Abstract
The title molecule, C10H10O4, is a functionalized carbonate used in the synthetic route to organic glasses. The central CH fragment of the allyl group is disordered over two positions, with occupancies in a 0.758 (10):0.242 (10)ratio. This disorder reflects the torsional flexibility of the oxygen–allyl group, although both disordered parts present the expected anticlinal conformation, with O—CH2—CH=CH2 torsion angles of −111 (2) and 119.1 (4)°. The crystal structure is based on chains parallel to [010], formed by O⋯H—O hydrogen bonds involving hydroxyl and carbonyl groups as donors and acceptors, respectively. The molecular packing is further stabilized by two weak C—H⋯π contacts from the benzene ring of the asymmetric unit with two benzene rings of neighboring molecules.
Related literature
The crystal structures of two allyl carbonates have been reported to date, see: Michelet et al. (2003 ▶); Burns & Forsyth (2008 ▶). For the use of allyl ester and allyl carbonate derivatives as precursors for organic glasses, see: Herrera et al. (2003 ▶); Herrera (2006 ▶).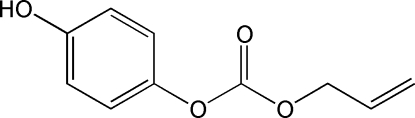
Experimental
Crystal data
C10H10O4
M r = 194.18
Monoclinic,

a = 5.8148 (7) Å
b = 7.5413 (11) Å
c = 11.4499 (17) Å
β = 93.515 (10)°
V = 501.15 (12) Å3
Z = 2
Mo Kα radiation
μ = 0.10 mm−1
T = 298 K
0.60 × 0.30 × 0.24 mm
Data collection
Bruker P4 diffractometer
Absorption correction: none
2559 measured reflections
1233 independent reflections
1042 reflections with I > 2σ(I)
R int = 0.025
3 standard reflections every 97 reflections intensity decay: 0.5%
Refinement
R[F 2 > 2σ(F 2)] = 0.037
wR(F 2) = 0.109
S = 1.06
1233 reflections
137 parameters
2 restraints
H-atom parameters constrained
Δρmax = 0.10 e Å−3
Δρmin = −0.17 e Å−3
Data collection: XSCANS (Siemens, 1996 ▶); cell refinement: XSCANS; data reduction: XSCANS; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: Mercury (Macrae et al., 2006 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809022387/bq2136sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809022387/bq2136Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O14—H14⋯O6i | 0.88 | 1.91 | 2.762 (2) | 160 |
| C10—H10A⋯Cgi | 0.93 | 2.90 | 3.612 (2) | 135 |
| C13—H13A⋯Cgii | 0.93 | 2.81 | 3.513 (3) | 133 |
Symmetry codes: (i)  ; (ii)
; (ii)  . Cg is the centroid of the benzene ring.
. Cg is the centroid of the benzene ring.
Acknowledgments
Financial support from VIEP-BUAP (grant 7-/I/NAT/05–06) is acknowledged. VHFA also express his sincere thanks to VIEP for a partial scholarship.
supplementary crystallographic information
Comment
We have synthesized some functionalized aromatic allyl ester (Herrera et al., 2003) and allyl carbonate compounds (Herrera, 2006) as intermediates in the preparation of new diallylcarbonate monomers, which are potential precursors of organic glasses. One representative example is the title molecule, which includes phenol and allyl functional groups as substitutents of the carbonate core (Fig. 1).
The allyl group presents a degree of flexibility, resulting in two disordered positions for the methylene group, C21 and C22, with site occupation factors 0.758 (10) and 0.242 (10), respectively (Fig. 1, inset). Regardless of the position for the CH2 fragment, O—allyl chain displays the expected anticlinal conformation, characterized by torsion angles O—CH2—CH═CH2 of -111 (2)° and 119.1 (4)°. A similar disorder was previously observed for a closely related phenol allylic derivative, allyl-4-hydroxybenzoate (Herrera et al., 2003). To date, only two acyclic allyl carbonate systems have been X ray characterized, both with non disordered allyl groups. The anticlinal conformation has been stabilized in the first molecule, with O—allyl torsion angle of -131.4° (Michelet et al., 2003), while in the most recent report, a synperiplanar O—allyl group is observed, the torsion angle being 9.7° (Burns & Forsyth, 2008). This very limited set of structurally characterized allyl carbonate derivatives does not allow to determine the factors influencing the stable conformation for these molecules in the solid state. In contrast, a number of allyl ester derivatives have been X-ray characterized, with conformations restricted to two attractors (Herrera et al., 2003).
The crystal structure of the title compound is based on chains running along [010], formed through O···H—O bonds, where carbonyl functionalities act as acceptor and hydroxyl functionalities as donor groups. Moreover, two aryl C—H groups of the benzene ring of the asymmetric unit form stabilizing C—H···π contacts with the centroids of two symmetry-related benzene rings (Fig. 2).
Experimental
Allyl 4-hydroxyphenyl-carbonate was synthesized by reacting hydroquinone and allylchloroformate in the presence of NaOH at 273 K for 2 h., affording a white powder (Yield: 43%). After recrystallization from a mixture of methylene chloride and hexane (7:3), colourless crystals were obtained. Anal. calcd for C10H10O4: C 61.85, H 5.19; found: C 61.85, H 5.25.
Refinement
Allylic CH group was found to be disordered over two sites, C21 and C22, and occupancies were refined with the sum constrained to unity. In order to get a sensible geometry for the minor part, bond length C3—C22 was restrained to 1.480 (15)Å. C-bonded H atoms were placed in idealized positions and refined as riding to their carrier C atoms, with bond lengths fixed to 0.93Å (aromatic and allylic H atoms) or 0.97Å (methylene CH2). Hydroxyl H atom H14 was found in a difference map and refined as riding to O14, with the O—H bond length fixed to the found value, 0.883Å. Isotropic displacement parameters for H atoms were calculated as Uiso(H) = 1.2Ueq(carrier atom) for C-bonded H atoms and Uiso(H) = 1.5Ueq(O14) for H14. Measured Friedel pairs were merged in the final refinement.
Figures
Fig. 1.
The structure of the title compound with displacement ellipsoids at the 30% probability level. C22 (minor part of the disorder) has been omitted for clarity. The inset shows the complete structure. Colour code: green for C21-disordered part; purple for the C22-disordered part.
Fig. 2.
A part of the crystal structure of the title compound, including hydrogen bonds depicted with blue dashed lines, and C—H···π contacts from the asymmetric unit, represented as red dashed lines. Colour code for symmetry-related molecules: blue -x, -1/2+y, -z; green -x, 1/2+y, -z; red 1-x, 1/2+y, -z; gold x, 1+y, z.
Crystal data
| C10H10O4 | F(000) = 204 |
| Mr = 194.18 | Dx = 1.287 Mg m−3 |
| Monoclinic, P21 | Melting point: 337 K |
| Hall symbol: P 2yb | Mo Kα radiation, λ = 0.71073 Å |
| a = 5.8148 (7) Å | Cell parameters from 52 reflections |
| b = 7.5413 (11) Å | θ = 4.7–13.6° |
| c = 11.4499 (17) Å | µ = 0.10 mm−1 |
| β = 93.515 (10)° | T = 298 K |
| V = 501.15 (12) Å3 | Prism, colorless |
| Z = 2 | 0.60 × 0.30 × 0.24 mm |
Data collection
| Bruker P4 diffractometer | Rint = 0.025 |
| Radiation source: fine-focus sealed tube | θmax = 27.5°, θmin = 3.2° |
| graphite | h = −7→5 |
| 2θ/ω scans | k = −9→1 |
| 2559 measured reflections | l = −14→14 |
| 1233 independent reflections | 3 standard reflections every 97 reflections |
| 1042 reflections with I > 2σ(I) | intensity decay: 0.5% |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.037 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.109 | H-atom parameters constrained |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.0619P)2 + 0.0247P] where P = (Fo2 + 2Fc2)/3 |
| 1233 reflections | (Δ/σ)max < 0.001 |
| 137 parameters | Δρmax = 0.10 e Å−3 |
| 2 restraints | Δρmin = −0.17 e Å−3 |
| 0 constraints |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| C1 | 0.9530 (6) | 0.3863 (7) | 0.5802 (3) | 0.1057 (12) | |
| H1A | 0.9781 | 0.4877 | 0.5365 | 0.127* | 0.758 (10) |
| H1B | 1.0579 | 0.3541 | 0.6411 | 0.127* | 0.758 (10) |
| H1C | 0.9365 | 0.3187 | 0.6471 | 0.127* | 0.242 (10) |
| H1D | 1.0856 | 0.4534 | 0.5729 | 0.127* | 0.242 (10) |
| C21 | 0.7760 (6) | 0.2923 (6) | 0.5569 (3) | 0.0724 (13) | 0.758 (10) |
| H21A | 0.7563 | 0.1919 | 0.6024 | 0.087* | 0.758 (10) |
| C22 | 0.825 (3) | 0.388 (3) | 0.4931 (14) | 0.136 (9) | 0.242 (10) |
| H22A | 0.8932 | 0.4395 | 0.4302 | 0.163* | 0.242 (10) |
| C3 | 0.5976 (6) | 0.3307 (5) | 0.4622 (3) | 0.0887 (9) | |
| H3A | 0.4484 | 0.3453 | 0.4945 | 0.106* | 0.758 (10) |
| H3B | 0.6350 | 0.4391 | 0.4219 | 0.106* | 0.758 (10) |
| H3C | 0.5069 | 0.4263 | 0.4262 | 0.106* | 0.242 (10) |
| H3D | 0.5241 | 0.2953 | 0.5324 | 0.106* | 0.242 (10) |
| O4 | 0.5923 (3) | 0.1818 (3) | 0.38141 (16) | 0.0764 (5) | |
| C5 | 0.4547 (4) | 0.2025 (3) | 0.2869 (2) | 0.0610 (5) | |
| O6 | 0.3334 (3) | 0.3258 (3) | 0.26428 (16) | 0.0802 (6) | |
| O7 | 0.4800 (3) | 0.0608 (2) | 0.21897 (15) | 0.0736 (5) | |
| C8 | 0.3616 (4) | 0.0614 (3) | 0.10692 (18) | 0.0563 (5) | |
| C9 | 0.1521 (4) | −0.0236 (3) | 0.0904 (2) | 0.0613 (6) | |
| H9A | 0.0817 | −0.0718 | 0.1539 | 0.074* | |
| C10 | 0.0481 (3) | −0.0368 (3) | −0.01992 (19) | 0.0569 (5) | |
| H10A | −0.0935 | −0.0934 | −0.0315 | 0.068* | |
| C11 | 0.1552 (4) | 0.0349 (3) | −0.11458 (18) | 0.0554 (5) | |
| C12 | 0.3637 (4) | 0.1230 (4) | −0.0966 (2) | 0.0628 (6) | |
| H12A | 0.4341 | 0.1734 | −0.1594 | 0.075* | |
| C13 | 0.4666 (4) | 0.1355 (3) | 0.0157 (2) | 0.0625 (6) | |
| H13A | 0.6066 | 0.1942 | 0.0286 | 0.075* | |
| O14 | 0.0629 (3) | 0.0249 (3) | −0.22658 (14) | 0.0836 (6) | |
| H14 | −0.0418 | −0.0598 | −0.2344 | 0.125* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.098 (2) | 0.127 (3) | 0.090 (2) | −0.013 (2) | −0.0110 (17) | −0.020 (2) |
| C21 | 0.083 (2) | 0.079 (3) | 0.0532 (18) | −0.0054 (19) | −0.0087 (14) | −0.0045 (17) |
| C22 | 0.23 (2) | 0.108 (13) | 0.073 (9) | −0.074 (15) | 0.022 (11) | −0.019 (9) |
| C3 | 0.0970 (19) | 0.0839 (19) | 0.0817 (16) | 0.0192 (17) | −0.0228 (14) | −0.0244 (16) |
| O4 | 0.0857 (11) | 0.0696 (11) | 0.0698 (9) | 0.0154 (9) | −0.0273 (8) | −0.0095 (9) |
| C5 | 0.0606 (11) | 0.0567 (12) | 0.0640 (12) | 0.0060 (10) | −0.0101 (9) | 0.0000 (10) |
| O6 | 0.0825 (11) | 0.0763 (12) | 0.0789 (11) | 0.0279 (10) | −0.0197 (9) | −0.0097 (10) |
| O7 | 0.0836 (10) | 0.0574 (9) | 0.0752 (10) | 0.0154 (9) | −0.0315 (8) | −0.0076 (8) |
| C8 | 0.0580 (11) | 0.0486 (11) | 0.0606 (11) | 0.0064 (10) | −0.0114 (9) | −0.0040 (10) |
| C9 | 0.0622 (12) | 0.0593 (13) | 0.0620 (12) | −0.0034 (10) | 0.0017 (10) | 0.0043 (10) |
| C10 | 0.0471 (10) | 0.0536 (12) | 0.0692 (13) | −0.0050 (9) | −0.0031 (9) | 0.0011 (10) |
| C11 | 0.0582 (10) | 0.0476 (11) | 0.0591 (10) | −0.0055 (10) | −0.0061 (9) | −0.0034 (10) |
| C12 | 0.0619 (12) | 0.0590 (13) | 0.0678 (13) | −0.0138 (11) | 0.0067 (10) | −0.0041 (11) |
| C13 | 0.0500 (11) | 0.0548 (12) | 0.0813 (14) | −0.0100 (10) | −0.0071 (10) | −0.0116 (11) |
| O14 | 0.0940 (12) | 0.0949 (15) | 0.0596 (9) | −0.0340 (12) | −0.0141 (8) | 0.0015 (10) |
Geometric parameters (Å, °)
| C1—C22 | 1.208 (16) | C5—O6 | 1.186 (3) |
| C1—C21 | 1.265 (5) | C5—O7 | 1.335 (3) |
| C1—H1A | 0.9300 | O7—C8 | 1.418 (2) |
| C1—H1B | 0.9300 | C8—C13 | 1.362 (4) |
| C1—H1C | 0.9300 | C8—C9 | 1.379 (3) |
| C1—H1D | 0.9300 | C9—C10 | 1.371 (3) |
| C21—C3 | 1.482 (4) | C9—H9A | 0.9300 |
| C21—H21A | 0.9300 | C10—C11 | 1.392 (3) |
| C22—C3 | 1.414 (13) | C10—H10A | 0.9300 |
| C22—H22A | 0.9300 | C11—O14 | 1.362 (2) |
| C3—O4 | 1.454 (4) | C11—C12 | 1.387 (3) |
| C3—H3A | 0.9700 | C12—C13 | 1.388 (3) |
| C3—H3B | 0.9700 | C12—H12A | 0.9300 |
| C3—H3C | 0.9700 | C13—H13A | 0.9300 |
| C3—H3D | 0.9700 | O14—H14 | 0.8830 |
| O4—C5 | 1.315 (3) | ||
| H1A—C1—H1B | 120.0 | C5—O4—C3 | 114.8 (2) |
| H1C—C1—H1D | 120.0 | O6—C5—O4 | 126.6 (2) |
| C21—C1—H1A | 120.0 | O6—C5—O7 | 125.9 (2) |
| C21—C1—H1B | 120.0 | O4—C5—O7 | 107.52 (19) |
| C22—C1—H1C | 126.5 | C5—O7—C8 | 117.36 (17) |
| C22—C1—H1D | 113.2 | C13—C8—C9 | 121.3 (2) |
| C1—C21—C3 | 124.7 (4) | C13—C8—O7 | 118.65 (19) |
| C1—C22—C3 | 136.4 (14) | C9—C8—O7 | 119.9 (2) |
| C1—C21—H21A | 117.7 | C10—C9—C8 | 119.7 (2) |
| C1—C22—H22A | 111.8 | C10—C9—H9A | 120.1 |
| C3—C21—H21A | 117.7 | C8—C9—H9A | 120.1 |
| C3—C22—H22A | 111.8 | C9—C10—C11 | 119.77 (19) |
| C22—C3—O4 | 112.2 (8) | C9—C10—H10A | 120.1 |
| O4—C3—C21 | 107.5 (3) | C11—C10—H10A | 120.1 |
| O4—C3—H3A | 110.2 | O14—C11—C12 | 117.2 (2) |
| O4—C3—H3B | 110.2 | O14—C11—C10 | 122.85 (18) |
| C21—C3—H3A | 110.2 | C12—C11—C10 | 119.95 (19) |
| C21—C3—H3B | 110.2 | C11—C12—C13 | 119.6 (2) |
| H3A—C3—H3B | 108.5 | C11—C12—H12A | 120.2 |
| C22—C3—H3C | 110.8 | C13—C12—H12A | 120.2 |
| C22—C3—H3D | 109.2 | C8—C13—C12 | 119.64 (19) |
| O4—C3—H3C | 108.3 | C8—C13—H13A | 120.2 |
| O4—C3—H3D | 108.8 | C12—C13—H13A | 120.2 |
| C21—C3—H3C | 142.5 | C11—O14—H14 | 111.4 |
| H3C—C3—H3D | 107.5 | ||
| C22—C1—C21—C3 | −15.6 (12) | C5—O7—C8—C13 | 87.7 (3) |
| C21—C1—C22—C3 | 19.6 (16) | C5—O7—C8—C9 | −97.1 (3) |
| C1—C22—C3—O4 | −111 (2) | C13—C8—C9—C10 | 1.0 (4) |
| C1—C22—C3—C21 | −19.0 (16) | O7—C8—C9—C10 | −174.1 (2) |
| C1—C21—C3—C22 | 15.1 (11) | C8—C9—C10—C11 | 0.3 (3) |
| C1—C21—C3—O4 | 119.1 (4) | C9—C10—C11—O14 | 179.3 (2) |
| C22—C3—O4—C5 | −128.5 (9) | C9—C10—C11—C12 | −1.6 (3) |
| C21—C3—O4—C5 | −174.7 (3) | O14—C11—C12—C13 | −179.3 (2) |
| C3—O4—C5—O6 | −2.4 (4) | C10—C11—C12—C13 | 1.5 (4) |
| C3—O4—C5—O7 | 176.2 (3) | C9—C8—C13—C12 | −1.1 (4) |
| O6—C5—O7—C8 | 3.8 (4) | O7—C8—C13—C12 | 174.0 (2) |
| O4—C5—O7—C8 | −174.8 (2) | C11—C12—C13—C8 | −0.1 (4) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O14—H14···O6i | 0.88 | 1.91 | 2.762 (2) | 160 |
| C10—H10A···Cgi | 0.93 | 2.90 | 3.612 (2) | 135 |
| C13—H13A···Cgii | 0.93 | 2.81 | 3.513 (3) | 133 |
Symmetry codes: (i) −x, y−1/2, −z; (ii) −x+1, y+1/2, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BQ2136).
References
- Burns, A. C. & Forsyth, C. J. (2008). Org. Lett 10, 97–100. [DOI] [PubMed]
- Herrera, A. M. (2006). PhD Thesis, Universidad Autónoma de Puebla, Mexico.
- Herrera, A. M., Bernès, S. & López, D. (2003). Acta Cryst. E59, o1522–o1524.
- Macrae, C. F., Edgington, P. R., McCabe, P., Pidcock, E., Shields, G. P., Taylor, R., Towler, M. & van de Streek, J. (2006). J. Appl. Cryst.39, 453–457.
- Michelet, V., Adiey, K., Tanier, S., Dujardin, G. & Genêt, J. P. (2003). Eur. J. Org. Chem pp. 2947–2958.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Siemens (1996). XSCANS Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809022387/bq2136sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809022387/bq2136Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




