Abstract
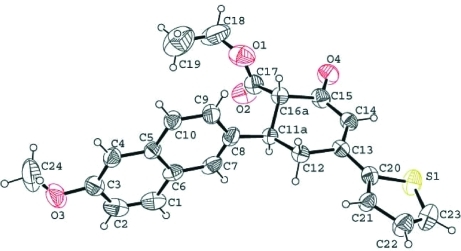
The title compound, C24H22O4S, was prepared by reaction between (2E)-3-(6-methoxy-2-naphthyl)-1-(2-thienyl)prop-2-en-1-one and ethyl acetoacetate. In the crystal, the cyclohexenone ring shows a distorted half-chair conformation. The length of the double bond in the cyclohexenone ring [1.343 (4) Å] is normal.
Related literature
For related structures, see: Fischer et al. (2007a ▶,b
▶; 2008a ▶,b
▶). For the use of cyclohexenones in organic synthesis, see: Padmavathi et al. (1999 ▶, 2001 ▶). For pharmaceutical applications of cyclohexenone derivatives, see: Hoye & Tennakoon (2000 ▶); Hiromichi et al. (2002 ▶).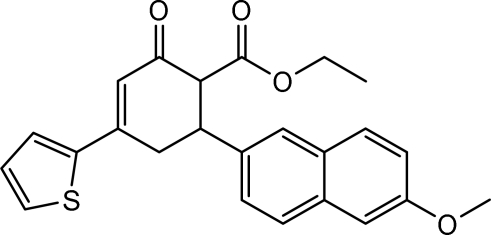
Experimental
Crystal data
C24H22O4S
M r = 406.48
Monoclinic,
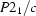
a = 18.2501 (4) Å
b = 11.7176 (2) Å
c = 9.6846 (2) Å
β = 93.048 (1)°
V = 2068.10 (7) Å3
Z = 4
Mo Kα radiation
μ = 0.18 mm−1
T = 296 K
0.45 × 0.29 × 0.16 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2004 ▶) T min = 0.922, T max = 0.972
17446 measured reflections
4035 independent reflections
2880 reflections with I > 2σ(I)
R int = 0.028
Refinement
R[F 2 > 2σ(F 2)] = 0.063
wR(F 2) = 0.202
S = 1.02
4035 reflections
282 parameters
H-atom parameters constrained
Δρmax = 0.26 e Å−3
Δρmin = −0.38 e Å−3
Data collection: SMART (Bruker, 2001 ▶); cell refinement: SAINT (Bruker, 2001 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809021308/sj2629sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809021308/sj2629Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
ANM thanks the University of Mysore for research facilities.
supplementary crystallographic information
Comment
Cyclohexenones are efficient synthons in building spiro compounds (Padmavathi et al., 2001) or intermediates in the synthesis of benzisoxazole or carbazole derivatives (Padmavathi et al., 1999). Cyclohexenone derivatives are well known lead compounds for the treatment of inflammation and autoimmune diseases (Hoye & Tennakoon, 2000; Hiromichi et al., 2002).
The crystal structures of a series of ethyl 6-substituted 2-oxocyclohex- 3-ene-1-carboxylates have been reported (Fischer et al., 2007; 2007a,b; 2008ab). In view of the importance of these derivatives and continuing our efforts in this field, the title compound, ethyl 6-(6- methoxynaphthalen-2-yl)-2-oxo-4-(2-thienyl)cyclohexa-3-ene-1- carboxylate, was synthesized and its crystal structure is reported in this paper.
Experimental
(2E)-3-(6-methoxy-2-naphthyl)-1-(2-thienyl)prop-2-en-1-one (1.51 g, 5 mmol) and ethyl acetoacetate (0.65 g, 5 mmol) were refluxed for 6 hr in 10–15 ml of ethanol in the presence of 0.8 ml 10% NaOH. The reaction mixture was cooled to room temperature and the resulting product was filtered and recrystallized from acetonitrile (m.p.: 415–418 K). Analysis % found (calculated): C, 71.19 (71.27); H, 4.94 (4.98); S, 7.89 (7.93).
Refinement
All H atoms were placed in idealized locations (C—H = 0.93–0.98 Å) and refined as riding with Uiso(H) = 1.2Ueq(C) or 1.5Ueq(methyl C). The methyl groups were allowed to rotate, but not to tip, to best fit the electron density. The large difference between min and max Uiso values for the H atoms is a result of unresolved disorder in the ethyl side chain.
Figures
Fig. 1.
A view of the crystal structure of the title compound.
Crystal data
| C24H22O4S | F(000) = 856 |
| Mr = 406.48 | Dx = 1.305 Mg m−3 |
| Monoclinic, P21/c | Melting point = 415–418 K |
| Hall symbol: -P 2ybc | Mo Kα radiation, λ = 0.71073 Å |
| a = 18.2501 (4) Å | Cell parameters from 8575 reflections |
| b = 11.7176 (2) Å | θ = 2.3–28.1° |
| c = 9.6846 (2) Å | µ = 0.18 mm−1 |
| β = 93.048 (1)° | T = 296 K |
| V = 2068.10 (7) Å3 | Block, colourless |
| Z = 4 | 0.45 × 0.29 × 0.16 mm |
Data collection
| Bruker SMART CCD area-detector diffractometer | 4035 independent reflections |
| Radiation source: fine-focus sealed tube | 2880 reflections with I > 2σ(I) |
| graphite | Rint = 0.028 |
| φ and ω scans | θmax = 26.0°, θmin = 2.2° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2004) | h = −22→22 |
| Tmin = 0.922, Tmax = 0.972 | k = −11→14 |
| 17446 measured reflections | l = −11→10 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.063 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.202 | H-atom parameters constrained |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.095P)2 + 0.9907P] where P = (Fo2 + 2Fc2)/3 |
| 4035 reflections | (Δ/σ)max = 0.001 |
| 282 parameters | Δρmax = 0.26 e Å−3 |
| 0 restraints | Δρmin = −0.37 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| C1 | 0.1379 (2) | 0.5193 (3) | 0.4085 (3) | 0.0917 (10) | |
| H1A | 0.1524 | 0.4568 | 0.4625 | 0.110* | |
| C2 | 0.0777 (2) | 0.5795 (4) | 0.4405 (4) | 0.0955 (12) | |
| H2A | 0.0516 | 0.5577 | 0.5160 | 0.115* | |
| C3 | 0.05423 (16) | 0.6739 (3) | 0.3619 (4) | 0.0845 (9) | |
| C4 | 0.09205 (15) | 0.7057 (3) | 0.2525 (3) | 0.0733 (8) | |
| H4A | 0.0762 | 0.7682 | 0.1997 | 0.088* | |
| C5 | 0.15546 (13) | 0.6457 (2) | 0.2168 (3) | 0.0593 (6) | |
| C6 | 0.17879 (16) | 0.5499 (2) | 0.2952 (3) | 0.0661 (7) | |
| C7 | 0.24126 (19) | 0.4896 (3) | 0.2584 (3) | 0.0810 (9) | |
| H7A | 0.2567 | 0.4267 | 0.3108 | 0.097* | |
| C8 | 0.28020 (16) | 0.5208 (3) | 0.1474 (3) | 0.0787 (9) | |
| C9 | 0.25672 (16) | 0.6164 (3) | 0.0705 (3) | 0.0768 (8) | |
| H9A | 0.2829 | 0.6392 | −0.0045 | 0.092* | |
| C10 | 0.19634 (15) | 0.6766 (3) | 0.1033 (3) | 0.0694 (7) | |
| H10A | 0.1818 | 0.7393 | 0.0497 | 0.083* | |
| C11A | 0.3387 (4) | 0.4303 (7) | 0.1361 (8) | 0.0567 (17) | 0.489 (11) |
| H11A | 0.3274 | 0.3649 | 0.1944 | 0.068* | 0.489 (11) |
| C11B | 0.3537 (3) | 0.4735 (6) | 0.0866 (7) | 0.0498 (15) | 0.511 (11) |
| H11B | 0.3642 | 0.5140 | 0.0013 | 0.060* | 0.511 (11) |
| C12 | 0.41517 (14) | 0.4837 (2) | 0.1877 (3) | 0.0587 (6) | |
| H12A | 0.4212 | 0.5568 | 0.1426 | 0.070* | |
| H12B | 0.4149 | 0.4974 | 0.2864 | 0.070* | |
| C13 | 0.47940 (14) | 0.4085 (2) | 0.1587 (3) | 0.0563 (6) | |
| C14 | 0.47374 (16) | 0.3283 (2) | 0.0596 (3) | 0.0654 (7) | |
| H14A | 0.5156 | 0.2875 | 0.0396 | 0.078* | |
| C15 | 0.40620 (18) | 0.3025 (3) | −0.0168 (3) | 0.0779 (8) | |
| C16A | 0.3403 (3) | 0.3921 (6) | −0.0137 (8) | 0.0659 (19) | 0.511 (11) |
| H16A | 0.3472 | 0.4565 | −0.0763 | 0.079* | 0.511 (11) |
| C16B | 0.3388 (3) | 0.3456 (5) | 0.0561 (7) | 0.0501 (17) | 0.489 (11) |
| H16B | 0.3323 | 0.3031 | 0.1418 | 0.060* | 0.489 (11) |
| C17 | 0.27068 (18) | 0.3302 (3) | −0.0483 (4) | 0.0771 (8) | |
| C18 | 0.1848 (5) | 0.3678 (7) | −0.2222 (6) | 0.190 (3) | |
| H18A | 0.1700 | 0.2910 | −0.1987 | 0.228* | |
| H18B | 0.1906 | 0.3710 | −0.3211 | 0.228* | |
| C19 | 0.1297 (5) | 0.4479 (7) | −0.1848 (8) | 0.212 (3) | |
| H19A | 0.0839 | 0.4294 | −0.2329 | 0.317* | |
| H19B | 0.1442 | 0.5236 | −0.2095 | 0.317* | |
| H19C | 0.1240 | 0.4443 | −0.0869 | 0.317* | |
| C20 | 0.54589 (14) | 0.4261 (2) | 0.2453 (3) | 0.0628 (7) | |
| C21 | 0.56318 (14) | 0.5200 (2) | 0.3318 (3) | 0.0689 (7) | |
| H21A | 0.5331 | 0.5831 | 0.3424 | 0.083* | |
| C22 | 0.63359 (19) | 0.5044 (3) | 0.4008 (4) | 0.0956 (10) | |
| H22A | 0.6556 | 0.5588 | 0.4595 | 0.115* | |
| C23 | 0.66455 (19) | 0.4056 (3) | 0.3736 (5) | 0.1036 (12) | |
| H23A | 0.7098 | 0.3828 | 0.4128 | 0.124* | |
| C24 | −0.0321 (3) | 0.8260 (6) | 0.3341 (8) | 0.169 (3) | |
| H24A | −0.0767 | 0.8524 | 0.3713 | 0.254* | |
| H24B | −0.0408 | 0.8082 | 0.2379 | 0.254* | |
| H24C | 0.0047 | 0.8844 | 0.3443 | 0.254* | |
| S1 | 0.61390 (5) | 0.32512 (7) | 0.26068 (12) | 0.0945 (4) | |
| O1 | 0.2523 (2) | 0.3953 (3) | −0.1501 (3) | 0.1250 (10) | |
| O2 | 0.23644 (17) | 0.2511 (2) | −0.0137 (3) | 0.1130 (9) | |
| O3 | −0.00726 (14) | 0.7259 (3) | 0.4069 (3) | 0.1238 (11) | |
| O4 | 0.40154 (14) | 0.2313 (2) | −0.1083 (2) | 0.0996 (8) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.127 (3) | 0.076 (2) | 0.070 (2) | −0.028 (2) | −0.0062 (19) | 0.0070 (16) |
| C2 | 0.106 (3) | 0.110 (3) | 0.071 (2) | −0.048 (2) | 0.0134 (18) | −0.006 (2) |
| C3 | 0.0610 (16) | 0.109 (3) | 0.084 (2) | −0.0190 (16) | 0.0062 (14) | −0.015 (2) |
| C4 | 0.0597 (15) | 0.080 (2) | 0.0794 (18) | 0.0007 (13) | −0.0029 (13) | −0.0023 (15) |
| C5 | 0.0576 (13) | 0.0555 (15) | 0.0637 (14) | −0.0041 (11) | −0.0067 (11) | −0.0018 (12) |
| C6 | 0.0832 (17) | 0.0517 (15) | 0.0614 (15) | −0.0115 (13) | −0.0138 (13) | −0.0029 (12) |
| C7 | 0.104 (2) | 0.0579 (17) | 0.077 (2) | 0.0198 (16) | −0.0339 (17) | −0.0135 (15) |
| C8 | 0.0733 (17) | 0.079 (2) | 0.080 (2) | 0.0214 (15) | −0.0240 (15) | −0.0337 (17) |
| C9 | 0.0673 (16) | 0.085 (2) | 0.0781 (18) | 0.0060 (15) | 0.0041 (14) | −0.0083 (16) |
| C10 | 0.0680 (16) | 0.0662 (18) | 0.0738 (17) | 0.0069 (13) | 0.0020 (13) | 0.0082 (14) |
| C11A | 0.067 (3) | 0.047 (4) | 0.056 (4) | −0.003 (3) | 0.002 (3) | 0.006 (3) |
| C11B | 0.062 (3) | 0.037 (3) | 0.050 (3) | 0.001 (2) | 0.003 (2) | 0.005 (2) |
| C12 | 0.0691 (14) | 0.0452 (13) | 0.0620 (14) | 0.0002 (11) | 0.0051 (11) | −0.0005 (11) |
| C13 | 0.0683 (14) | 0.0429 (13) | 0.0588 (14) | 0.0007 (10) | 0.0134 (11) | 0.0065 (11) |
| C14 | 0.0757 (16) | 0.0535 (15) | 0.0682 (16) | 0.0077 (12) | 0.0151 (13) | −0.0001 (13) |
| C15 | 0.089 (2) | 0.0624 (17) | 0.0824 (19) | 0.0045 (15) | 0.0099 (15) | −0.0215 (16) |
| C16A | 0.085 (4) | 0.052 (4) | 0.061 (4) | −0.002 (3) | 0.003 (3) | 0.002 (3) |
| C16B | 0.068 (3) | 0.037 (3) | 0.045 (3) | −0.002 (2) | 0.001 (2) | 0.001 (3) |
| C17 | 0.0830 (19) | 0.0625 (19) | 0.086 (2) | −0.0068 (15) | 0.0024 (16) | −0.0232 (17) |
| C18 | 0.266 (8) | 0.195 (6) | 0.103 (4) | 0.108 (6) | −0.067 (5) | −0.030 (4) |
| C19 | 0.222 (8) | 0.195 (7) | 0.209 (7) | 0.017 (7) | −0.066 (6) | −0.024 (6) |
| C20 | 0.0628 (14) | 0.0547 (15) | 0.0719 (16) | 0.0001 (11) | 0.0139 (12) | 0.0094 (13) |
| C21 | 0.0631 (14) | 0.0668 (17) | 0.0753 (17) | 0.0019 (12) | −0.0092 (12) | −0.0095 (14) |
| C22 | 0.084 (2) | 0.090 (2) | 0.110 (3) | −0.0001 (19) | −0.0164 (19) | −0.011 (2) |
| C23 | 0.0742 (19) | 0.091 (3) | 0.144 (3) | −0.0013 (18) | −0.015 (2) | 0.014 (2) |
| C24 | 0.101 (3) | 0.190 (6) | 0.220 (6) | 0.053 (4) | 0.038 (4) | −0.022 (5) |
| S1 | 0.0799 (5) | 0.0638 (5) | 0.1391 (9) | 0.0073 (4) | −0.0014 (5) | 0.0047 (5) |
| O1 | 0.171 (3) | 0.105 (2) | 0.100 (2) | 0.004 (2) | 0.022 (2) | 0.0166 (17) |
| O2 | 0.140 (2) | 0.0862 (18) | 0.1104 (19) | −0.0330 (17) | −0.0168 (17) | 0.0061 (15) |
| O3 | 0.0724 (15) | 0.181 (3) | 0.120 (2) | −0.0047 (18) | 0.0278 (15) | −0.032 (2) |
| O4 | 0.1168 (18) | 0.0868 (16) | 0.0945 (16) | 0.0164 (13) | −0.0023 (13) | −0.0425 (14) |
Geometric parameters (Å, °)
| C1—C2 | 1.354 (5) | C14—C15 | 1.436 (4) |
| C1—C6 | 1.406 (4) | C14—H14A | 0.9300 |
| C1—H1A | 0.9300 | C15—O4 | 1.217 (3) |
| C2—C3 | 1.396 (5) | C15—C16B | 1.536 (6) |
| C2—H2A | 0.9300 | C15—C16A | 1.599 (7) |
| C3—C4 | 1.347 (4) | C16A—C17 | 1.486 (7) |
| C3—O3 | 1.369 (4) | C16A—H16A | 0.9800 |
| C4—C5 | 1.413 (4) | C16B—C17 | 1.571 (6) |
| C4—H4A | 0.9300 | C16B—H16B | 0.9800 |
| C5—C10 | 1.408 (4) | C17—O2 | 1.176 (4) |
| C5—C6 | 1.409 (4) | C17—O1 | 1.277 (4) |
| C6—C7 | 1.403 (4) | C18—O1 | 1.420 (7) |
| C7—C8 | 1.370 (5) | C18—C19 | 1.437 (9) |
| C7—H7A | 0.9300 | C18—H18A | 0.9700 |
| C8—C9 | 1.400 (5) | C18—H18B | 0.9700 |
| C8—C11A | 1.513 (7) | C19—H19A | 0.9600 |
| C8—C11B | 1.593 (6) | C19—H19B | 0.9600 |
| C9—C10 | 1.360 (4) | C19—H19C | 0.9600 |
| C9—H9A | 0.9300 | C20—C21 | 1.409 (4) |
| C10—H10A | 0.9300 | C20—S1 | 1.715 (3) |
| C11A—C16A | 1.520 (8) | C21—C22 | 1.428 (4) |
| C11A—C12 | 1.586 (7) | C21—H21A | 0.9300 |
| C11A—H11A | 0.9800 | C22—C23 | 1.321 (5) |
| C11B—C12 | 1.455 (6) | C22—H22A | 0.9300 |
| C11B—C16B | 1.549 (7) | C23—S1 | 1.683 (4) |
| C11B—H11B | 0.9800 | C23—H23A | 0.9300 |
| C12—C13 | 1.505 (3) | C24—O3 | 1.429 (7) |
| C12—H12A | 0.9700 | C24—H24A | 0.9600 |
| C12—H12B | 0.9700 | C24—H24B | 0.9600 |
| C13—C14 | 1.343 (4) | C24—H24C | 0.9600 |
| C13—C20 | 1.453 (4) | ||
| C2—C1—C6 | 121.1 (3) | C13—C14—H14A | 118.4 |
| C2—C1—H1A | 119.5 | C15—C14—H14A | 118.4 |
| C6—C1—H1A | 119.5 | O4—C15—C14 | 123.0 (3) |
| C1—C2—C3 | 121.1 (3) | O4—C15—C16B | 122.3 (3) |
| C1—C2—H2A | 119.4 | C14—C15—C16B | 112.3 (3) |
| C3—C2—H2A | 119.4 | O4—C15—C16A | 116.1 (3) |
| C4—C3—O3 | 126.1 (4) | C14—C15—C16A | 118.6 (3) |
| C4—C3—C2 | 119.5 (3) | C16B—C15—C16A | 32.2 (2) |
| O3—C3—C2 | 114.4 (3) | C17—C16A—C11A | 107.4 (5) |
| C3—C4—C5 | 121.0 (3) | C17—C16A—C15 | 108.1 (4) |
| C3—C4—H4A | 119.5 | C11A—C16A—C15 | 105.3 (5) |
| C5—C4—H4A | 119.5 | C17—C16A—H16A | 111.9 |
| C10—C5—C6 | 117.9 (2) | C11A—C16A—H16A | 111.9 |
| C10—C5—C4 | 122.6 (3) | C15—C16A—H16A | 111.9 |
| C6—C5—C4 | 119.5 (3) | C15—C16B—C11B | 105.6 (4) |
| C7—C6—C1 | 122.8 (3) | C15—C16B—C17 | 107.0 (4) |
| C7—C6—C5 | 119.4 (3) | C11B—C16B—C17 | 111.0 (4) |
| C1—C6—C5 | 117.8 (3) | C15—C16B—H16B | 111.0 |
| C8—C7—C6 | 121.8 (3) | C11B—C16B—H16B | 111.0 |
| C8—C7—H7A | 119.1 | C17—C16B—H16B | 111.0 |
| C6—C7—H7A | 119.1 | O2—C17—O1 | 124.7 (3) |
| C7—C8—C9 | 118.3 (3) | O2—C17—C16A | 141.4 (5) |
| C7—C8—C11A | 105.5 (5) | O1—C17—C16A | 93.9 (5) |
| C9—C8—C11A | 136.0 (5) | O2—C17—C16B | 108.7 (4) |
| C7—C8—C11B | 132.8 (4) | O1—C17—C16B | 126.5 (4) |
| C9—C8—C11B | 108.8 (4) | C16A—C17—C16B | 32.9 (3) |
| C11A—C8—C11B | 28.1 (2) | O1—C18—C19 | 109.2 (5) |
| C10—C9—C8 | 121.3 (3) | O1—C18—H18A | 109.8 |
| C10—C9—H9A | 119.3 | C19—C18—H18A | 109.8 |
| C8—C9—H9A | 119.3 | O1—C18—H18B | 109.8 |
| C9—C10—C5 | 121.2 (3) | C19—C18—H18B | 109.8 |
| C9—C10—H10A | 119.4 | H18A—C18—H18B | 108.3 |
| C5—C10—H10A | 119.4 | C18—C19—H19A | 109.5 |
| C8—C11A—C16A | 108.9 (5) | C18—C19—H19B | 109.5 |
| C8—C11A—C12 | 108.2 (5) | H19A—C19—H19B | 109.5 |
| C16A—C11A—C12 | 110.9 (5) | C18—C19—H19C | 109.5 |
| C8—C11A—H11A | 109.6 | H19A—C19—H19C | 109.5 |
| C16A—C11A—H11A | 109.6 | H19B—C19—H19C | 109.5 |
| C12—C11A—H11A | 109.6 | C21—C20—C13 | 127.4 (2) |
| C12—C11B—C16B | 109.2 (4) | C21—C20—S1 | 110.4 (2) |
| C12—C11B—C8 | 110.9 (4) | C13—C20—S1 | 122.1 (2) |
| C16B—C11B—C8 | 105.4 (5) | C20—C21—C22 | 110.3 (3) |
| C12—C11B—H11B | 110.4 | C20—C21—H21A | 124.9 |
| C16B—C11B—H11B | 110.4 | C22—C21—H21A | 124.9 |
| C8—C11B—H11B | 110.4 | C23—C22—C21 | 113.7 (3) |
| C11B—C12—C13 | 114.0 (3) | C23—C22—H22A | 123.2 |
| C11B—C12—C11A | 28.5 (2) | C21—C22—H22A | 123.2 |
| C13—C12—C11A | 113.0 (3) | C22—C23—S1 | 113.3 (3) |
| C11B—C12—H12A | 82.6 | C22—C23—H23A | 123.4 |
| C13—C12—H12A | 109.0 | S1—C23—H23A | 123.4 |
| C11A—C12—H12A | 109.0 | O3—C24—H24A | 109.5 |
| C11B—C12—H12B | 129.3 | O3—C24—H24B | 109.5 |
| C13—C12—H12B | 109.0 | H24A—C24—H24B | 109.5 |
| C11A—C12—H12B | 109.0 | O3—C24—H24C | 109.5 |
| H12A—C12—H12B | 107.8 | H24A—C24—H24C | 109.5 |
| C14—C13—C20 | 122.8 (2) | H24B—C24—H24C | 109.5 |
| C14—C13—C12 | 120.8 (2) | C23—S1—C20 | 92.28 (17) |
| C20—C13—C12 | 116.4 (2) | C17—O1—C18 | 115.4 (4) |
| C13—C14—C15 | 123.2 (3) | C3—O3—C24 | 116.9 (3) |
| C6—C1—C2—C3 | 0.1 (5) | C13—C14—C15—C16B | −18.7 (5) |
| C1—C2—C3—C4 | −0.1 (5) | C13—C14—C15—C16A | 16.4 (6) |
| C1—C2—C3—O3 | −180.0 (3) | C8—C11A—C16A—C17 | −68.1 (6) |
| O3—C3—C4—C5 | 179.3 (3) | C12—C11A—C16A—C17 | 172.9 (6) |
| C2—C3—C4—C5 | −0.5 (5) | C8—C11A—C16A—C15 | 176.9 (6) |
| C3—C4—C5—C10 | −180.0 (3) | C12—C11A—C16A—C15 | 57.9 (6) |
| C3—C4—C5—C6 | 1.1 (4) | O4—C15—C16A—C17 | 39.2 (7) |
| C2—C1—C6—C7 | −179.8 (3) | C14—C15—C16A—C17 | −157.4 (4) |
| C2—C1—C6—C5 | 0.5 (4) | C16B—C15—C16A—C17 | −70.5 (7) |
| C10—C5—C6—C7 | 0.3 (4) | O4—C15—C16A—C11A | 153.7 (4) |
| C4—C5—C6—C7 | 179.2 (2) | C14—C15—C16A—C11A | −42.9 (6) |
| C10—C5—C6—C1 | 179.9 (3) | C16B—C15—C16A—C11A | 44.1 (5) |
| C4—C5—C6—C1 | −1.1 (4) | O4—C15—C16B—C11B | −144.5 (4) |
| C1—C6—C7—C8 | 179.9 (3) | C14—C15—C16B—C11B | 52.6 (5) |
| C5—C6—C7—C8 | −0.4 (4) | C16A—C15—C16B—C11B | −55.9 (5) |
| C6—C7—C8—C9 | 0.7 (4) | O4—C15—C16B—C17 | −26.2 (7) |
| C6—C7—C8—C11A | −175.6 (3) | C14—C15—C16B—C17 | 170.9 (3) |
| C6—C7—C8—C11B | 176.6 (3) | C16A—C15—C16B—C17 | 62.4 (6) |
| C7—C8—C9—C10 | −0.7 (4) | C12—C11B—C16B—C15 | −66.7 (5) |
| C11A—C8—C9—C10 | 174.1 (4) | C8—C11B—C16B—C15 | 174.1 (5) |
| C11B—C8—C9—C10 | −177.6 (3) | C12—C11B—C16B—C17 | 177.7 (5) |
| C8—C9—C10—C5 | 0.6 (5) | C8—C11B—C16B—C17 | 58.5 (5) |
| C6—C5—C10—C9 | −0.4 (4) | C11A—C16A—C17—O2 | −58.3 (7) |
| C4—C5—C10—C9 | −179.3 (3) | C15—C16A—C17—O2 | 54.8 (8) |
| C7—C8—C11A—C16A | 132.9 (5) | C11A—C16A—C17—O1 | 123.3 (5) |
| C9—C8—C11A—C16A | −42.3 (8) | C15—C16A—C17—O1 | −123.5 (5) |
| C11B—C8—C11A—C16A | −59.2 (7) | C11A—C16A—C17—C16B | −48.7 (5) |
| C7—C8—C11A—C12 | −106.5 (5) | C15—C16A—C17—C16B | 64.4 (6) |
| C9—C8—C11A—C12 | 78.3 (6) | C15—C16B—C17—O2 | 104.7 (5) |
| C11B—C8—C11A—C12 | 61.4 (7) | C11B—C16B—C17—O2 | −140.6 (4) |
| C7—C8—C11B—C12 | −60.7 (7) | C15—C16B—C17—O1 | −78.9 (5) |
| C9—C8—C11B—C12 | 115.5 (5) | C11B—C16B—C17—O1 | 35.9 (6) |
| C11A—C8—C11B—C12 | −76.8 (8) | C15—C16B—C17—C16A | −69.0 (6) |
| C7—C8—C11B—C16B | 57.3 (5) | C11B—C16B—C17—C16A | 45.8 (5) |
| C9—C8—C11B—C16B | −126.5 (4) | C14—C13—C20—C21 | 166.3 (3) |
| C11A—C8—C11B—C16B | 41.2 (6) | C12—C13—C20—C21 | −15.2 (4) |
| C16B—C11B—C12—C13 | 45.4 (6) | C14—C13—C20—S1 | −17.2 (3) |
| C8—C11B—C12—C13 | 161.1 (4) | C12—C13—C20—S1 | 161.28 (18) |
| C16B—C11B—C12—C11A | −49.0 (6) | C13—C20—C21—C22 | 179.4 (3) |
| C8—C11B—C12—C11A | 66.7 (8) | S1—C20—C21—C22 | 2.6 (3) |
| C8—C11A—C12—C11B | −71.9 (8) | C20—C21—C22—C23 | −2.8 (5) |
| C16A—C11A—C12—C11B | 47.5 (6) | C21—C22—C23—S1 | 1.7 (5) |
| C8—C11A—C12—C13 | −170.2 (4) | C22—C23—S1—C20 | −0.1 (3) |
| C16A—C11A—C12—C13 | −50.8 (6) | C21—C20—S1—C23 | −1.5 (2) |
| C11B—C12—C13—C14 | −9.4 (5) | C13—C20—S1—C23 | −178.5 (2) |
| C11A—C12—C13—C14 | 21.7 (5) | O2—C17—O1—C18 | 0.9 (6) |
| C11B—C12—C13—C20 | 172.1 (4) | C16A—C17—O1—C18 | 179.6 (4) |
| C11A—C12—C13—C20 | −156.9 (4) | C16B—C17—O1—C18 | −175.0 (4) |
| C20—C13—C14—C15 | 173.4 (3) | C19—C18—O1—C17 | 104.5 (7) |
| C12—C13—C14—C15 | −5.0 (4) | C4—C3—O3—C24 | −2.5 (6) |
| C13—C14—C15—O4 | 178.6 (3) | C2—C3—O3—C24 | 177.4 (4) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SJ2629).
References
- Bruker (2001). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Fischer, A., Swamy, M. T., Narayana, B. & Yathirajan, H. S. (2008a). Acta Cryst. E64, o2152. [DOI] [PMC free article] [PubMed]
- Fischer, A., Yathirajan, H. S., Ashalatha, B. V., Narayana, B. & Sarojini, B. K. (2007a). Acta Cryst. E63, o254–o255. [DOI] [PMC free article] [PubMed]
- Fischer, A., Yathirajan, H. S., Ashalatha, B. V., Narayana, B. & Sarojini, B. K. (2007b). Acta Cryst. E63, o3616. [DOI] [PMC free article] [PubMed]
- Fischer, A., Yathirajan, H. S., Ashalatha, B. V., Narayana, B. & Sarojini, B. K. (2008b). Acta Cryst. E64, o560. [DOI] [PMC free article] [PubMed]
- Hiromichi, F., Naoyuki, K., Yoshinari, S., Yasushi, N. & Yasuyuki, K. (2002). Tetrahedron Lett.43, 4825–4828.
- Hoye, T. R. & Tennakoon, M. A. (2000). Org. Lett.2, 1481–1483. [DOI] [PubMed]
- Padmavathi, V., Sharmila, K., Padmaja, A. & Bhaskar Reddy, D. (1999). Heterocycl. Commun.5, 451–456.
- Padmavathi, V., Sharmila, K., Somashekara Reddy, A. & Bhaskar Reddy, D. (2001). Ind. J. Chem. Sect. B, 40, 11–14.
- Sheldrick, G. M. (2004). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809021308/sj2629sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809021308/sj2629Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report