Abstract
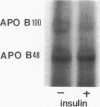
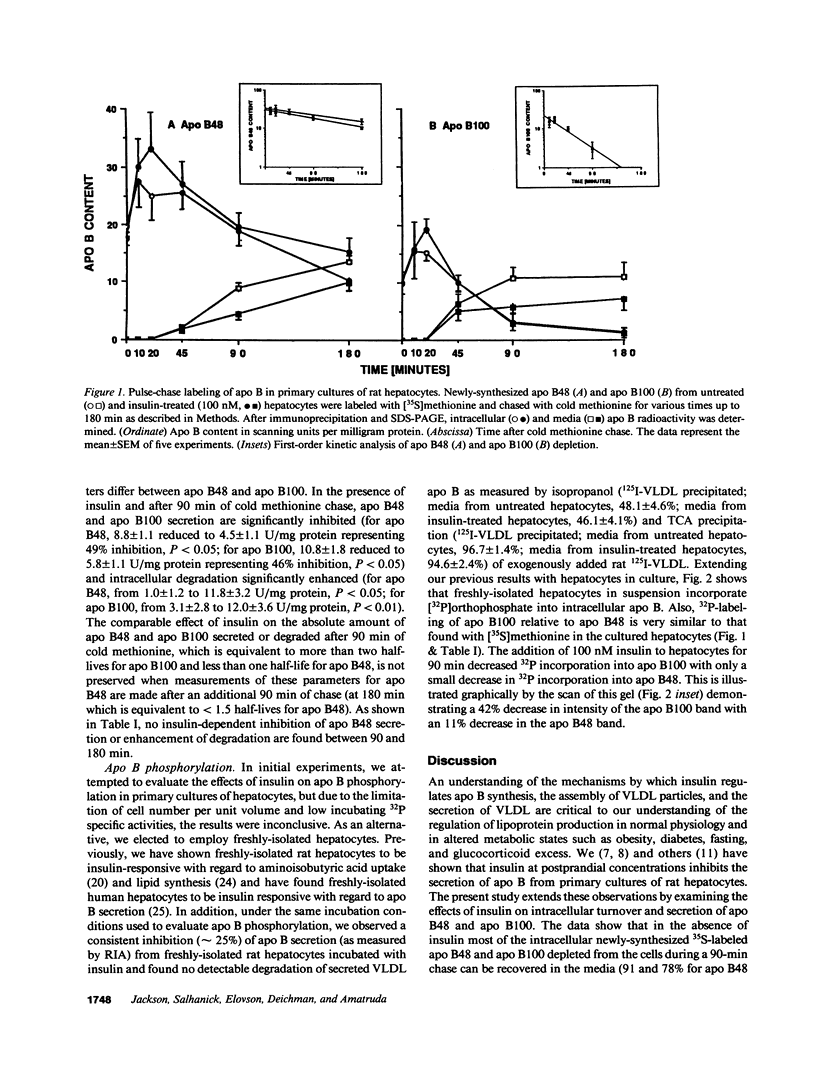
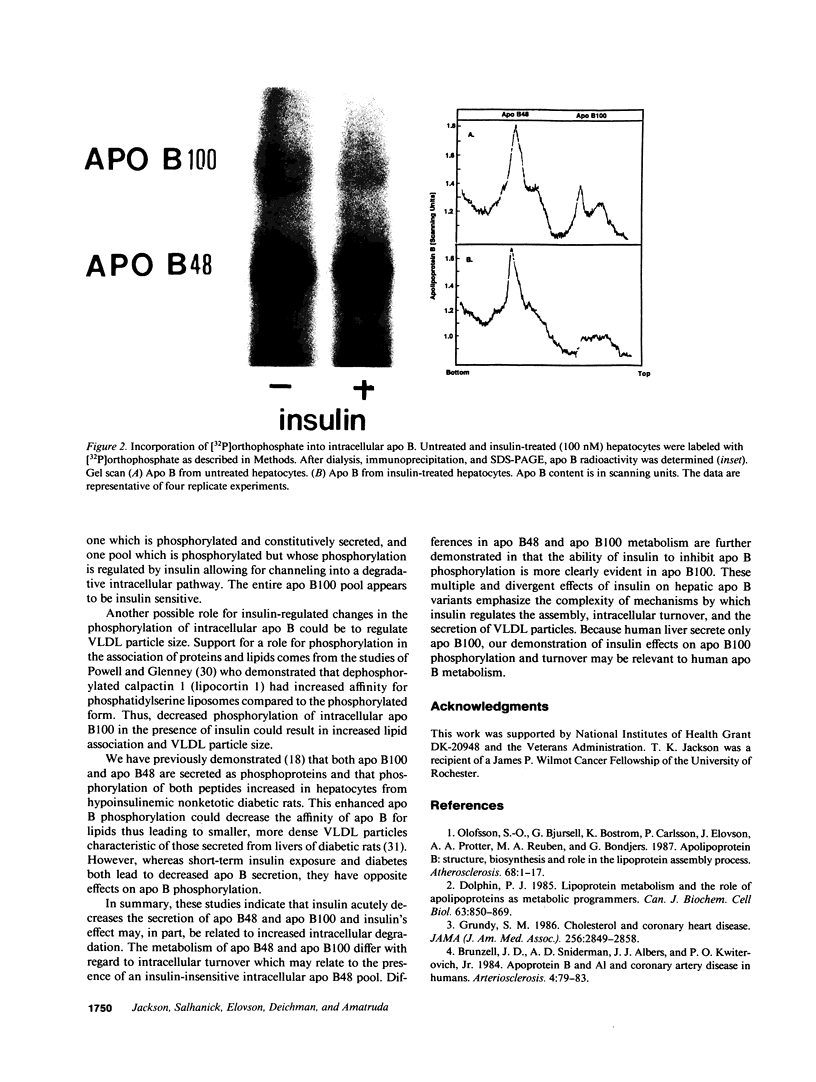
Our laboratory has previously shown that insulin inhibits the secretion of newly-synthesized and immunoreactive apo B from rat hepatocytes. We have also shown that apo B is secreted as a phosphoprotein and that phosphorylation is increased in hypoinsulinemic nonketotic diabetes. The present studies were conducted to determine whether the ability of insulin to inhibit apo B secretion is related to alterations in apo B turnover and whether insulin itself affects apo B phosphorylation. Pulse-chase studies with [35S]methionine in primary cultures of hepatocytes from normal rats in the absence and presence of insulin show that the secretion of apo B100 and apo B48 are inhibited by insulin and that this inhibition may be due in part to enhanced intracellular degradation. In addition, there is a second intracellular apo B48 pool which is not insulin regulated or degraded. In experiments in which hepatocytes were incubated with [32P]orthophosphate, insulin decreased 32P incorporation into apo B100 (42%) with only small effects on apo B48 (11%). The small insulin effect on apo B48 may relate to an insulin-insensitive apo B48 intracellular pool. These studies show that insulin can affect the intracellular turnover, secretion, degradation, and phosphorylation of apo B and emphasize the differential regulation of apo B100 and apo B48 with regard to these parameters in rat liver.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett S. M., Gibbons G. F. Short- and longer-term regulation of very-low-density lipoprotein secretion by insulin, dexamethasone and lipogenic substrates in cultured hepatocytes. A biphasic effect of insulin. Biochem J. 1988 Jan 1;249(1):37–43. doi: 10.1042/bj2490037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry E. M., Ziv E., Bar-On H. Lipoprotein secretion by isolated perfused livers from streptozotocin-diabetic rats. Diabetologia. 1981 Oct;21(4):402–408. doi: 10.1007/BF00252689. [DOI] [PubMed] [Google Scholar]
- Borchardt R. A., Davis R. A. Intrahepatic assembly of very low density lipoproteins. Rate of transport out of the endoplasmic reticulum determines rate of secretion. J Biol Chem. 1987 Dec 5;262(34):16394–16402. [PubMed] [Google Scholar]
- Brunzell J. D., Sniderman A. D., Albers J. J., Kwiterovich P. O., Jr Apoproteins B and A-I and coronary artery disease in humans. Arteriosclerosis. 1984 Mar-Apr;4(2):79–83. doi: 10.1161/01.atv.4.2.79. [DOI] [PubMed] [Google Scholar]
- Capasso J. M., Keenan T. W., Abeijon C., Hirschberg C. B. Mechanism of phosphorylation in the lumen of the Golgi apparatus. Translocation of adenosine 5'-triphosphate into Golgi vesicles from rat liver and mammary gland. J Biol Chem. 1989 Mar 25;264(9):5233–5240. [PubMed] [Google Scholar]
- Caro J. F., Amatruda J. M. Evidence for modulation of insulin action and degradation independently of insulin binding. Am J Physiol. 1981 Mar;240(3):E325–E332. doi: 10.1152/ajpendo.1981.240.3.E325. [DOI] [PubMed] [Google Scholar]
- Cech J. M., Freeman R. B., Jr, Caro J. F., Amatruda J. M. Insulin action and binding in isolated hepatocytes from fasted, streptozotocin-diabetic, and older, spontaneously obese rats. Biochem J. 1980 Jun 15;188(3):839–845. doi: 10.1042/bj1880839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti N., Wolfbauer G. Secretion of lipids, apolipoproteins, and lipoproteins by human hepatoma cell line, HepG2: effects of oleic acid and insulin. J Lipid Res. 1987 Apr;28(4):423–436. [PubMed] [Google Scholar]
- Davis R. A., Boogaerts J. R., Borchardt R. A., Malone-McNeal M., Archambault-Schexnayder J. Intrahepatic assembly of very low density lipoproteins. Varied synthetic response of individual apolipoproteins to fasting. J Biol Chem. 1985 Nov 15;260(26):14137–14144. [PubMed] [Google Scholar]
- Davis R. A., Clinton G. M., Borchardt R. A., Malone-McNeal M., Tan T., Lattier G. R. Intrahepatic assembly of very low density lipoproteins. Phosphorylation of small molecular weight apolipoprotein B. J Biol Chem. 1984 Mar 25;259(6):3383–3386. [PubMed] [Google Scholar]
- Dolphin P. J. Lipoprotein metabolism and the role of apolipoproteins as metabolic programmers. Can J Biochem Cell Biol. 1985 Aug;63(8):850–869. doi: 10.1139/o85-107. [DOI] [PubMed] [Google Scholar]
- Durrington P. N., Newton R. S., Weinstein D. B., Steinberg D. Effects of insulin and glucose on very low density lipoprotein triglyceride secretion by cultured rat hepatocytes. J Clin Invest. 1982 Jul;70(1):63–73. doi: 10.1172/JCI110604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons G. F. Insulin, diabetes and hepatic very-low-density lipoprotein metabolism. Biochem Soc Trans. 1989 Feb;17(1):49–51. doi: 10.1042/bst0170049. [DOI] [PubMed] [Google Scholar]
- Grundy S. M. Cholesterol and coronary heart disease. A new era. JAMA. 1986 Nov 28;256(20):2849–2858. [PubMed] [Google Scholar]
- Jackson T. K., Salhanick A. I., Sparks J. D., Sparks C. E., Bolognino M., Amatruda J. M. Insulin-mimetic effects of vanadate in primary cultures of rat hepatocytes. Diabetes. 1988 Sep;37(9):1234–1240. doi: 10.2337/diab.37.9.1234. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Use of protein A-bearing staphylococci for the immunoprecipitation and isolation of antigens from cells. Methods Enzymol. 1981;73(Pt B):442–459. doi: 10.1016/0076-6879(81)73084-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Olofsson S. O., Bjursell G., Boström K., Carlsson P., Elovson J., Protter A. A., Reuben M. A., Bondjers G. Apolipoprotein B: structure, biosynthesis and role in the lipoprotein assembly process. Atherosclerosis. 1987 Nov;68(1-2):1–17. doi: 10.1016/0021-9150(87)90088-8. [DOI] [PubMed] [Google Scholar]
- Patsch W., Franz S., Schonfeld G. Role of insulin in lipoprotein secretion by cultured rat hepatocytes. J Clin Invest. 1983 May;71(5):1161–1174. doi: 10.1172/JCI110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietri A. O., Dunn F. L., Grundy S. M., Raskin P. The effect of continuous subcutaneous insulin infusion on very-low-density lipoprotein triglyceride metabolism in type I diabetes mellitus. Diabetes. 1983 Jan;32(1):75–81. doi: 10.2337/diab.32.1.75. [DOI] [PubMed] [Google Scholar]
- Powell M. A., Glenney J. R. Regulation of calpactin I phospholipid binding by calpactin I light-chain binding and phosphorylation by p60v-src. Biochem J. 1987 Oct 15;247(2):321–328. doi: 10.1042/bj2470321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullinger C. R., North J. D., Teng B. B., Rifici V. A., Ronhild de Brito A. E., Scott J. The apolipoprotein B gene is constitutively expressed in HepG2 cells: regulation of secretion by oleic acid, albumin, and insulin, and measurement of the mRNA half-life. J Lipid Res. 1989 Jul;30(7):1065–1077. [PubMed] [Google Scholar]
- Reuben M. A., Svenson K. L., Doolittle M. H., Johnson D. F., Lusis A. J., Elovson J. Biosynthetic relationships between three rat apolipoprotein B peptides. J Lipid Res. 1988 Oct;29(10):1337–1347. [PubMed] [Google Scholar]
- Sparks C. E., Sparks J. D., Bolognino M., Salhanick A., Strumph P. S., Amatruda J. M. Insulin effects on apolipoprotein B lipoprotein synthesis and secretion by primary cultures of rat hepatocytes. Metabolism. 1986 Dec;35(12):1128–1136. doi: 10.1016/0026-0495(86)90026-0. [DOI] [PubMed] [Google Scholar]
- Sparks J. D., Sparks C. E., Bolognino M., Roncone A. M., Jackson T. K., Amatruda J. M. Effects of nonketotic streptozotocin diabetes on apolipoprotein B synthesis and secretion by primary cultures of rat hepatocytes. J Clin Invest. 1988 Jul;82(1):37–43. doi: 10.1172/JCI113597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks J. D., Sparks C. E., Roncone A. M., Amatruda J. M. Secretion of high and low molecular weight phosphorylated apolipoprotein B by hepatocytes from control and diabetic rats. Phosphorylation of APO BH and APO BL. J Biol Chem. 1988 Apr 15;263(11):5001–5004. [PubMed] [Google Scholar]
- Swift L. L., Padley R. J., Getz G. S. Differential labeling of rat hepatic Golgi and serum very low density lipoprotein apoprotein B variants. J Lipid Res. 1987 Feb;28(2):207–215. [PubMed] [Google Scholar]
- Vogelberg K. H., Gries F. A., Moschinski D. Hepatic production of VLDL-triglycerides. Dependence of portal substrate and insulin concentration. Horm Metab Res. 1980 Dec;12(12):688–694. doi: 10.1055/s-2007-999233. [DOI] [PubMed] [Google Scholar]
- Wang S. Y., Williams D. L. Biosynthesis of the vitellogenins. Identification and characterization of nonphosphorylated precursors to avian vitellogenin I and vitellogenin II. J Biol Chem. 1982 Apr 10;257(7):3837–3846. [PubMed] [Google Scholar]
- Zick Y. The insulin receptor: structure and function. Crit Rev Biochem Mol Biol. 1989;24(3):217–269. doi: 10.3109/10409238909082554. [DOI] [PubMed] [Google Scholar]