Abstract
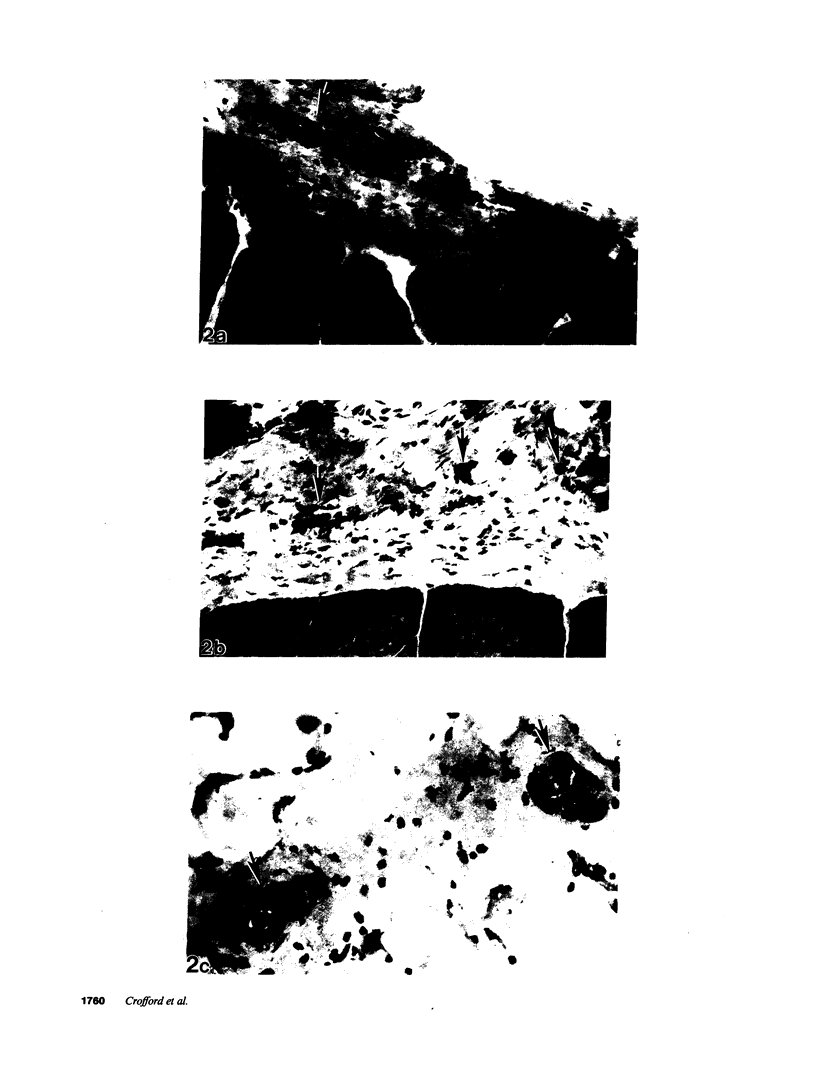
Tryptophan-associated eosinophilia-myalgia syndrome (L-TRP-EMS) is a newly described syndrome which occurred in epidemic fashion in the United States in the summer and fall of 1989. Epidemiologic data has linked the syndrome to intake of L-tryptophan (L-TRP) from one specific manufacturer, but the precise etiologic compound(s) must be established by replication of the syndrome in an appropriate animal model. In this study, implicated L-TRP, United States Pharmacopeia (USP) grade L-TRP, or vehicle was administered by gavage in a blinded fashion for 38 d to female Lewis rats at doses comparable with those ingested by patients who developed the eosinophilia-myalgia syndrome. Animals receiving implicated L-TRP, but not those receiving USP grade L-TRP or vehicle, developed histologic signs consistent with fasciitis and perimyositis, specific pathologic features of human L-TRP-EMS. Peripheral blood eosinophilia was not observed. Hypothalamic corticotropin releasing hormone mRNA levels were lower and plasma corticosterone levels tended to be lower in the animals that received implicated L-TRP. Plasma L-kynurenine was higher in both L-TRP-treated groups compared to the vehicle-treated animals. The female Lewis rat is known to be susceptible to a wide variety of inflammatory diseases. Identification of specific inflammatory changes in this rat following exposure to implicated L-TRP indicates that this animal model will be important in subsequent investigations into the etiology, pathogenesis, and treatment of human L-TRP-EMS.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arriza J. L., Simerly R. B., Swanson L. W., Evans R. M. The neuronal mineralocorticoid receptor as a mediator of glucocorticoid response. Neuron. 1988 Nov;1(9):887–900. doi: 10.1016/0896-6273(88)90136-5. [DOI] [PubMed] [Google Scholar]
- Belongia E. A., Hedberg C. W., Gleich G. J., White K. E., Mayeno A. N., Loegering D. A., Dunnette S. L., Pirie P. L., MacDonald K. L., Osterholm M. T. An investigation of the cause of the eosinophilia-myalgia syndrome associated with tryptophan use. N Engl J Med. 1990 Aug 9;323(6):357–365. doi: 10.1056/NEJM199008093230601. [DOI] [PubMed] [Google Scholar]
- Bender D. A. Biochemistry of tryptophan in health and disease. Mol Aspects Med. 1983;6(2):101–197. doi: 10.1016/0098-2997(83)90005-5. [DOI] [PubMed] [Google Scholar]
- Buckholtz N. S. Neurobiology of tetrahydro-beta-carbolines. Life Sci. 1980 Sep 15;27(11):893–903. doi: 10.1016/0024-3205(80)90098-3. [DOI] [PubMed] [Google Scholar]
- Capron M., Bazin H., Joseph M., Capron A. Evidence for IgE-dependent cytotoxicity by rat eosinophils. J Immunol. 1981 May;126(5):1764–1768. [PubMed] [Google Scholar]
- Capron M., Capron A., Dessaint J. P., Torpier G., Johansson S. G., Prin L. Fc receptors for IgE on human and rat eosinophils. J Immunol. 1981 Jun;126(6):2087–2092. [PubMed] [Google Scholar]
- Clauw D. J., Nashel D. J., Umhau A., Katz P. Tryptophan-associated eosinophilic connective-tissue disease. A new clinical entity? JAMA. 1990 Mar 16;263(11):1502–1506. [PubMed] [Google Scholar]
- Fernstrom J. D., Wurtman R. J. Brain serotonin content: physiological dependence on plasma tryptophan levels. Science. 1971 Jul 9;173(3992):149–152. doi: 10.1126/science.173.3992.149. [DOI] [PubMed] [Google Scholar]
- Hertzman P. A., Blevins W. L., Mayer J., Greenfield B., Ting M., Gleich G. J. Association of the eosinophilia-myalgia syndrome with the ingestion of tryptophan. N Engl J Med. 1990 Mar 29;322(13):869–873. doi: 10.1056/NEJM199003293221301. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Quearry B. J., Markey S. P. Systemic endotoxin increases L-tryptophan, 5-hydroxyindoleacetic acid, 3-hydroxykynurenine and quinolinic acid content of mouse cerebral cortex. Brain Res. 1989 Jul 3;491(1):173–179. doi: 10.1016/0006-8993(89)90101-7. [DOI] [PubMed] [Google Scholar]
- Hong C. B., Chow C. K. Induction of eosinophilic enteritis and eosinophilia in rats by vitamin E and selenium deficiency. Exp Mol Pathol. 1988 Apr;48(2):182–192. doi: 10.1016/0014-4800(88)90055-x. [DOI] [PubMed] [Google Scholar]
- Jingami H., Mizuno N., Takahashi H., Shibahara S., Furutani Y., Imura H., Numa S. Cloning and sequence analysis of cDNA for rat corticotropin-releasing factor precursor. FEBS Lett. 1985 Oct 21;191(1):63–66. doi: 10.1016/0014-5793(85)80994-7. [DOI] [PubMed] [Google Scholar]
- Kalogeras K. T., Calogero A. E., Kuribayiashi T., Khan I., Gallucci W. T., Kling M. A., Chrousos G. P., Gold P. W. In vitro and in vivo effects of the triazolobenzodiazepine alprazolam on hypothalamic-pituitary-adrenal function: pharmacological and clinical implications. J Clin Endocrinol Metab. 1990 May;70(5):1462–1471. doi: 10.1210/jcem-70-5-1462. [DOI] [PubMed] [Google Scholar]
- Kilbourne E. M., Bernert J. T., Jr, Posada de la Paz M., Hill R. H., Jr, Abaitua Borda I., Kilbourne B. W., Zack M. M. Chemical correlates of pathogenicity of oils related to the toxic oil syndrome epidemic in Spain. Am J Epidemiol. 1988 Jun;127(6):1210–1227. doi: 10.1093/oxfordjournals.aje.a114914. [DOI] [PubMed] [Google Scholar]
- Kilbourne E. M., Rigau-Perez J. G., Heath C. W., Jr, Zack M. M., Falk H., Martin-Marcos M., de Carlos A. Clinical epidemiology of toxic-oil syndrome. Manifestations of a New Illness. N Engl J Med. 1983 Dec 8;309(23):1408–1414. doi: 10.1056/NEJM198312083092302. [DOI] [PubMed] [Google Scholar]
- Lakhanpal S., Duffy J., Engel A. G. Eosinophilia associated with perimyositis and pneumonitis. Mayo Clin Proc. 1988 Jan;63(1):37–41. doi: 10.1016/s0025-6196(12)62663-9. [DOI] [PubMed] [Google Scholar]
- Laycock S. M., Smith H., Spicer B. A. Airway hyper-reactivity and blood, lung and airway eosinophilia in rats treated with Sephadex particles. Int Arch Allergy Appl Immunol. 1986;81(4):363–367. doi: 10.1159/000234164. [DOI] [PubMed] [Google Scholar]
- Martin R. W., Duffy J., Engel A. G., Lie J. T., Bowles C. A., Moyer T. P., Gleich G. J. The clinical spectrum of the eosinophilia-myalgia syndrome associated with L-tryptophan ingestion. Clinical features in 20 patients and aspects of pathophysiology. Ann Intern Med. 1990 Jul 15;113(2):124–134. doi: 10.7326/0003-4819-113-2-124. [DOI] [PubMed] [Google Scholar]
- Miesfeld R., Rusconi S., Godowski P. J., Maler B. A., Okret S., Wikström A. C., Gustafsson J. A., Yamamoto K. R. Genetic complementation of a glucocorticoid receptor deficiency by expression of cloned receptor cDNA. Cell. 1986 Aug 1;46(3):389–399. doi: 10.1016/0092-8674(86)90659-8. [DOI] [PubMed] [Google Scholar]
- Moutsopoulos H. M., Webber B. L., Pavlidis N. A., Fostiropoulos G., Goules D., Shulman L. E. Diffuse fasciitis with eosinophilia. A clinicopathologic study. Am J Med. 1980 May;68(5):701–709. doi: 10.1016/0002-9343(80)90257-0. [DOI] [PubMed] [Google Scholar]
- Seuwen K., Magnaldo I., Pouysségur J. Serotonin stimulates DNA synthesis in fibroblasts acting through 5-HT1B receptors coupled to a Gi-protein. Nature. 1988 Sep 15;335(6187):254–256. doi: 10.1038/335254a0. [DOI] [PubMed] [Google Scholar]
- Silver R. M., Heyes M. P., Maize J. C., Quearry B., Vionnet-Fuasset M., Sternberg E. M. Scleroderma, fasciitis, and eosinophilia associated with the ingestion of tryptophan. N Engl J Med. 1990 Mar 29;322(13):874–881. doi: 10.1056/NEJM199003293221302. [DOI] [PubMed] [Google Scholar]
- Slutsker L., Hoesly F. C., Miller L., Williams L. P., Watson J. C., Fleming D. W. Eosinophilia-myalgia syndrome associated with exposure to tryptophan from a single manufacturer. JAMA. 1990 Jul 11;264(2):213–217. [PubMed] [Google Scholar]
- Sternberg E. M., Hill J. M., Chrousos G. P., Kamilaris T., Listwak S. J., Gold P. W., Wilder R. L. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2374–2378. doi: 10.1073/pnas.86.7.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg E. M., Van Woert M. H., Young S. N., Magnussen I., Baker H., Gauthier S., Osterland C. K. Development of a scleroderma-like illness during therapy with L-5-hydroxytryptophan and carbidopa. N Engl J Med. 1980 Oct 2;303(14):782–787. doi: 10.1056/NEJM198010023031403. [DOI] [PubMed] [Google Scholar]
- Sternberg E. M., Young W. S., 3rd, Bernardini R., Calogero A. E., Chrousos G. P., Gold P. W., Wilder R. L. A central nervous system defect in biosynthesis of corticotropin-releasing hormone is associated with susceptibility to streptococcal cell wall-induced arthritis in Lewis rats. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4771–4775. doi: 10.1073/pnas.86.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield H. J., Jr, Brady L. S., Smith M. A., Mamalaki E., Fox R. J., Herkenham M. Optimization of cRNA probe in situ hybridization methodology for localization of glucocorticoid receptor mRNA in rat brain: a detailed protocol. Cell Mol Neurobiol. 1990 Mar;10(1):145–157. doi: 10.1007/BF00733641. [DOI] [PMC free article] [PubMed] [Google Scholar]








