 1979 Jan;17(1):38–54. doi: 10.1016/0026-2862(79)90006-2. [DOI] [PubMed] [Google Scholar]
- Turitto V. T., Weiss H. J., Baumgartner H. R. Decreased platelet adhesion on vessel segments in von Willebrand's disease: a defect in initial platelet attachment. J Lab Clin Med. 1983 Oct;102(4):551–564. [PubMed] [Google Scholar]
- Weiss H. J., Hawiger J., Ruggeri Z. M., Turitto V. T., Thiagarajan P., Hoffmann T. Fibrinogen-independent platelet adhesion and thrombus formation on subendothelium mediated by glycoprotein IIb-IIIa complex at high shear rate. J Clin Invest. 1989 Jan;83(1):288–297. doi: 10.1172/JCI113871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss H. J., Turitto V. T., Baumgartner H. R. Platelet adhesion and thrombus formation on subendothelium in platelets deficient in glycoproteins IIb-IIIa, Ib, and storage granules. Blood. 1986 Feb;67(2):322–330. [PubMed] [Google Scholar]
- Williams S., Gralnick H. Inhibition of von Willebrand factor binding to platelets by two recognition site peptides: the pentadecapeptide of the carboxy terminus of the fibrinogen gamma chain and the tetrapeptide arg-gly-asp-ser. Thromb Res. 1987 May 1;46(3):457–471. doi: 10.1016/0049-3848(87)90133-2. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W. Amino acid sequence specificities of an adhesive recognition signal. J Cell Biochem. 1985;28(2):99–104. doi: 10.1002/jcb.240280203. [DOI] [PubMed] [Google Scholar]



