Abstract
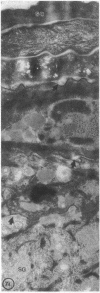
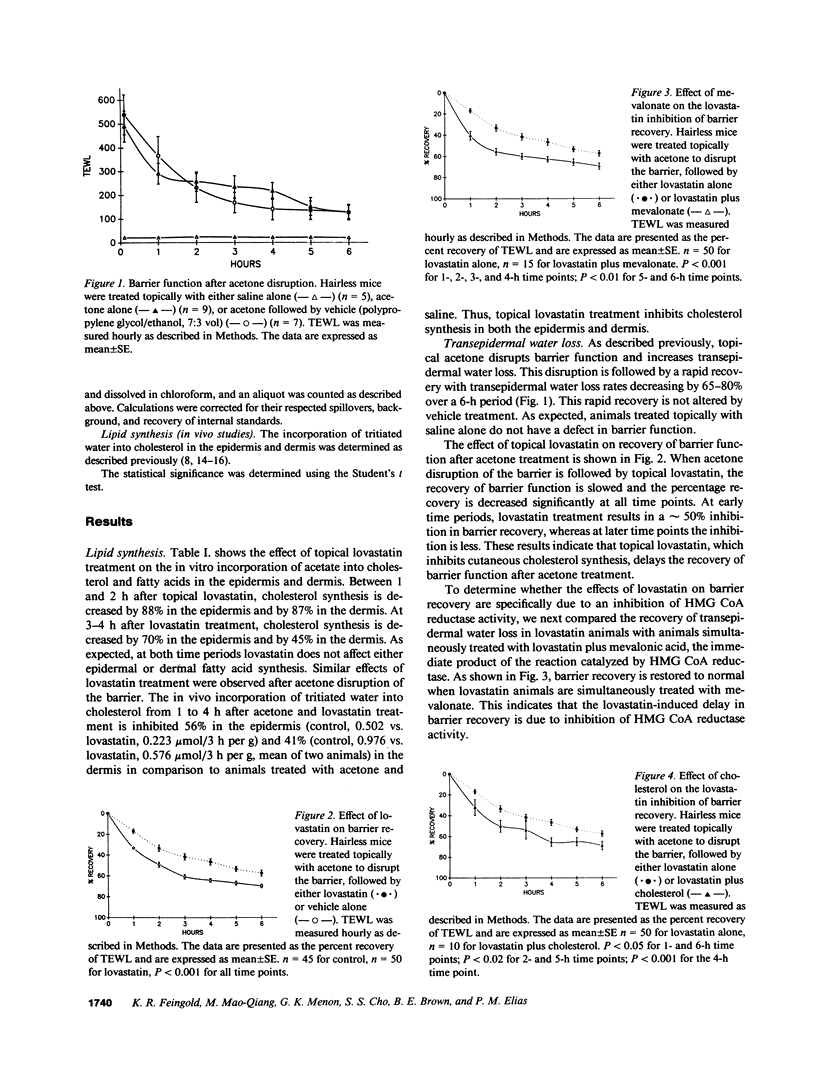
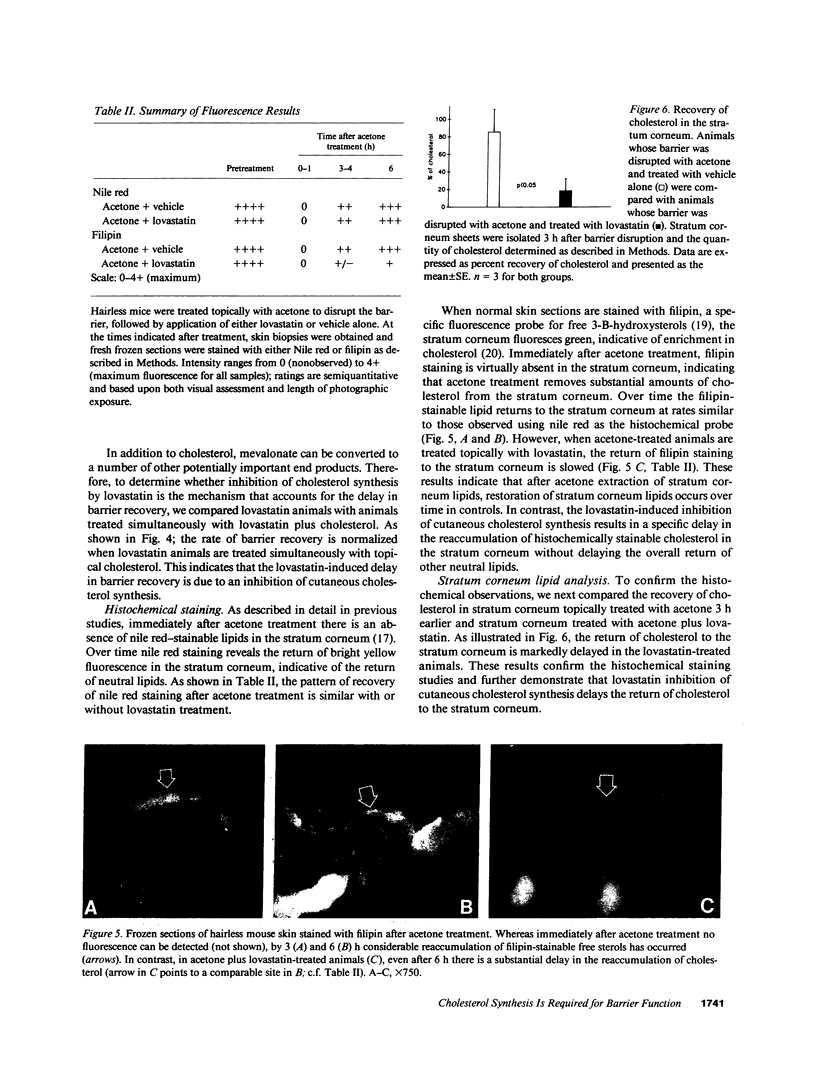
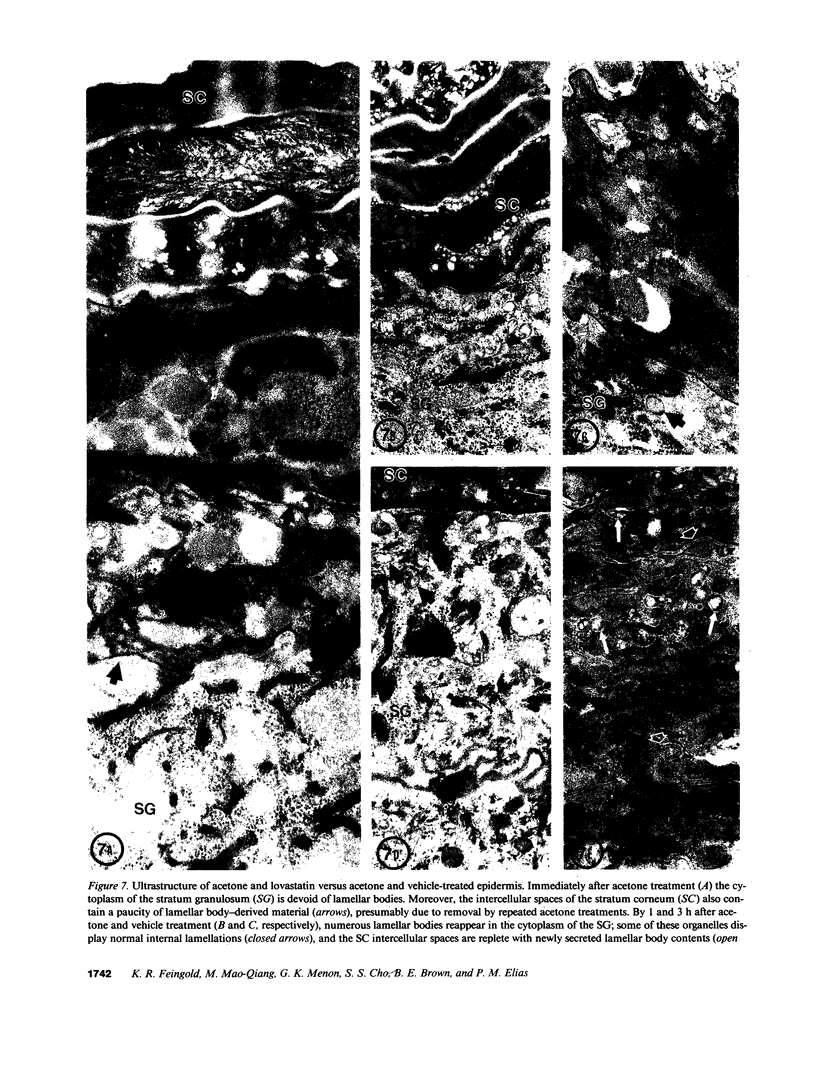
Previous studies have shown that topical acetone treatment results in the removal of stratum corneum lipids and disruption of the permeability barrier. This disruption stimulates epidermal lipid synthesis which is associated with the rapid restoration of stratum corneum lipids and barrier function. The aim of this study was to determine the role of cutaneous cholesterol synthesis in the barrier recovery. Here we show that topical lovastatin, a competitive inhibitor of HMG CoA reductase, inhibits cholesterol synthesis. After acetone disruption of the barrier, the normal rapid return of cholesterol to the stratum corneum and recovery of barrier function is impaired in animals treated topically with lovastatin. When lovastatin animals are simultaneously treated topically with either mevalonate, the immediate product of HMG CoA reductase, or cholesterol, the final end product of the pathway, the recovery of the barrier is normalized. Lovastatin resulted in the delayed secretion and abnormal appearance of lamellar bodies. These results provide the first evidence demonstrating that cholesterol synthesis is required for the maintenance of barrier structure and function and suggests a crucial role for cholesterol synthesis in allowing for terrestrial existence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts A. W., Chen J., Kuron G., Hunt V., Huff J., Hoffman C., Rothrock J., Lopez M., Joshua H., Harris E. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J. M., Dietschy J. M. Regulation of sterol synthesis in 15 tissues of rat. II. Role of rat and human high and low density plasma lipoproteins and of rat chylomicron remnants. J Biol Chem. 1977 Jun 10;252(11):3652–3659. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bittman R., Clejan S., Rottem S. Transbilayer distribution of sterols in mycoplasma membranes: a review. Yale J Biol Med. 1983 Sep-Dec;56(5-6):397–403. [PMC free article] [PubMed] [Google Scholar]
- Brannan P. G., Goldstein J. L., Brown M. S. 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human hair roots. J Lipid Res. 1975 Jan;16(1):7–11. [PubMed] [Google Scholar]
- Elias P. M., Feingold K. R. Lipid-related barriers and gradients in the epidermis. Ann N Y Acad Sci. 1988;548:4–13. doi: 10.1111/j.1749-6632.1988.tb18788.x. [DOI] [PubMed] [Google Scholar]
- Elias P. M., Menon G. K., Grayson S., Brown B. E. Membrane structural alterations in murine stratum corneum: relationship to the localization of polar lipids and phospholipases. J Invest Dermatol. 1988 Jul;91(1):3–10. doi: 10.1111/1523-1747.ep12463279. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Brown B. E., Lear S. R., Moser A. H., Elias P. M. Effect of essential fatty acid deficiency on cutaneous sterol synthesis. J Invest Dermatol. 1986 Nov;87(5):588–591. doi: 10.1111/1523-1747.ep12455835. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Brown B. E., Lear S. R., Moser A. H., Elias P. M. Localization of de novo sterologenesis in mammalian skin. J Invest Dermatol. 1983 Oct;81(4):365–369. doi: 10.1111/1523-1747.ep12519974. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., MacRae G., Moser A. H., Wu J., Siperstein M. D., Wiley M. H. Differences in de novo cholesterol synthesis between the intact male and female rat. Endocrinology. 1983 Jan;112(1):96–103. doi: 10.1210/endo-112-1-96. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Wiley M. H., MacRae G., Lear S., Moser A. H., Zsigmond G., Siperstein M. D. De novo sterologenesis in the intact rat. Metabolism. 1983 Jan;32(1):75–81. doi: 10.1016/0026-0495(83)90160-9. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Wiley M. H., Moser A. H., Lau D. T., Lear S. R., Siperstein M. D. De novo sterologenesis in intact primates. J Lab Clin Med. 1982 Sep;100(3):405–410. [PubMed] [Google Scholar]
- Grubauer G., Elias P. M., Feingold K. R. Transepidermal water loss: the signal for recovery of barrier structure and function. J Lipid Res. 1989 Mar;30(3):323–333. [PubMed] [Google Scholar]
- Grubauer G., Feingold K. R., Elias P. M. Relationship of epidermal lipogenesis to cutaneous barrier function. J Lipid Res. 1987 Jun;28(6):746–752. [PubMed] [Google Scholar]
- Grubauer G., Feingold K. R., Harris R. M., Elias P. M. Lipid content and lipid type as determinants of the epidermal permeability barrier. J Lipid Res. 1989 Jan;30(1):89–96. [PubMed] [Google Scholar]
- Ham A. B. A new reagent for the determination of true cholesterol. Am J Med Technol. 1971 Aug;37(8):319–324. [PubMed] [Google Scholar]
- Menon G. K., Feingold K. R., Moser A. H., Brown B. E., Elias P. M. De novo sterologenesis in the skin. II. Regulation by cutaneous barrier requirements. J Lipid Res. 1985 Apr;26(4):418–427. [PubMed] [Google Scholar]
- Mommaas-Kienhuis A. M., Grayson S., Wijsman M. C., Vermeer B. J., Elias P. M. Low density lipoprotein receptor expression on keratinocytes in normal and psoriatic epidermis. J Invest Dermatol. 1987 Nov;89(5):513–517. doi: 10.1111/1523-1747.ep12461024. [DOI] [PubMed] [Google Scholar]
- Ponec M., Havekes L., Kempenaar J., Vermeer B. J. Cultured human skin fibroblasts and keratinocytes: differences in the regulation of cholesterol synthesis. J Invest Dermatol. 1983 Aug;81(2):125–130. doi: 10.1111/1523-1747.ep12542979. [DOI] [PubMed] [Google Scholar]
- Turley S. D., Andersen J. M., Dietschy J. M. Rates of sterol synthesis and uptake in the major organs of the rat in vivo. J Lipid Res. 1981 May;22(4):551–569. [PubMed] [Google Scholar]
- Williams M. L., Mommaas-Kienhuis A. M., Rutherford S. L., Grayson S., Vermeer B. J., Elias P. M. Free sterol metabolism and low density lipoprotein receptor expression as differentiation markers of cultured human keratinocytes. J Cell Physiol. 1987 Sep;132(3):428–440. doi: 10.1002/jcp.1041320305. [DOI] [PubMed] [Google Scholar]