Abstract
The title compound, C8H10N2O2, is stabilized by three intermolecular hydrogen bonds of the N—H⋯O and N—H⋯N types. Two intramolecular interactions of the N—H⋯O and C—H⋯O types are also observed.
Related literature
For related structures see: Ashiq, Jamal et al. (2008 ▶); Jamal et al. (2008 ▶), Kallel et al. (1992 ▶); Saraogi et al. (2002 ▶); For the biological activity of hydrazides, see: Ara et al. (2007 ▶); Ashiq, Ara et al. (2008 ▶); El-Emam et al. (2004 ▶); Maqsood et al. (2006 ▶).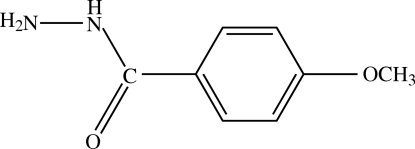
Experimental
Crystal data
C8H10N2O2
M r = 166.18
Orthorhombic,
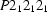
a = 3.9887 (1) Å
b = 6.1487 (2) Å
c = 32.8919 (9) Å
V = 806.68 (4) Å3
Z = 4
Mo Kα radiation
μ = 0.10 mm−1
T = 296 K
0.22 × 0.12 × 0.10 mm
Data collection
Bruker Kappa APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2005 ▶) T min = 0.979, T max = 0.992
17597 measured reflections
1288 independent reflections
1052 reflections with I > 2σ(I)
R int = 0.035
Refinement
R[F 2 > 2σ(F 2)] = 0.043
wR(F 2) = 0.147
S = 1.03
1288 reflections
119 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.39 e Å−3
Δρmin = −0.24 e Å−3
Data collection: APEX2 (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809021345/pv2163sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809021345/pv2163Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1⋯N2i | 0.83 (4) | 2.16 (4) | 2.961 (3) | 162 (3) |
| N2—H2A⋯O1 | 0.92 (5) | 2.42 (4) | 2.729 (2) | 100 (3) |
| N2—H2A⋯O1ii | 0.92 (5) | 2.44 (4) | 3.026 (2) | 122 (3) |
| N2—H2B⋯O1iii | 0.93 (4) | 2.07 (4) | 2.991 (2) | 170 (4) |
| C7—H7⋯O1 | 0.93 | 2.47 | 2.781 (3) | 100 |
Symmetry codes: (i) 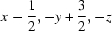 ; (ii)
; (ii) 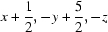 ; (iii)
; (iii) 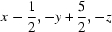 .
.
Acknowledgments
The authors thank the Higher Education Commission Pakistan for providing the diffractometer at GCU, Lahore, and BANA International for their support in collecting the crystallographic data.
supplementary crystallographic information
Comment
Hydrazides are known to have different biological activities and have been used for the synthesis of various heterocyclic compounds (El-Emam et al., 2004). The title compound was found to be antileishmanial, antibacterial and antifungal (Maqsood et al., 2006). Vanadium complex of the title compound was found to ba a good inhibitor of urease (Ara et al., 2007) and alpha-glucosidase (Ashiq, Ara et al., 2008). In order to study the biological behaviour of 4-methoxybenzhydrazide and to investigate the change in activity due to complexation with vanadium center, we have synthesized (I) and report its crystal structure in this paper. The structures of benzhydrazide (Kallel et al., 1992), para-chloro (Saraogi et al., 2002), para-bromo (Ashiq, Jamal et al., 2008) and para-iodo (Jamal et al., 2008) analogues of (I) have already been reported.
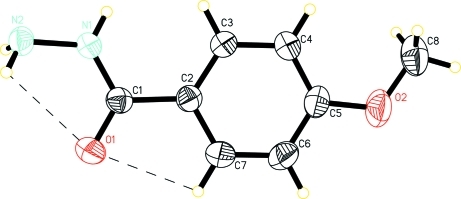
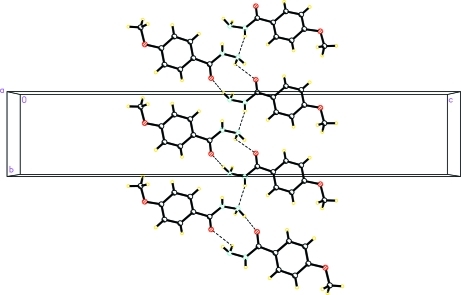
The crystal structure of the title compound is presented in Fig. 1. The bond distances and bond angles in (I) are similar to the corresponding distances and angles reported in the structures quoted above. The phenyl group (C2—C7) and hydrazide moiety, O1/N1/N2/C1, in (I) are each planar with a dihedral angle between their least square planes being 7.08 (14)%. In the crystal structure, the molecules of I are linked by the N1—H1···N2, N2—H2A···O1 and N2—H2B···O1 intermolecular hydrogen bonds to form chains (details are given in Table 1, Fig 2). The geometry of 4-methoxybenzhydrazide is stabilized by N2—H2A···O1 and C7—H7···O1 intramolecular hydrogen interactions.
Experimental
All reagent-grade chemicals were obtained from Aldrich and Sigma Chemical companies and were used without further purification. To a solution of ethyl-4-methoxybenzoate (3.6 g, 20 mmol) in 75 ml ethanol, hydrazine hydrate (5.0 ml, 100 mmol) was added. The mixture was refluxed for 5 h and a solid was obtained upon removal of the solvent by rotary evaporation. The resulting solid was washed with hexane to afford 4-methoxybenzhydrazide (yield 64%). (Maqsood et al., 2006).
Refinement
An absolute structure was not established due to lack of sufficient anomalous dispersion effects. Therefore, Friedel pairs (236) were merged. H atoms were positioned geometrically, with C—H = 0.93 and 0.96 Å for aromatic and methyl C-atoms and constrained to ride on their parent atoms. The H-atoms attached to N1 and N2 atoms were taken from Fourier synthesis and their coordinates were refined. The thermal parameter of H-atoms were: Uiso(H) = 1.5Ueq(methyl C) and 1.2Ueq(the rest of the parent atoms).
Figures
Fig. 1.
The molecular structure of (I) with displacement ellipsoids drawn at 50% probability level. The dashed lines indicates the intramolecular interactions.
Fig. 2.
A packing diagram of (I). Hydrogen bonds are shown by dashed lines.
Crystal data
| C8H10N2O2 | F(000) = 352 |
| Mr = 166.18 | Dx = 1.368 Mg m−3 |
| Orthorhombic, P212121 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 1288 reflections |
| a = 3.9887 (1) Å | θ = 1.2–28.7° |
| b = 6.1487 (2) Å | µ = 0.10 mm−1 |
| c = 32.8919 (9) Å | T = 296 K |
| V = 806.68 (4) Å3 | Needle, colourless |
| Z = 4 | 0.22 × 0.12 × 0.10 mm |
Data collection
| Bruker Kappa APEXII CCD diffractometer | 1288 independent reflections |
| Radiation source: fine-focus sealed tube | 1052 reflections with I > 2σ(I) |
| graphite | Rint = 0.035 |
| Detector resolution: 7.40 pixels mm-1 | θmax = 28.7°, θmin = 1.2° |
| ω scans | h = −5→5 |
| Absorption correction: multi-scan (SADABS; Bruker, 2005) | k = −8→8 |
| Tmin = 0.979, Tmax = 0.992 | l = −44→44 |
| 17597 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.043 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.147 | w = 1/[σ2(Fo2) + (0.1097P)2 + 0.027P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.03 | (Δ/σ)max < 0.001 |
| 1288 reflections | Δρmax = 0.39 e Å−3 |
| 119 parameters | Δρmin = −0.24 e Å−3 |
| 0 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.058 (15) |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell e.s.d.'s are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.9709 (4) | 1.2503 (3) | 0.05610 (4) | 0.0459 (5) | |
| O2 | 0.2810 (5) | 0.8530 (3) | 0.21180 (5) | 0.0600 (6) | |
| N1 | 0.8024 (5) | 0.9374 (3) | 0.02782 (5) | 0.0384 (5) | |
| N2 | 0.9644 (5) | 0.9802 (3) | −0.00972 (5) | 0.0376 (5) | |
| C1 | 0.8272 (5) | 1.0744 (3) | 0.05921 (5) | 0.0320 (5) | |
| C2 | 0.6754 (5) | 1.0039 (3) | 0.09850 (5) | 0.0325 (5) | |
| C3 | 0.5396 (7) | 0.8001 (4) | 0.10519 (6) | 0.0427 (6) | |
| C4 | 0.4053 (6) | 0.7433 (4) | 0.14251 (6) | 0.0436 (7) | |
| C5 | 0.4061 (6) | 0.8925 (4) | 0.17369 (6) | 0.0415 (6) | |
| C6 | 0.5410 (8) | 1.0960 (4) | 0.16743 (6) | 0.0505 (8) | |
| C7 | 0.6748 (6) | 1.1507 (4) | 0.13034 (7) | 0.0441 (7) | |
| C8 | 0.1357 (9) | 0.6492 (5) | 0.21996 (8) | 0.0627 (10) | |
| H1 | 0.694 (10) | 0.822 (6) | 0.0282 (9) | 0.0752* | |
| H2A | 1.157 (12) | 1.055 (6) | −0.0037 (11) | 0.0940* | |
| H2B | 0.832 (12) | 1.068 (6) | −0.0262 (10) | 0.0940* | |
| H3 | 0.53858 | 0.69896 | 0.08416 | 0.0513* | |
| H4 | 0.31513 | 0.60542 | 0.14648 | 0.0523* | |
| H6 | 0.54148 | 1.19702 | 0.18846 | 0.0606* | |
| H7 | 0.76633 | 1.28832 | 0.12659 | 0.0529* | |
| H8A | 0.05128 | 0.64745 | 0.24729 | 0.0940* | |
| H8B | 0.30181 | 0.53746 | 0.21686 | 0.0940* | |
| H8C | −0.04508 | 0.62368 | 0.20129 | 0.0940* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0595 (10) | 0.0336 (8) | 0.0445 (8) | −0.0109 (8) | 0.0000 (7) | 0.0023 (7) |
| O2 | 0.0770 (12) | 0.0652 (12) | 0.0379 (9) | 0.0043 (11) | 0.0195 (8) | 0.0041 (8) |
| N1 | 0.0521 (10) | 0.0313 (9) | 0.0317 (8) | −0.0065 (9) | 0.0048 (7) | 0.0016 (7) |
| N2 | 0.0451 (9) | 0.0348 (10) | 0.0329 (8) | 0.0030 (8) | 0.0062 (7) | 0.0040 (7) |
| C1 | 0.0350 (9) | 0.0285 (9) | 0.0325 (9) | 0.0020 (8) | −0.0038 (7) | 0.0031 (8) |
| C2 | 0.0352 (9) | 0.0317 (9) | 0.0306 (9) | 0.0028 (8) | −0.0027 (7) | 0.0018 (8) |
| C3 | 0.0593 (12) | 0.0363 (11) | 0.0326 (9) | −0.0064 (11) | 0.0021 (10) | −0.0029 (8) |
| C4 | 0.0544 (12) | 0.0384 (12) | 0.0379 (10) | −0.0068 (11) | 0.0031 (9) | 0.0044 (9) |
| C5 | 0.0457 (10) | 0.0469 (13) | 0.0318 (10) | 0.0076 (10) | 0.0033 (8) | 0.0031 (9) |
| C6 | 0.0708 (16) | 0.0418 (13) | 0.0390 (11) | 0.0024 (13) | 0.0038 (11) | −0.0096 (10) |
| C7 | 0.0566 (12) | 0.0318 (11) | 0.0438 (11) | −0.0025 (11) | 0.0022 (11) | −0.0023 (9) |
| C8 | 0.0665 (16) | 0.0722 (19) | 0.0493 (14) | 0.0039 (17) | 0.0159 (12) | 0.0163 (13) |
Geometric parameters (Å, °)
| O1—C1 | 1.228 (3) | C3—C4 | 1.384 (3) |
| O2—C5 | 1.371 (3) | C4—C5 | 1.376 (3) |
| O2—C8 | 1.407 (4) | C5—C6 | 1.378 (4) |
| N1—N2 | 1.418 (2) | C6—C7 | 1.373 (3) |
| N1—C1 | 1.336 (2) | C3—H3 | 0.9300 |
| N1—H1 | 0.83 (4) | C4—H4 | 0.9300 |
| N2—H2A | 0.92 (5) | C6—H6 | 0.9300 |
| N2—H2B | 0.93 (4) | C7—H7 | 0.9300 |
| C1—C2 | 1.492 (2) | C8—H8A | 0.9600 |
| C2—C7 | 1.383 (3) | C8—H8B | 0.9600 |
| C2—C3 | 1.383 (3) | C8—H8C | 0.9600 |
| C5—O2—C8 | 118.84 (19) | O2—C5—C6 | 116.1 (2) |
| N2—N1—C1 | 121.47 (17) | C5—C6—C7 | 120.5 (2) |
| C1—N1—H1 | 124 (2) | C2—C7—C6 | 120.9 (2) |
| N2—N1—H1 | 114 (2) | C2—C3—H3 | 119.00 |
| N1—N2—H2B | 111 (3) | C4—C3—H3 | 119.00 |
| H2A—N2—H2B | 108 (4) | C3—C4—H4 | 120.00 |
| N1—N2—H2A | 107 (2) | C5—C4—H4 | 120.00 |
| O1—C1—N1 | 121.69 (17) | C5—C6—H6 | 120.00 |
| N1—C1—C2 | 117.14 (17) | C7—C6—H6 | 120.00 |
| O1—C1—C2 | 121.17 (16) | C2—C7—H7 | 120.00 |
| C1—C2—C7 | 117.86 (18) | C6—C7—H7 | 120.00 |
| C3—C2—C7 | 118.06 (18) | O2—C8—H8A | 109.00 |
| C1—C2—C3 | 124.08 (16) | O2—C8—H8B | 109.00 |
| C2—C3—C4 | 121.4 (2) | O2—C8—H8C | 109.00 |
| C3—C4—C5 | 119.5 (2) | H8A—C8—H8B | 109.00 |
| O2—C5—C4 | 124.2 (2) | H8A—C8—H8C | 109.00 |
| C4—C5—C6 | 119.7 (2) | H8B—C8—H8C | 109.00 |
| C8—O2—C5—C4 | −1.3 (4) | C7—C2—C3—C4 | 0.2 (4) |
| C8—O2—C5—C6 | 179.2 (3) | C1—C2—C7—C6 | −179.6 (2) |
| N2—N1—C1—O1 | 5.3 (3) | C3—C2—C7—C6 | −0.5 (4) |
| N2—N1—C1—C2 | −174.18 (18) | C2—C3—C4—C5 | 0.1 (4) |
| O1—C1—C2—C3 | −173.4 (2) | C3—C4—C5—O2 | −179.7 (2) |
| O1—C1—C2—C7 | 5.7 (3) | C3—C4—C5—C6 | −0.2 (4) |
| N1—C1—C2—C3 | 6.1 (3) | O2—C5—C6—C7 | 179.6 (2) |
| N1—C1—C2—C7 | −174.9 (2) | C4—C5—C6—C7 | −0.1 (4) |
| C1—C2—C3—C4 | 179.3 (2) | C5—C6—C7—C2 | 0.4 (4) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1···N2i | 0.83 (4) | 2.16 (4) | 2.961 (3) | 162 (3) |
| N2—H2A···O1 | 0.92 (5) | 2.42 (4) | 2.729 (2) | 100 (3) |
| N2—H2A···O1ii | 0.92 (5) | 2.44 (4) | 3.026 (2) | 122 (3) |
| N2—H2B···O1iii | 0.93 (4) | 2.07 (4) | 2.991 (2) | 170 (4) |
| C7—H7···O1 | 0.93 | 2.47 | 2.781 (3) | 100 |
Symmetry codes: (i) x−1/2, −y+3/2, −z; (ii) x+1/2, −y+5/2, −z; (iii) x−1/2, −y+5/2, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: PV2163).
References
- Ara, R., Ashiq, U., Mahroof-Tahir, M., Maqsood, Z. T., Khan, K. M., Lodhi, M. A. & Choudhary, M. I. (2007). Chem. Biodivers.4, 58–71. [DOI] [PubMed]
- Ashiq, U., Ara, R., Mahroof-Tahir, M., Maqsood, Z. T., Khan, K. M., Khan, S. N., Siddiqui, H. & Choudhary, M. I. (2008). Chem. Biodivers.5, 82–92. [DOI] [PubMed]
- Ashiq, U., Jamal, R. A., Mahroof-Tahir, M., Keramidas, A. D., Maqsood, Z. T., Khan, K. M. & Tahir, M. N. (2008). Anal. Sci X, 24, 103–104.
- Bruker (2005). SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2007). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- El-Emam, A. A., Al-Deeb, O. A., Al-Omar, M. & Lehmann, J. (2004). Bioorg. Med. Chem.12, 5107–5113. [DOI] [PubMed]
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Jamal, R. A., Ashiq, U., Arshad, M. N., Maqsood, Z. T. & Khan, I. U. (2008). Acta Cryst. E64, o2188. [DOI] [PMC free article] [PubMed]
- Kallel, A., Amor, B. H., Svoboda, I. & Fuess, H. (1992). Z. Kristallogr.198, 137–140.
- Maqsood, Z. T., Khan, K. M., Ashiq, U., Jamal, R. A., Chohan, Z. H., Mahroof-Tahir, M. & Supuran, C. T. (2006). J. Enz. Inhib. Med. Chem.21, 37–42. [DOI] [PubMed]
- Saraogi, I., Mruthyunjayaswamy, B. H. M., Ijare, O. B., Jadegoud, Y. & Guru Row, T. N. (2002). Acta Cryst. E58, o1341–o1342.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809021345/pv2163sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809021345/pv2163Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report