Abstract
A membrane-bound cytochrome b, a heterodimer formed by a 91-kD glycoprotein (heavy chain) and a 22-kD polypeptide (light chain), is an essential component of the phagocyte NADPH-oxidase responsible for superoxide generation. Cytochrome b is absent in two subgroups of chronic granulomatous disease (CGD), an inherited disorder characterized by the lack of oxidase activity. Mutations in the cytochrome heavy chain gene, encoded by the CYBB locus in Xp21.1, result in the X-linked form of CGD. A rare subgroup of autosomal recessive CGD also lacks cytochrome b (A- CGD), but the genetic defect has not previously been identified. In order to search for possible mutations in the cytochrome light chain locus, CYBA, the structure of this gene was characterized. The CYBA locus was localized to 16q24, and the approximately 600-bp open reading frame determined to be encoded by six exons that span approximately 8.5 kb. Three unrelated patients with A- CGD were studied for evidence of mutations in the light chain gene. One patient, whose parents were first cousins, was homozygous for a large deletion that removed all but the extreme 5' coding sequence of the gene. The other two patients had a grossly normal light chain transcript on Northern blot of mononuclear cell RNA. The light chain transcript was amplified by the polymerase chain reaction and sequenced. One patient was a compound heterozygote for two alleles containing point mutations in the open reading frame that predict a frame shift and a nonconservative amino acid replacement, respectively. The second patient, whose parents were second cousins, was homozygous for a different single-base substitution resulting in another nonconservative amino acid change. These results indicate that A- CGD can results from defects in the gene encoding the 22-kD light chain of the phagocyte cytochrome b.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehner R. L., Kunkel L. M., Monaco A. P., Haines J. L., Conneally P. M., Palmer C., Heerema N., Orkin S. H. DNA linkage analysis of X chromosome-linked chronic granulomatous disease. Proc Natl Acad Sci U S A. 1986 May;83(10):3398–3401. doi: 10.1073/pnas.83.10.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Quantitative nitroblue tetrazolium test in chronic granulomatous disease. N Engl J Med. 1968 May 2;278(18):971–976. doi: 10.1056/NEJM196805022781801. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolscher B. G., van Zwieten R., Kramer I. M., Weening R. S., Verhoeven A. J., Roos D. A phosphoprotein of Mr 47,000, defective in autosomal chronic granulomatous disease, copurifies with one of two soluble components required for NADPH:O2 oxidoreductase activity in human neutrophils. J Clin Invest. 1989 Mar;83(3):757–763. doi: 10.1172/JCI113954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthron D. T., Morton C. C., Orkin S. H., Collins T. Platelet-derived growth factor A chain: gene structure, chromosomal location, and basis for alternative mRNA splicing. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1492–1496. doi: 10.1073/pnas.85.5.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Bruns G. A., Mintz B. J., Leary A. C., Regina V. M., Gerald P. S. Human lysosomal genes: arylsulfatase A and beta-galactosidase. Biochem Genet. 1979 Dec;17(11-12):1031–1059. doi: 10.1007/BF00504344. [DOI] [PubMed] [Google Scholar]
- Bruns G., Stroh H., Veldman G. M., Latt S. A., Floros J. The 35 kd pulmonary surfactant-associated protein is encoded on chromosome 10. Hum Genet. 1987 May;76(1):58–62. doi: 10.1007/BF00283051. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Malech H. L., Gallin J. I., Nunoi H., Volpp B. D., Pearson D. W., Nauseef W. M., Curnutte J. T. Genetic variants of chronic granulomatous disease: prevalence of deficiencies of two cytosolic components of the NADPH oxidase system. N Engl J Med. 1989 Sep 7;321(10):647–652. doi: 10.1056/NEJM198909073211005. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Chronic granulomatous disease. Adv Hum Genet. 1987;16:229–297. doi: 10.1007/978-1-4757-0620-8_4. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Berkow R. L., Roberts R. L., Shurin S. B., Scott P. J. Chronic granulomatous disease due to a defect in the cytosolic factor required for nicotinamide adenine dinucleotide phosphate oxidase activation. J Clin Invest. 1988 Feb;81(2):606–610. doi: 10.1172/JCI113360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Kuver R., Scott P. J. Activation of neutrophil NADPH oxidase in a cell-free system. Partial purification of components and characterization of the activation process. J Biol Chem. 1987 Apr 25;262(12):5563–5569. [PubMed] [Google Scholar]
- Curnutte J. T., Scott P. J., Mayo L. A. Cytosolic components of the respiratory burst oxidase: resolution of four components, two of which are missing in complementing types of chronic granulomatous disease. Proc Natl Acad Sci U S A. 1989 Feb;86(3):825–829. doi: 10.1073/pnas.86.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinauer M. C., Curnutte J. T., Rosen H., Orkin S. H. A missense mutation in the neutrophil cytochrome b heavy chain in cytochrome-positive X-linked chronic granulomatous disease. J Clin Invest. 1989 Dec;84(6):2012–2016. doi: 10.1172/JCI114393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinauer M. C., Orkin S. H., Brown R., Jesaitis A. J., Parkos C. A. The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. 1987 Jun 25-Jul 1Nature. 327(6124):717–720. doi: 10.1038/327717a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Francke U., Ochs H. D., de Martinville B., Giacalone J., Lindgren V., Distèche C., Pagon R. A., Hofker M. H., van Ommen G. J., Pearson P. L. Minor Xp21 chromosome deletion in a male associated with expression of Duchenne muscular dystrophy, chronic granulomatous disease, retinitis pigmentosa, and McLeod syndrome. Am J Hum Genet. 1985 Mar;37(2):250–267. [PMC free article] [PubMed] [Google Scholar]
- Ginsburg D., Handin R. I., Bonthron D. T., Donlon T. A., Bruns G. A., Latt S. A., Orkin S. H. Human von Willebrand factor (vWF): isolation of complementary DNA (cDNA) clones and chromosomal localization. Science. 1985 Jun 21;228(4706):1401–1406. doi: 10.1126/science.3874428. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., Hollander N., Roberts T. M., Anderson D. C., Springer T. A. Heterogeneous mutations in the beta subunit common to the LFA-1, Mac-1, and p150,95 glycoproteins cause leukocyte adhesion deficiency. Cell. 1987 Jul 17;50(2):193–202. doi: 10.1016/0092-8674(87)90215-7. [DOI] [PubMed] [Google Scholar]
- McConlogue L., Brow M. A., Innis M. A. Structure-independent DNA amplification by PCR using 7-deaza-2'-deoxyguanosine. Nucleic Acids Res. 1988 Oct 25;16(20):9869–9869. doi: 10.1093/nar/16.20.9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P. Biogenesis of the acetylcholine receptor, a multisubunit integral membrane protein. Cell. 1984 Mar;36(3):573–575. doi: 10.1016/0092-8674(84)90335-0. [DOI] [PubMed] [Google Scholar]
- Minami Y., Weissman A. M., Samelson L. E., Klausner R. D. Building a multichain receptor: synthesis, degradation, and assembly of the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1987 May;84(9):2688–2692. doi: 10.1073/pnas.84.9.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent J. H., Gratzer W., Segal A. W. Identification of the haem-binding subunit of cytochrome b-245. Biochem J. 1989 Dec 15;264(3):921–924. doi: 10.1042/bj2640921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoi H., Rotrosen D., Gallin J. I., Malech H. L. Two forms of autosomal chronic granulomatous disease lack distinct neutrophil cytosol factors. Science. 1988 Dec 2;242(4883):1298–1301. doi: 10.1126/science.2848319. [DOI] [PubMed] [Google Scholar]
- Okamura N., Malawista S. E., Roberts R. L., Rosen H., Ochs H. D., Babior B. M., Curnutte J. T. Phosphorylation of the oxidase-related 48K phosphoprotein family in the unusual autosomal cytochrome-negative and X-linked cytochrome-positive types of chronic granulomatous disease. Blood. 1988 Aug;72(2):811–816. [PubMed] [Google Scholar]
- Parkos C. A., Allen R. A., Cochrane C. G., Jesaitis A. J. Purified cytochrome b from human granulocyte plasma membrane is comprised of two polypeptides with relative molecular weights of 91,000 and 22,000. J Clin Invest. 1987 Sep;80(3):732–742. doi: 10.1172/JCI113128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkos C. A., Dinauer M. C., Jesaitis A. J., Orkin S. H., Curnutte J. T. Absence of both the 91kD and 22kD subunits of human neutrophil cytochrome b in two genetic forms of chronic granulomatous disease. Blood. 1989 May 1;73(6):1416–1420. [PubMed] [Google Scholar]
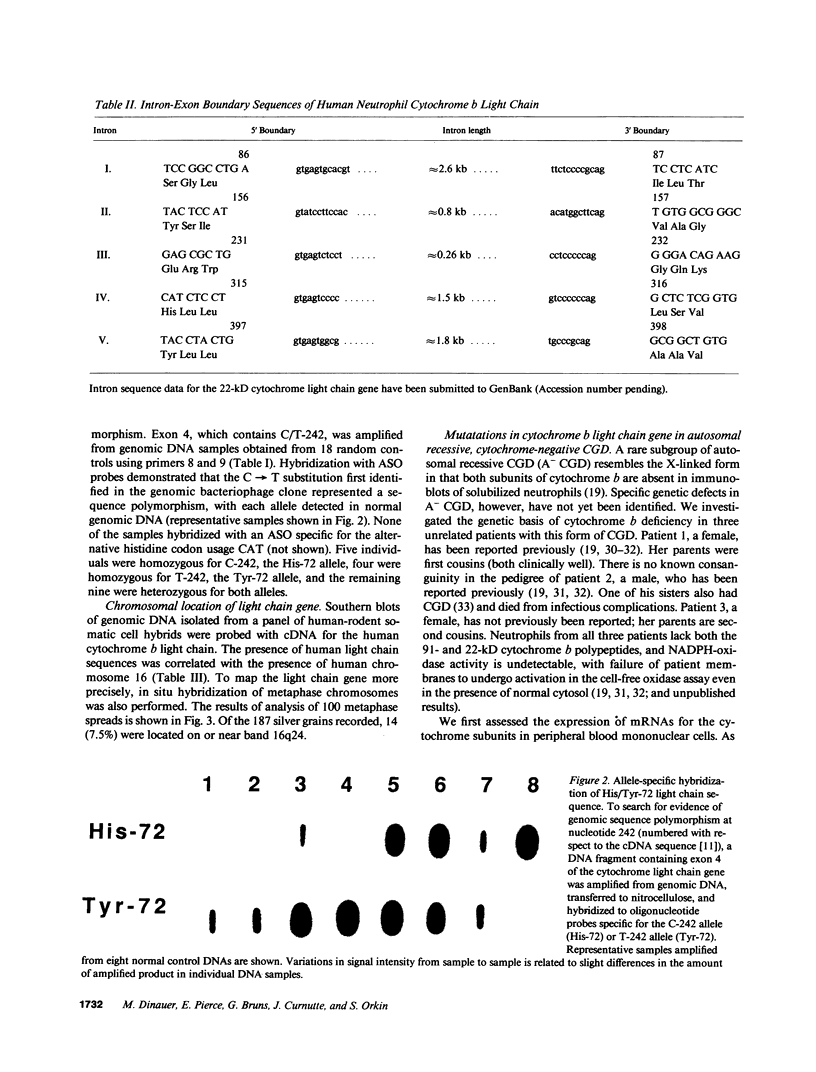
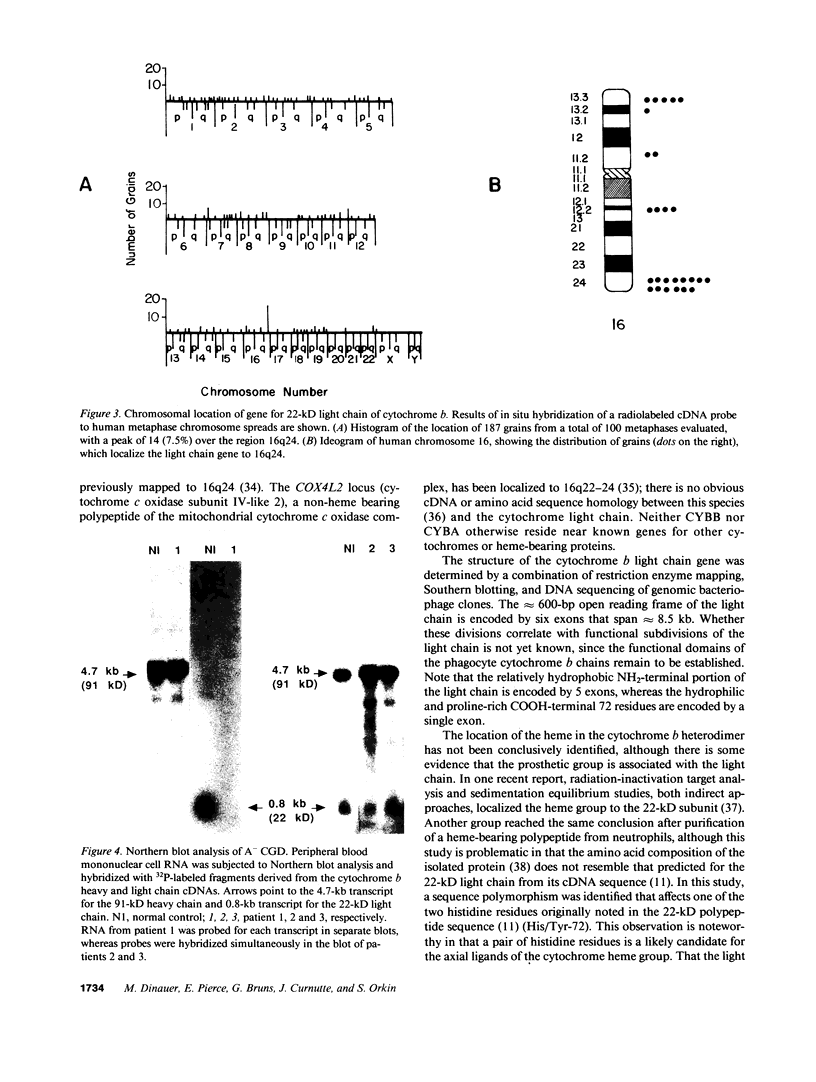
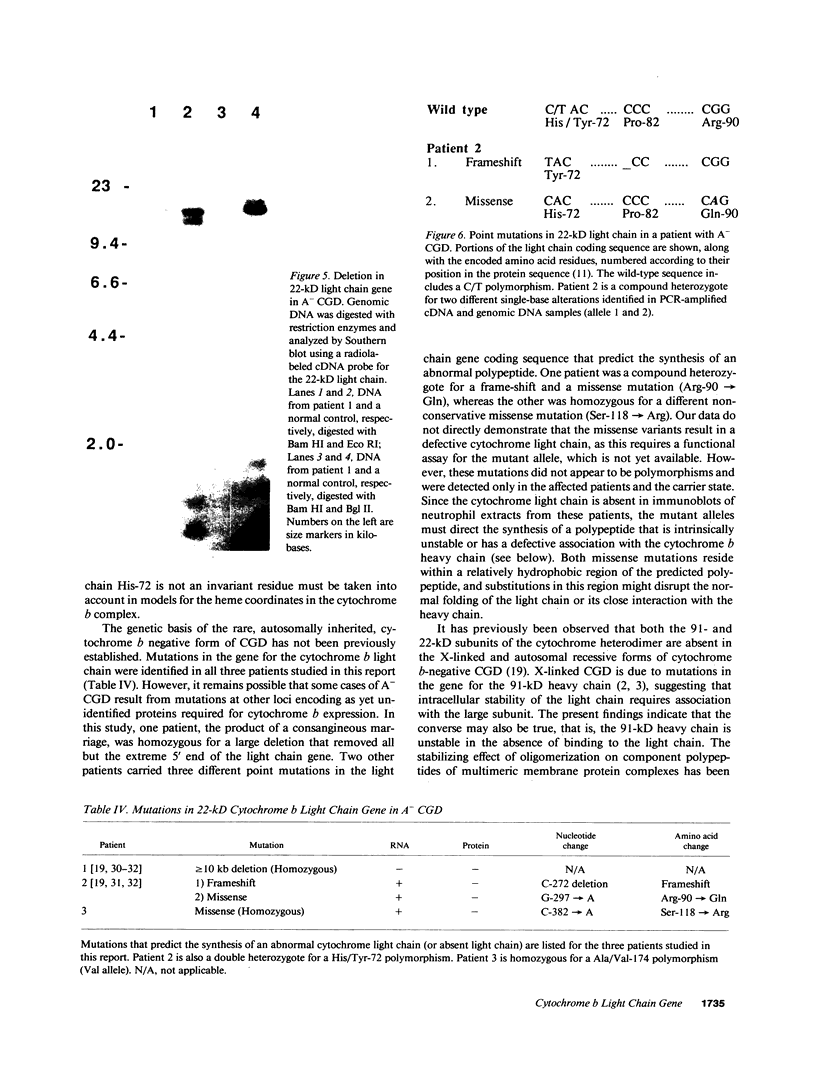
- Parkos C. A., Dinauer M. C., Walker L. E., Allen R. A., Jesaitis A. J., Orkin S. H. Primary structure and unique expression of the 22-kilodalton light chain of human neutrophil cytochrome b. Proc Natl Acad Sci U S A. 1988 May;85(10):3319–3323. doi: 10.1073/pnas.85.10.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quie P. G., Kaplan E. L., Page A. R., Gruskay F. L., Malawista S. E. Defective polymorphonuclear-leukocyte function and chronic granulomatous disease in two female children. N Engl J Med. 1968 May 2;278(18):976–980. doi: 10.1056/NEJM196805022781802. [DOI] [PubMed] [Google Scholar]
- Reeders S. T., Hildebrand C. E. Report of the committee on the genetic constitution of chromosome 16. Cytogenet Cell Genet. 1989;51(1-4):299–318. doi: 10.1159/000132796. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Kunkel L. M., Monaco A. P., Goff S. C., Newburger P. E., Baehner R. L., Cole F. S., Curnutte J. T., Orkin S. H. Cloning the gene for an inherited human disorder--chronic granulomatous disease--on the basis of its chromosomal location. Nature. 1986 Jul 3;322(6074):32–38. doi: 10.1038/322032a0. [DOI] [PubMed] [Google Scholar]
- Segal A. W. Absence of both cytochrome b-245 subunits from neutrophils in X-linked chronic granulomatous disease. Nature. 1987 Mar 5;326(6108):88–91. doi: 10.1038/326088a0. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Cross A. R., Garcia R. C., Borregaard N., Valerius N. H., Soothill J. F., Jones O. T. Absence of cytochrome b-245 in chronic granulomatous disease. A multicenter European evaluation of its incidence and relevance. N Engl J Med. 1983 Feb 3;308(5):245–251. doi: 10.1056/NEJM198302033080503. [DOI] [PubMed] [Google Scholar]
- Segal A. W. The electron transport chain of the microbicidal oxidase of phagocytic cells and its involvement in the molecular pathology of chronic granulomatous disease. J Clin Invest. 1989 Jun;83(6):1785–1793. doi: 10.1172/JCI114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber A. I., Borregaard N., Simons E., Wright J. Chronic granulomatous disease: a syndrome of phagocyte oxidase deficiencies. Medicine (Baltimore) 1983 Sep;62(5):286–309. [PubMed] [Google Scholar]
- Teahan C., Rowe P., Parker P., Totty N., Segal A. W. The X-linked chronic granulomatous disease gene codes for the beta-chain of cytochrome b-245. 1987 Jun 25-Jul 1Nature. 327(6124):720–721. doi: 10.1038/327720a0. [DOI] [PubMed] [Google Scholar]
- Volpp B. D., Nauseef W. M., Clark R. A. Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease. Science. 1988 Dec 2;242(4883):1295–1297. doi: 10.1126/science.2848318. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Hayakawa T., Kaneda M., Kakinuma K., Yoshikawa A. Purification and some properties of the small subunit of cytochrome b558 from human neutrophils. J Biol Chem. 1989 Jan 5;264(1):112–118. [PubMed] [Google Scholar]
- Zeviani M., Nakagawa M., Herbert J., Lomax M. I., Grossman L. I., Sherbany A. A., Miranda A. F., DiMauro S., Schon E. A. Isolation of a cDNA clone encoding subunit IV of human cytochrome c oxidase. Gene. 1987;55(2-3):205–217. doi: 10.1016/0378-1119(87)90281-2. [DOI] [PubMed] [Google Scholar]