Abstract
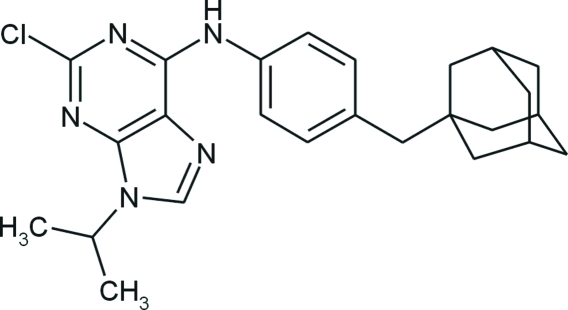
The asymmetric unit of the title compound, C25H30ClN5, consists of two molecules with slightly different geometrical parameters. The dihedral angles between the purine and benzene rings are 39.54 (5) and 23.69 (5)° in the two molecules. The adamantane cages consist of three fused cyclohexane rings in classical chair conformations, with C—C—C angles in the range 108 (2)–111 (2)°. In the crystal, molecules are linked into dimers via two N—H⋯N hydrogen bonds.
Related literature
The title compound was prepared according to a modification of the procedure of Fiorini & Abel (1998 ▶). For the synthesis and/or biological activity of related compounds, see: Hardcastle et al. (2002 ▶); Villhauer et al., (2003 ▶). For related structures, see: Trávníček & Zatloukal (2004 ▶); Trávníček & Popa (2007a
▶,b
▶); Rouchal et al. (2009a
▶,b
▶).
Experimental
Crystal data
C25H30ClN5
M r = 435.99
Triclinic,

a = 11.731 (1) Å
b = 13.421 (1) Å
c = 15.531 (1) Å
α = 72.002 (7)°
β = 81.912 (7)°
γ = 79.688 (7)°
V = 2278.4 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.19 mm−1
T = 120 K
0.20 × 0.20 × 0.10 mm
Data collection
Kuma KM-4-CCD diffractometer
Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2006 ▶) T min = 0.962, T max = 0.981
17173 measured reflections
7983 independent reflections
4502 reflections with I > 2σ(I)
R int = 0.030
Refinement
R[F 2 > 2σ(F 2)] = 0.033
wR(F 2) = 0.081
S = 0.85
7983 reflections
563 parameters
H-atom parameters constrained
Δρmax = 0.19 e Å−3
Δρmin = −0.28 e Å−3
Data collection: CrysAlis CCD (Oxford Diffraction, 2006 ▶); cell refinement: CrysAlis CCD; data reduction: CrysAlis RED (Oxford Diffraction, 2006 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809023629/im2124sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809023629/im2124Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1A⋯N54 | 0.88 | 2.14 | 2.940 (2) | 152 |
| N51—H51A⋯N4 | 0.88 | 2.27 | 3.026 (2) | 144 |
Acknowledgments
The financial support of this work by the Science Foundation of Czech Republic (grant No. 203/06/P362) and by the Czech Ministry of Education (project No. MSM 7088352101) is gratefully acknowledged.
supplementary crystallographic information
Comment
The published structure represents a typical member of a new trisubstituted purine series currently synthesized in our laboratory. These molecules belong to the family of purine derivatives that exhibit a wide range of biological activities. Purine based molecules with suitable substituents at the most active centers can present low-molecular-weight inhibitors of cyclin-dependent kinases playing a crucial role in the regulation of the cell division cycle (Hardcastle et al., 2002). A unique adamantane moiety is frequently linked into known medicaments or drug candidates with the aim to improve the biological properties of these structures, e.g. a novel potent hypoglycemic agent reported by Villhauer et al. (2003).
The title compound (Fig. 1) crystallizes with two geometrically slightly different molecules in the asymmetric unit that are linked into dimers by two N–H···N hydrogen bonds (Table 1). The dihedral angles between purine and benzene rings are 39.54 (5)° and 23.69 (5)°. The torsion angles describing the orientation of isopropyl, purine, benzene and adamantane moiety C22–N5–C23–H23A, C21–C18–N1–C15 and C17–C12–C11–C1 are -177.4 (2), 174.9 (2) and -94.6 (3)° respectively. The corresponding values of torsion angles for the second conformer are 144.0 (2), 173.9 (2) and -98.4 (2)° respectively.
Experimental
The title compound was prepared according to a slightly modified literature procedure (Fiorini & Abel, 1998). 2,6-Dichloro-9-(propan-2-yl)-9H-purine (0.85 mmol, 196 mg) and 4-[(1-adamantyl)methyl]aniline hydrochloride (0.90 mmol, 250 mg) were dissolved in a mixture of DMF (2.5 ml) and triethylamine (1.70 mmol, 0.24 ml). The resulting solution was stirred at 363 K for required time (according to TLC). After the reaction was complete, the mixture was diluted with water and extracted with diethyl ether (5 times 15 ml). The connected organic layers were washed twice with brine and dried over sodium sulfate. The desired product was obtained by evaporation of the solvent in vacuum followed by purification of the crude product using column chromatography (silica gel; light petroleum/ethyl acetate (1:1 v/v) as a colorless crystalline powder (152 mg, 41%, mp 453–457 K). The single crystals suitable for X-ray analysis were grown by liquid diffusion (acetone/hexane, 1:3 v/v) at room temperature within 24 h.
Refinement
Hydrogen atoms were positioned geometrically and refined as riding using standard SHELXTL facilities, with their Uiso set to either 1.2Ueq or 1.5Ueq (methyl) of their parent atoms.
Figures
Fig. 1.
ORTEP plot of the asymmetric unit with atoms represented as 50% probability ellipsoids (H-bonds are denoted as dashed lines).
Crystal data
| C25H30ClN5 | Z = 4 |
| Mr = 435.99 | F(000) = 928 |
| Triclinic, P1 | Dx = 1.271 Mg m−3 |
| Hall symbol: -P 1 | Melting point: 455 K |
| a = 11.731 (1) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 13.421 (1) Å | Cell parameters from 7983 reflections |
| c = 15.531 (1) Å | θ = 2.8–25.0° |
| α = 72.002 (7)° | µ = 0.19 mm−1 |
| β = 81.912 (7)° | T = 120 K |
| γ = 79.688 (7)° | Plate, colourless |
| V = 2278.4 (3) Å3 | 0.20 × 0.20 × 0.10 mm |
Data collection
| Kuma KM-4-CCD diffractometer | 7983 independent reflections |
| Radiation source: fine-focus sealed tube | 4502 reflections with I > 2σ(I) |
| graphite | Rint = 0.030 |
| Detector resolution: 0.06 pixels mm-1 | θmax = 25.0°, θmin = 2.8° |
| ω scans | h = −13→13 |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2006) | k = −15→10 |
| Tmin = 0.962, Tmax = 0.981 | l = −18→18 |
| 17173 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.033 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.081 | H-atom parameters constrained |
| S = 0.85 | w = 1/[σ2(Fo2) + (0.0366P)2] where P = (Fo2 + 2Fc2)/3 |
| 7983 reflections | (Δ/σ)max = 0.002 |
| 563 parameters | Δρmax = 0.19 e Å−3 |
| 0 restraints | Δρmin = −0.28 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | 0.66480 (5) | 0.02196 (4) | 1.09703 (4) | 0.02989 (16) | |
| N1 | 0.47703 (14) | 0.31228 (13) | 0.84935 (11) | 0.0212 (4) | |
| H1A | 0.4662 | 0.3352 | 0.7911 | 0.025* | |
| N2 | 0.56519 (14) | 0.16913 (14) | 0.96467 (11) | 0.0213 (4) | |
| N3 | 0.64870 (14) | 0.00011 (13) | 0.93892 (11) | 0.0203 (4) | |
| N4 | 0.54147 (15) | 0.16914 (14) | 0.72689 (11) | 0.0253 (5) | |
| N5 | 0.62661 (14) | −0.00033 (14) | 0.78420 (11) | 0.0233 (4) | |
| C1 | 0.15565 (17) | 0.67309 (16) | 1.02665 (14) | 0.0192 (5) | |
| C2 | 0.07127 (18) | 0.59104 (16) | 1.05850 (14) | 0.0228 (5) | |
| H2B | 0.0799 | 0.5510 | 1.1230 | 0.027* | |
| H2C | 0.0905 | 0.5403 | 1.0222 | 0.027* | |
| C3 | −0.05581 (18) | 0.64537 (17) | 1.04799 (14) | 0.0230 (5) | |
| H3B | −0.1093 | 0.5906 | 1.0689 | 0.028* | |
| C4 | −0.06925 (18) | 0.70646 (17) | 0.94756 (14) | 0.0252 (6) | |
| H4B | −0.0509 | 0.6567 | 0.9103 | 0.030* | |
| H4C | −0.1507 | 0.7411 | 0.9404 | 0.030* | |
| C5 | 0.01297 (18) | 0.79041 (17) | 0.91466 (14) | 0.0258 (6) | |
| H5A | 0.0042 | 0.8296 | 0.8492 | 0.031* | |
| C6 | −0.01802 (19) | 0.86830 (16) | 0.97223 (14) | 0.0261 (6) | |
| H6A | 0.0340 | 0.9233 | 0.9514 | 0.031* | |
| H6B | −0.0992 | 0.9037 | 0.9652 | 0.031* | |
| C7 | −0.00410 (18) | 0.80685 (17) | 1.07278 (14) | 0.0256 (6) | |
| H7A | −0.0234 | 0.8571 | 1.1104 | 0.031* | |
| C8 | 0.12254 (18) | 0.75150 (17) | 1.08348 (14) | 0.0237 (5) | |
| H8A | 0.1759 | 0.8054 | 1.0637 | 0.028* | |
| H8B | 0.1314 | 0.7131 | 1.1483 | 0.028* | |
| C9 | 0.13970 (17) | 0.73551 (17) | 0.92593 (13) | 0.0221 (5) | |
| H9A | 0.1930 | 0.7894 | 0.9047 | 0.027* | |
| H9B | 0.1599 | 0.6865 | 0.8881 | 0.027* | |
| C10 | −0.08652 (18) | 0.72313 (16) | 1.10517 (14) | 0.0239 (5) | |
| H10A | −0.1680 | 0.7579 | 1.0987 | 0.029* | |
| H10B | −0.0789 | 0.6845 | 1.1701 | 0.029* | |
| C11 | 0.28362 (18) | 0.62051 (17) | 1.03993 (14) | 0.0247 (5) | |
| H11A | 0.3334 | 0.6773 | 1.0202 | 0.030* | |
| H11B | 0.2898 | 0.5866 | 1.1058 | 0.030* | |
| C12 | 0.33367 (17) | 0.53798 (18) | 0.99061 (14) | 0.0216 (5) | |
| C13 | 0.38638 (17) | 0.56703 (17) | 0.90161 (14) | 0.0222 (5) | |
| H13A | 0.3888 | 0.6400 | 0.8713 | 0.027* | |
| C14 | 0.43523 (17) | 0.49333 (17) | 0.85604 (14) | 0.0221 (5) | |
| H14A | 0.4703 | 0.5159 | 0.7956 | 0.027* | |
| C15 | 0.43260 (17) | 0.38575 (17) | 0.89960 (14) | 0.0186 (5) | |
| C16 | 0.38099 (18) | 0.35447 (17) | 0.98847 (14) | 0.0237 (5) | |
| H16A | 0.3788 | 0.2815 | 1.0188 | 0.028* | |
| C17 | 0.33275 (18) | 0.42993 (17) | 1.03266 (14) | 0.0251 (6) | |
| H17A | 0.2981 | 0.4073 | 1.0932 | 0.030* | |
| C18 | 0.53343 (17) | 0.21243 (17) | 0.87816 (14) | 0.0183 (5) | |
| C19 | 0.62045 (18) | 0.06941 (18) | 0.98556 (14) | 0.0210 (5) | |
| C20 | 0.61511 (18) | 0.04658 (17) | 0.85336 (14) | 0.0205 (5) | |
| C21 | 0.56177 (17) | 0.15023 (17) | 0.81755 (14) | 0.0198 (5) | |
| C22 | 0.58096 (18) | 0.07720 (17) | 0.71132 (14) | 0.0250 (6) | |
| H22A | 0.5781 | 0.0655 | 0.6544 | 0.030* | |
| C23 | 0.67589 (19) | −0.11099 (17) | 0.78976 (15) | 0.0260 (6) | |
| H23A | 0.6980 | −0.1473 | 0.8530 | 0.031* | |
| C24 | 0.7855 (2) | −0.11393 (19) | 0.72457 (17) | 0.0487 (8) | |
| H24A | 0.8410 | −0.0757 | 0.7383 | 0.073* | |
| H24B | 0.7653 | −0.0803 | 0.6620 | 0.073* | |
| H24C | 0.8205 | −0.1876 | 0.7315 | 0.073* | |
| C25 | 0.5850 (2) | −0.16786 (18) | 0.77139 (17) | 0.0418 (7) | |
| H25A | 0.5161 | −0.1638 | 0.8146 | 0.063* | |
| H25B | 0.6172 | −0.2423 | 0.7785 | 0.063* | |
| H25C | 0.5631 | −0.1342 | 0.7092 | 0.063* | |
| Cl51 | 0.28331 (5) | 0.58057 (5) | 0.30212 (4) | 0.02837 (15) | |
| N51 | 0.52129 (14) | 0.32715 (13) | 0.54043 (11) | 0.0190 (4) | |
| H51A | 0.5363 | 0.3069 | 0.5977 | 0.023* | |
| N52 | 0.40140 (14) | 0.44895 (13) | 0.43181 (11) | 0.0181 (4) | |
| N53 | 0.22379 (14) | 0.55319 (13) | 0.47526 (11) | 0.0181 (4) | |
| N54 | 0.35856 (14) | 0.41024 (13) | 0.68229 (11) | 0.0186 (4) | |
| N55 | 0.19479 (14) | 0.52510 (13) | 0.63989 (11) | 0.0175 (4) | |
| C51 | 0.84156 (17) | −0.02070 (16) | 0.35781 (13) | 0.0178 (5) | |
| C52 | 0.72001 (17) | −0.04891 (16) | 0.35767 (14) | 0.0221 (5) | |
| H52B | 0.6931 | −0.0179 | 0.2961 | 0.027* | |
| H52C | 0.6640 | −0.0188 | 0.4003 | 0.027* | |
| C53 | 0.72440 (19) | −0.17002 (17) | 0.38639 (15) | 0.0266 (6) | |
| H53B | 0.6452 | −0.1873 | 0.3855 | 0.032* | |
| C54 | 0.76341 (19) | −0.21829 (17) | 0.48350 (14) | 0.0288 (6) | |
| H54B | 0.7077 | −0.1889 | 0.5267 | 0.035* | |
| H54C | 0.7648 | −0.2960 | 0.5025 | 0.035* | |
| C55 | 0.88519 (19) | −0.19224 (16) | 0.48462 (14) | 0.0242 (5) | |
| H55A | 0.9108 | −0.2227 | 0.5474 | 0.029* | |
| C56 | 0.97131 (19) | −0.23935 (17) | 0.41818 (14) | 0.0261 (6) | |
| H56A | 1.0503 | −0.2236 | 0.4192 | 0.031* | |
| H56B | 0.9741 | −0.3172 | 0.4368 | 0.031* | |
| C57 | 0.93224 (18) | −0.19142 (16) | 0.32160 (14) | 0.0241 (5) | |
| H57A | 0.9883 | −0.2218 | 0.2783 | 0.029* | |
| C58 | 0.92764 (18) | −0.06962 (16) | 0.29220 (14) | 0.0255 (6) | |
| H58A | 1.0062 | −0.0519 | 0.2917 | 0.031* | |
| H58B | 0.9031 | −0.0394 | 0.2298 | 0.031* | |
| C59 | 0.88055 (18) | −0.07101 (16) | 0.45488 (14) | 0.0227 (5) | |
| H59A | 0.9585 | −0.0534 | 0.4566 | 0.027* | |
| H59B | 0.8255 | −0.0410 | 0.4980 | 0.027* | |
| C60 | 0.81062 (19) | −0.21660 (17) | 0.31982 (15) | 0.0291 (6) | |
| H60A | 0.8121 | −0.2942 | 0.3373 | 0.035* | |
| H60B | 0.7856 | −0.1860 | 0.2576 | 0.035* | |
| C61 | 0.84161 (18) | 0.10059 (15) | 0.32633 (14) | 0.0228 (5) | |
| H61A | 0.9212 | 0.1143 | 0.3283 | 0.027* | |
| H61B | 0.8234 | 0.1279 | 0.2621 | 0.027* | |
| C62 | 0.75770 (17) | 0.16313 (15) | 0.38065 (14) | 0.0183 (5) | |
| C63 | 0.78513 (18) | 0.17167 (15) | 0.46234 (14) | 0.0206 (5) | |
| H63A | 0.8586 | 0.1388 | 0.4839 | 0.025* | |
| C64 | 0.70683 (17) | 0.22741 (15) | 0.51275 (14) | 0.0195 (5) | |
| H64A | 0.7273 | 0.2323 | 0.5682 | 0.023* | |
| C65 | 0.59856 (17) | 0.27620 (15) | 0.48277 (14) | 0.0174 (5) | |
| C66 | 0.56996 (18) | 0.27038 (16) | 0.40016 (14) | 0.0233 (5) | |
| H66A | 0.4969 | 0.3039 | 0.3782 | 0.028* | |
| C67 | 0.65035 (19) | 0.21459 (16) | 0.35056 (14) | 0.0234 (5) | |
| H67A | 0.6311 | 0.2116 | 0.2941 | 0.028* | |
| C68 | 0.42732 (17) | 0.40287 (16) | 0.51939 (14) | 0.0179 (5) | |
| C69 | 0.30520 (18) | 0.52051 (16) | 0.41747 (14) | 0.0185 (5) | |
| C70 | 0.25453 (17) | 0.50790 (15) | 0.56185 (13) | 0.0160 (5) | |
| C71 | 0.35421 (17) | 0.43674 (15) | 0.58869 (13) | 0.0157 (5) | |
| C72 | 0.26190 (18) | 0.46506 (16) | 0.70871 (14) | 0.0199 (5) | |
| H72A | 0.2406 | 0.4631 | 0.7705 | 0.024* | |
| C73 | 0.08037 (17) | 0.59161 (16) | 0.64500 (14) | 0.0192 (5) | |
| H73A | 0.0789 | 0.6554 | 0.5905 | 0.023* | |
| C74 | 0.06466 (19) | 0.62912 (17) | 0.72953 (14) | 0.0261 (6) | |
| H74A | 0.1307 | 0.6644 | 0.7301 | 0.039* | |
| H74B | 0.0607 | 0.5682 | 0.7839 | 0.039* | |
| H74C | −0.0075 | 0.6790 | 0.7290 | 0.039* | |
| C75 | −0.01608 (18) | 0.53080 (18) | 0.64186 (15) | 0.0304 (6) | |
| H75A | −0.0031 | 0.5097 | 0.5858 | 0.046* | |
| H75B | −0.0914 | 0.5761 | 0.6432 | 0.046* | |
| H75C | −0.0159 | 0.4676 | 0.6945 | 0.046* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.0341 (4) | 0.0329 (4) | 0.0208 (3) | −0.0003 (3) | −0.0101 (3) | −0.0043 (3) |
| N1 | 0.0266 (11) | 0.0203 (11) | 0.0170 (10) | 0.0020 (9) | −0.0042 (8) | −0.0079 (9) |
| N2 | 0.0215 (10) | 0.0241 (12) | 0.0188 (10) | −0.0026 (9) | −0.0046 (8) | −0.0064 (9) |
| N3 | 0.0234 (11) | 0.0186 (11) | 0.0179 (10) | 0.0003 (8) | −0.0045 (8) | −0.0044 (9) |
| N4 | 0.0317 (11) | 0.0236 (12) | 0.0217 (11) | 0.0007 (9) | −0.0068 (9) | −0.0088 (9) |
| N5 | 0.0262 (11) | 0.0203 (11) | 0.0227 (11) | 0.0001 (9) | −0.0042 (8) | −0.0061 (9) |
| C1 | 0.0206 (12) | 0.0184 (13) | 0.0202 (12) | 0.0007 (10) | −0.0006 (9) | −0.0105 (10) |
| C2 | 0.0313 (14) | 0.0195 (13) | 0.0170 (12) | −0.0022 (11) | −0.0026 (10) | −0.0049 (10) |
| C3 | 0.0229 (13) | 0.0242 (14) | 0.0213 (13) | −0.0050 (11) | −0.0002 (10) | −0.0056 (11) |
| C4 | 0.0210 (13) | 0.0306 (15) | 0.0244 (13) | −0.0016 (11) | −0.0030 (10) | −0.0096 (11) |
| C5 | 0.0269 (13) | 0.0289 (14) | 0.0168 (12) | −0.0010 (11) | −0.0040 (10) | −0.0005 (11) |
| C6 | 0.0266 (14) | 0.0204 (14) | 0.0263 (13) | 0.0013 (11) | −0.0001 (10) | −0.0036 (11) |
| C7 | 0.0308 (14) | 0.0257 (14) | 0.0221 (13) | −0.0022 (11) | 0.0027 (10) | −0.0128 (11) |
| C8 | 0.0282 (14) | 0.0253 (14) | 0.0195 (13) | −0.0032 (11) | −0.0015 (10) | −0.0096 (11) |
| C9 | 0.0222 (13) | 0.0255 (14) | 0.0184 (12) | −0.0059 (11) | 0.0003 (10) | −0.0056 (10) |
| C10 | 0.0227 (13) | 0.0244 (14) | 0.0202 (13) | 0.0045 (11) | −0.0006 (10) | −0.0052 (11) |
| C11 | 0.0244 (13) | 0.0293 (14) | 0.0239 (13) | −0.0016 (11) | −0.0056 (10) | −0.0127 (11) |
| C12 | 0.0149 (12) | 0.0286 (15) | 0.0235 (13) | −0.0011 (10) | −0.0070 (10) | −0.0096 (11) |
| C13 | 0.0220 (13) | 0.0167 (13) | 0.0253 (14) | 0.0000 (10) | −0.0032 (10) | −0.0033 (11) |
| C14 | 0.0217 (13) | 0.0233 (14) | 0.0174 (12) | −0.0013 (11) | −0.0016 (10) | −0.0012 (11) |
| C15 | 0.0156 (12) | 0.0224 (14) | 0.0175 (12) | −0.0003 (10) | −0.0057 (9) | −0.0051 (11) |
| C16 | 0.0250 (13) | 0.0174 (13) | 0.0253 (14) | 0.0007 (11) | −0.0049 (10) | −0.0020 (11) |
| C17 | 0.0271 (14) | 0.0274 (15) | 0.0173 (12) | 0.0004 (11) | −0.0022 (10) | −0.0040 (11) |
| C18 | 0.0158 (12) | 0.0171 (13) | 0.0227 (13) | −0.0029 (10) | −0.0023 (10) | −0.0063 (11) |
| C19 | 0.0184 (12) | 0.0255 (15) | 0.0177 (12) | −0.0045 (11) | −0.0048 (10) | −0.0020 (11) |
| C20 | 0.0192 (12) | 0.0255 (14) | 0.0182 (13) | −0.0058 (10) | 0.0002 (10) | −0.0077 (11) |
| C21 | 0.0197 (12) | 0.0188 (13) | 0.0215 (13) | −0.0012 (10) | −0.0058 (10) | −0.0061 (11) |
| C22 | 0.0356 (14) | 0.0191 (14) | 0.0184 (13) | 0.0027 (11) | −0.0086 (11) | −0.0038 (11) |
| C23 | 0.0312 (14) | 0.0193 (14) | 0.0252 (13) | 0.0056 (11) | −0.0042 (11) | −0.0074 (11) |
| C24 | 0.0436 (17) | 0.0399 (18) | 0.0519 (18) | 0.0125 (14) | 0.0044 (14) | −0.0121 (14) |
| C25 | 0.0486 (17) | 0.0218 (15) | 0.0589 (18) | −0.0019 (13) | −0.0133 (14) | −0.0153 (13) |
| Cl51 | 0.0277 (3) | 0.0355 (4) | 0.0161 (3) | 0.0008 (3) | −0.0039 (2) | −0.0012 (3) |
| N51 | 0.0185 (10) | 0.0229 (11) | 0.0162 (10) | 0.0018 (9) | −0.0042 (8) | −0.0082 (8) |
| N52 | 0.0168 (10) | 0.0177 (11) | 0.0201 (10) | −0.0023 (8) | −0.0016 (8) | −0.0060 (8) |
| N53 | 0.0177 (10) | 0.0173 (10) | 0.0193 (10) | −0.0014 (8) | −0.0012 (8) | −0.0062 (8) |
| N54 | 0.0199 (10) | 0.0188 (11) | 0.0164 (10) | 0.0014 (8) | −0.0025 (8) | −0.0063 (8) |
| N55 | 0.0177 (10) | 0.0186 (11) | 0.0142 (10) | 0.0006 (8) | −0.0036 (8) | −0.0027 (8) |
| C51 | 0.0188 (12) | 0.0155 (13) | 0.0193 (12) | −0.0001 (10) | 0.0017 (9) | −0.0082 (10) |
| C52 | 0.0225 (13) | 0.0222 (14) | 0.0210 (12) | 0.0010 (10) | −0.0059 (10) | −0.0061 (10) |
| C53 | 0.0234 (13) | 0.0270 (15) | 0.0327 (14) | −0.0081 (11) | −0.0049 (11) | −0.0098 (12) |
| C54 | 0.0354 (15) | 0.0186 (14) | 0.0304 (14) | −0.0040 (11) | 0.0003 (11) | −0.0059 (11) |
| C55 | 0.0352 (14) | 0.0189 (14) | 0.0171 (12) | 0.0014 (11) | −0.0118 (10) | −0.0020 (10) |
| C56 | 0.0284 (14) | 0.0183 (13) | 0.0331 (14) | 0.0020 (11) | −0.0105 (11) | −0.0094 (11) |
| C57 | 0.0270 (13) | 0.0208 (14) | 0.0273 (13) | 0.0018 (11) | 0.0005 (10) | −0.0153 (11) |
| C58 | 0.0256 (13) | 0.0259 (14) | 0.0268 (13) | −0.0050 (11) | 0.0024 (10) | −0.0116 (11) |
| C59 | 0.0251 (13) | 0.0212 (14) | 0.0247 (13) | −0.0019 (10) | −0.0037 (10) | −0.0110 (11) |
| C60 | 0.0370 (15) | 0.0225 (14) | 0.0325 (14) | −0.0040 (12) | −0.0104 (11) | −0.0118 (12) |
| C61 | 0.0233 (13) | 0.0183 (13) | 0.0250 (13) | −0.0029 (10) | 0.0023 (10) | −0.0058 (11) |
| C62 | 0.0217 (12) | 0.0105 (12) | 0.0200 (12) | −0.0032 (10) | 0.0038 (10) | −0.0025 (10) |
| C63 | 0.0194 (12) | 0.0147 (13) | 0.0272 (13) | −0.0001 (10) | −0.0017 (10) | −0.0069 (11) |
| C64 | 0.0183 (12) | 0.0199 (13) | 0.0218 (12) | −0.0027 (10) | −0.0042 (10) | −0.0074 (11) |
| C65 | 0.0178 (12) | 0.0110 (12) | 0.0224 (13) | −0.0033 (10) | 0.0014 (10) | −0.0043 (10) |
| C66 | 0.0224 (13) | 0.0227 (14) | 0.0229 (13) | 0.0030 (11) | −0.0083 (10) | −0.0047 (11) |
| C67 | 0.0334 (14) | 0.0195 (13) | 0.0160 (12) | 0.0000 (11) | −0.0045 (10) | −0.0044 (10) |
| C68 | 0.0156 (12) | 0.0160 (13) | 0.0238 (13) | −0.0080 (10) | −0.0025 (10) | −0.0046 (10) |
| C69 | 0.0188 (12) | 0.0191 (13) | 0.0180 (12) | −0.0032 (10) | −0.0044 (10) | −0.0045 (10) |
| C70 | 0.0173 (12) | 0.0150 (12) | 0.0159 (12) | −0.0058 (10) | −0.0012 (9) | −0.0029 (10) |
| C71 | 0.0176 (12) | 0.0138 (12) | 0.0144 (12) | −0.0008 (10) | −0.0022 (9) | −0.0028 (10) |
| C72 | 0.0218 (13) | 0.0247 (14) | 0.0161 (12) | −0.0043 (11) | 0.0001 (10) | −0.0103 (11) |
| C73 | 0.0169 (12) | 0.0177 (13) | 0.0204 (12) | 0.0027 (10) | −0.0006 (9) | −0.0051 (10) |
| C74 | 0.0273 (13) | 0.0223 (14) | 0.0258 (13) | 0.0020 (11) | 0.0008 (10) | −0.0073 (11) |
| C75 | 0.0236 (13) | 0.0323 (15) | 0.0367 (15) | −0.0048 (11) | −0.0045 (11) | −0.0110 (12) |
Geometric parameters (Å, °)
| Cl1—C19 | 1.768 (2) | Cl51—C69 | 1.757 (2) |
| N1—C18 | 1.352 (2) | N51—C68 | 1.360 (2) |
| N1—C15 | 1.425 (2) | N51—C65 | 1.421 (2) |
| N1—H1A | 0.8800 | N51—H51A | 0.8800 |
| N2—C19 | 1.340 (2) | N52—C69 | 1.340 (2) |
| N2—C18 | 1.365 (2) | N52—C68 | 1.362 (2) |
| N3—C19 | 1.318 (2) | N53—C69 | 1.327 (2) |
| N3—C20 | 1.363 (2) | N53—C70 | 1.364 (2) |
| N4—C22 | 1.321 (2) | N54—C72 | 1.324 (2) |
| N4—C21 | 1.398 (2) | N54—C71 | 1.392 (2) |
| N5—C22 | 1.376 (2) | N55—C72 | 1.374 (2) |
| N5—C20 | 1.384 (2) | N55—C70 | 1.375 (2) |
| N5—C23 | 1.475 (2) | N55—C73 | 1.480 (2) |
| C1—C2 | 1.534 (3) | C51—C52 | 1.540 (3) |
| C1—C8 | 1.539 (3) | C51—C58 | 1.541 (3) |
| C1—C9 | 1.549 (3) | C51—C59 | 1.547 (3) |
| C1—C11 | 1.553 (3) | C51—C61 | 1.548 (3) |
| C2—C3 | 1.545 (3) | C52—C53 | 1.540 (3) |
| C2—H2B | 0.9900 | C52—H52B | 0.9900 |
| C2—H2C | 0.9900 | C52—H52C | 0.9900 |
| C3—C10 | 1.535 (3) | C53—C60 | 1.537 (3) |
| C3—C4 | 1.537 (3) | C53—C54 | 1.545 (3) |
| C3—H3B | 1.0000 | C53—H53B | 1.0000 |
| C4—C5 | 1.535 (3) | C54—C55 | 1.534 (3) |
| C4—H4B | 0.9900 | C54—H54B | 0.9900 |
| C4—H4C | 0.9900 | C54—H54C | 0.9900 |
| C5—C6 | 1.541 (3) | C55—C56 | 1.538 (3) |
| C5—C9 | 1.544 (3) | C55—C59 | 1.541 (3) |
| C5—H5A | 1.0000 | C55—H55A | 1.0000 |
| C6—C7 | 1.541 (3) | C56—C57 | 1.538 (3) |
| C6—H6A | 0.9900 | C56—H56A | 0.9900 |
| C6—H6B | 0.9900 | C56—H56B | 0.9900 |
| C7—C10 | 1.534 (3) | C57—C60 | 1.530 (3) |
| C7—C8 | 1.544 (3) | C57—C58 | 1.548 (3) |
| C7—H7A | 1.0000 | C57—H57A | 1.0000 |
| C8—H8A | 0.9900 | C58—H58A | 0.9900 |
| C8—H8B | 0.9900 | C58—H58B | 0.9900 |
| C9—H9A | 0.9900 | C59—H59A | 0.9900 |
| C9—H9B | 0.9900 | C59—H59B | 0.9900 |
| C10—H10A | 0.9900 | C60—H60A | 0.9900 |
| C10—H10B | 0.9900 | C60—H60B | 0.9900 |
| C11—C12 | 1.524 (3) | C61—C62 | 1.514 (3) |
| C11—H11A | 0.9900 | C61—H61A | 0.9900 |
| C11—H11B | 0.9900 | C61—H61B | 0.9900 |
| C12—C17 | 1.396 (3) | C62—C67 | 1.391 (3) |
| C12—C13 | 1.399 (3) | C62—C63 | 1.394 (3) |
| C13—C14 | 1.387 (3) | C63—C64 | 1.389 (3) |
| C13—H13A | 0.9500 | C63—H63A | 0.9500 |
| C14—C15 | 1.396 (3) | C64—C65 | 1.392 (3) |
| C14—H14A | 0.9500 | C64—H64A | 0.9500 |
| C15—C16 | 1.395 (3) | C65—C66 | 1.399 (3) |
| C16—C17 | 1.390 (3) | C66—C67 | 1.394 (3) |
| C16—H16A | 0.9500 | C66—H66A | 0.9500 |
| C17—H17A | 0.9500 | C67—H67A | 0.9500 |
| C18—C21 | 1.411 (3) | C68—C71 | 1.415 (3) |
| C20—C21 | 1.394 (3) | C70—C71 | 1.394 (3) |
| C22—H22A | 0.9500 | C72—H72A | 0.9500 |
| C23—C25 | 1.521 (3) | C73—C74 | 1.523 (3) |
| C23—C24 | 1.523 (3) | C73—C75 | 1.523 (3) |
| C23—H23A | 1.0000 | C73—H73A | 1.0000 |
| C24—H24A | 0.9800 | C74—H74A | 0.9800 |
| C24—H24B | 0.9800 | C74—H74B | 0.9800 |
| C24—H24C | 0.9800 | C74—H74C | 0.9800 |
| C25—H25A | 0.9800 | C75—H75A | 0.9800 |
| C25—H25B | 0.9800 | C75—H75B | 0.9800 |
| C25—H25C | 0.9800 | C75—H75C | 0.9800 |
| C18—N1—C15 | 129.36 (18) | C68—N51—C65 | 129.02 (17) |
| C18—N1—H1A | 115.3 | C68—N51—H51A | 115.5 |
| C15—N1—H1A | 115.3 | C65—N51—H51A | 115.5 |
| C19—N2—C18 | 116.78 (18) | C69—N52—C68 | 117.47 (17) |
| C19—N3—C20 | 108.67 (17) | C69—N53—C70 | 109.58 (16) |
| C22—N4—C21 | 103.30 (17) | C72—N54—C71 | 102.93 (16) |
| C22—N5—C20 | 105.48 (17) | C72—N55—C70 | 105.18 (16) |
| C22—N5—C23 | 128.16 (17) | C72—N55—C73 | 129.36 (17) |
| C20—N5—C23 | 126.35 (17) | C70—N55—C73 | 125.42 (16) |
| C2—C1—C8 | 108.20 (17) | C52—C51—C58 | 108.83 (16) |
| C2—C1—C9 | 108.59 (16) | C52—C51—C59 | 108.02 (16) |
| C8—C1—C9 | 108.03 (17) | C58—C51—C59 | 108.37 (16) |
| C2—C1—C11 | 111.68 (17) | C52—C51—C61 | 111.60 (16) |
| C8—C1—C11 | 108.59 (16) | C58—C51—C61 | 108.54 (16) |
| C9—C1—C11 | 111.63 (17) | C59—C51—C61 | 111.40 (16) |
| C1—C2—C3 | 110.83 (17) | C51—C52—C53 | 110.02 (16) |
| C1—C2—H2B | 109.5 | C51—C52—H52B | 109.7 |
| C3—C2—H2B | 109.5 | C53—C52—H52B | 109.7 |
| C1—C2—H2C | 109.5 | C51—C52—H52C | 109.7 |
| C3—C2—H2C | 109.5 | C53—C52—H52C | 109.7 |
| H2B—C2—H2C | 108.1 | H52B—C52—H52C | 108.2 |
| C10—C3—C4 | 109.15 (17) | C60—C53—C52 | 109.65 (18) |
| C10—C3—C2 | 109.64 (16) | C60—C53—C54 | 109.59 (17) |
| C4—C3—C2 | 109.33 (17) | C52—C53—C54 | 109.72 (17) |
| C10—C3—H3B | 109.6 | C60—C53—H53B | 109.3 |
| C4—C3—H3B | 109.6 | C52—C53—H53B | 109.3 |
| C2—C3—H3B | 109.6 | C54—C53—H53B | 109.3 |
| C5—C4—C3 | 109.98 (17) | C55—C54—C53 | 109.22 (17) |
| C5—C4—H4B | 109.7 | C55—C54—H54B | 109.8 |
| C3—C4—H4B | 109.7 | C53—C54—H54B | 109.8 |
| C5—C4—H4C | 109.7 | C55—C54—H54C | 109.8 |
| C3—C4—H4C | 109.7 | C53—C54—H54C | 109.8 |
| H4B—C4—H4C | 108.2 | H54B—C54—H54C | 108.3 |
| C4—C5—C6 | 109.03 (18) | C54—C55—C56 | 109.45 (17) |
| C4—C5—C9 | 109.24 (17) | C54—C55—C59 | 108.79 (17) |
| C6—C5—C9 | 109.56 (17) | C56—C55—C59 | 109.54 (17) |
| C4—C5—H5A | 109.7 | C54—C55—H55A | 109.7 |
| C6—C5—H5A | 109.7 | C56—C55—H55A | 109.7 |
| C9—C5—H5A | 109.7 | C59—C55—H55A | 109.7 |
| C7—C6—C5 | 109.09 (17) | C57—C56—C55 | 109.45 (17) |
| C7—C6—H6A | 109.9 | C57—C56—H56A | 109.8 |
| C5—C6—H6A | 109.9 | C55—C56—H56A | 109.8 |
| C7—C6—H6B | 109.9 | C57—C56—H56B | 109.8 |
| C5—C6—H6B | 109.9 | C55—C56—H56B | 109.8 |
| H6A—C6—H6B | 108.3 | H56A—C56—H56B | 108.2 |
| C10—C7—C6 | 109.60 (17) | C60—C57—C56 | 109.76 (18) |
| C10—C7—C8 | 109.20 (17) | C60—C57—C58 | 108.50 (17) |
| C6—C7—C8 | 109.73 (17) | C56—C57—C58 | 109.97 (17) |
| C10—C7—H7A | 109.4 | C60—C57—H57A | 109.5 |
| C6—C7—H7A | 109.4 | C56—C57—H57A | 109.5 |
| C8—C7—H7A | 109.4 | C58—C57—H57A | 109.5 |
| C1—C8—C7 | 111.05 (16) | C51—C58—C57 | 110.60 (17) |
| C1—C8—H8A | 109.4 | C51—C58—H58A | 109.5 |
| C7—C8—H8A | 109.4 | C57—C58—H58A | 109.5 |
| C1—C8—H8B | 109.4 | C51—C58—H58B | 109.5 |
| C7—C8—H8B | 109.4 | C57—C58—H58B | 109.5 |
| H8A—C8—H8B | 108.0 | H58A—C58—H58B | 108.1 |
| C5—C9—C1 | 110.86 (17) | C55—C59—C51 | 111.33 (16) |
| C5—C9—H9A | 109.5 | C55—C59—H59A | 109.4 |
| C1—C9—H9A | 109.5 | C51—C59—H59A | 109.4 |
| C5—C9—H9B | 109.5 | C55—C59—H59B | 109.4 |
| C1—C9—H9B | 109.5 | C51—C59—H59B | 109.4 |
| H9A—C9—H9B | 108.1 | H59A—C59—H59B | 108.0 |
| C7—C10—C3 | 109.30 (17) | C57—C60—C53 | 109.58 (17) |
| C7—C10—H10A | 109.8 | C57—C60—H60A | 109.8 |
| C3—C10—H10A | 109.8 | C53—C60—H60A | 109.8 |
| C7—C10—H10B | 109.8 | C57—C60—H60B | 109.8 |
| C3—C10—H10B | 109.8 | C53—C60—H60B | 109.8 |
| H10A—C10—H10B | 108.3 | H60A—C60—H60B | 108.2 |
| C12—C11—C1 | 117.28 (16) | C62—C61—C51 | 116.11 (17) |
| C12—C11—H11A | 108.0 | C62—C61—H61A | 108.3 |
| C1—C11—H11A | 108.0 | C51—C61—H61A | 108.3 |
| C12—C11—H11B | 108.0 | C62—C61—H61B | 108.3 |
| C1—C11—H11B | 108.0 | C51—C61—H61B | 108.3 |
| H11A—C11—H11B | 107.2 | H61A—C61—H61B | 107.4 |
| C17—C12—C13 | 116.8 (2) | C67—C62—C63 | 117.53 (18) |
| C17—C12—C11 | 121.9 (2) | C67—C62—C61 | 121.10 (19) |
| C13—C12—C11 | 121.3 (2) | C63—C62—C61 | 121.38 (18) |
| C14—C13—C12 | 122.5 (2) | C64—C63—C62 | 121.09 (19) |
| C14—C13—H13A | 118.8 | C64—C63—H63A | 119.5 |
| C12—C13—H13A | 118.8 | C62—C63—H63A | 119.5 |
| C13—C14—C15 | 119.6 (2) | C63—C64—C65 | 120.62 (19) |
| C13—C14—H14A | 120.2 | C63—C64—H64A | 119.7 |
| C15—C14—H14A | 120.2 | C65—C64—H64A | 119.7 |
| C16—C15—C14 | 119.2 (2) | C64—C65—C66 | 119.31 (19) |
| C16—C15—N1 | 122.3 (2) | C64—C65—N51 | 117.14 (18) |
| C14—C15—N1 | 118.37 (19) | C66—C65—N51 | 123.53 (18) |
| C17—C16—C15 | 120.1 (2) | C67—C66—C65 | 118.95 (19) |
| C17—C16—H16A | 120.0 | C67—C66—H66A | 120.5 |
| C15—C16—H16A | 120.0 | C65—C66—H66A | 120.5 |
| C16—C17—C12 | 121.9 (2) | C62—C67—C66 | 122.47 (19) |
| C16—C17—H17A | 119.0 | C62—C67—H67A | 118.8 |
| C12—C17—H17A | 119.0 | C66—C67—H67A | 118.8 |
| N1—C18—N2 | 122.07 (19) | N51—C68—N52 | 121.44 (19) |
| N1—C18—C21 | 119.73 (19) | N51—C68—C71 | 120.41 (18) |
| N2—C18—C21 | 118.20 (19) | N52—C68—C71 | 118.15 (18) |
| N3—C19—N2 | 132.44 (19) | N53—C69—N52 | 131.12 (18) |
| N3—C19—Cl1 | 113.81 (16) | N53—C69—Cl51 | 114.79 (15) |
| N2—C19—Cl1 | 113.75 (16) | N52—C69—Cl51 | 114.09 (15) |
| N3—C20—N5 | 126.69 (19) | N53—C70—N55 | 126.88 (18) |
| N3—C20—C21 | 127.4 (2) | N53—C70—C71 | 126.86 (19) |
| N5—C20—C21 | 105.88 (18) | N55—C70—C71 | 106.25 (17) |
| C20—C21—N4 | 110.77 (18) | N54—C71—C70 | 110.81 (17) |
| C20—C21—C18 | 116.36 (19) | N54—C71—C68 | 132.71 (18) |
| N4—C21—C18 | 132.77 (19) | C70—C71—C68 | 116.44 (18) |
| N4—C22—N5 | 114.56 (18) | N54—C72—N55 | 114.83 (18) |
| N4—C22—H22A | 122.7 | N54—C72—H72A | 122.6 |
| N5—C22—H22A | 122.7 | N55—C72—H72A | 122.6 |
| N5—C23—C25 | 110.12 (17) | N55—C73—C74 | 110.12 (16) |
| N5—C23—C24 | 109.93 (18) | N55—C73—C75 | 109.84 (16) |
| C25—C23—C24 | 112.2 (2) | C74—C73—C75 | 112.51 (18) |
| N5—C23—H23A | 108.1 | N55—C73—H73A | 108.1 |
| C25—C23—H23A | 108.1 | C74—C73—H73A | 108.1 |
| C24—C23—H23A | 108.1 | C75—C73—H73A | 108.1 |
| C23—C24—H24A | 109.5 | C73—C74—H74A | 109.5 |
| C23—C24—H24B | 109.5 | C73—C74—H74B | 109.5 |
| H24A—C24—H24B | 109.5 | H74A—C74—H74B | 109.5 |
| C23—C24—H24C | 109.5 | C73—C74—H74C | 109.5 |
| H24A—C24—H24C | 109.5 | H74A—C74—H74C | 109.5 |
| H24B—C24—H24C | 109.5 | H74B—C74—H74C | 109.5 |
| C23—C25—H25A | 109.5 | C73—C75—H75A | 109.5 |
| C23—C25—H25B | 109.5 | C73—C75—H75B | 109.5 |
| H25A—C25—H25B | 109.5 | H75A—C75—H75B | 109.5 |
| C23—C25—H25C | 109.5 | C73—C75—H75C | 109.5 |
| H25A—C25—H25C | 109.5 | H75A—C75—H75C | 109.5 |
| H25B—C25—H25C | 109.5 | H75B—C75—H75C | 109.5 |
| C8—C1—C2—C3 | 58.5 (2) | C58—C51—C52—C53 | 58.5 (2) |
| C9—C1—C2—C3 | −58.6 (2) | C59—C51—C52—C53 | −59.0 (2) |
| C11—C1—C2—C3 | 177.92 (16) | C61—C51—C52—C53 | 178.21 (16) |
| C1—C2—C3—C10 | −59.9 (2) | C51—C52—C53—C60 | −59.7 (2) |
| C1—C2—C3—C4 | 59.7 (2) | C51—C52—C53—C54 | 60.7 (2) |
| C10—C3—C4—C5 | 60.3 (2) | C60—C53—C54—C55 | 59.9 (2) |
| C2—C3—C4—C5 | −59.6 (2) | C52—C53—C54—C55 | −60.6 (2) |
| C3—C4—C5—C6 | −60.2 (2) | C53—C54—C55—C56 | −60.1 (2) |
| C3—C4—C5—C9 | 59.5 (2) | C53—C54—C55—C59 | 59.5 (2) |
| C4—C5—C6—C7 | 60.0 (2) | C54—C55—C56—C57 | 60.2 (2) |
| C9—C5—C6—C7 | −59.5 (2) | C59—C55—C56—C57 | −59.0 (2) |
| C5—C6—C7—C10 | −60.5 (2) | C55—C56—C57—C60 | −59.9 (2) |
| C5—C6—C7—C8 | 59.4 (2) | C55—C56—C57—C58 | 59.4 (2) |
| C2—C1—C8—C7 | −58.8 (2) | C52—C51—C58—C57 | −59.2 (2) |
| C9—C1—C8—C7 | 58.6 (2) | C59—C51—C58—C57 | 58.0 (2) |
| C11—C1—C8—C7 | 179.78 (17) | C61—C51—C58—C57 | 179.16 (17) |
| C10—C7—C8—C1 | 60.1 (2) | C60—C57—C58—C51 | 60.4 (2) |
| C6—C7—C8—C1 | −60.0 (2) | C56—C57—C58—C51 | −59.7 (2) |
| C4—C5—C9—C1 | −59.2 (2) | C54—C55—C59—C51 | −60.2 (2) |
| C6—C5—C9—C1 | 60.1 (2) | C56—C55—C59—C51 | 59.4 (2) |
| C2—C1—C9—C5 | 58.4 (2) | C52—C51—C59—C55 | 59.4 (2) |
| C8—C1—C9—C5 | −58.7 (2) | C58—C51—C59—C55 | −58.4 (2) |
| C11—C1—C9—C5 | −178.01 (17) | C61—C51—C59—C55 | −177.72 (17) |
| C6—C7—C10—C3 | 60.6 (2) | C56—C57—C60—C53 | 59.7 (2) |
| C8—C7—C10—C3 | −59.6 (2) | C58—C57—C60—C53 | −60.5 (2) |
| C4—C3—C10—C7 | −60.1 (2) | C52—C53—C60—C57 | 60.8 (2) |
| C2—C3—C10—C7 | 59.7 (2) | C54—C53—C60—C57 | −59.7 (2) |
| C2—C1—C11—C12 | 59.7 (2) | C52—C51—C61—C62 | 57.5 (2) |
| C8—C1—C11—C12 | 178.91 (18) | C58—C51—C61—C62 | 177.43 (17) |
| C9—C1—C11—C12 | −62.1 (2) | C59—C51—C61—C62 | −63.3 (2) |
| C1—C11—C12—C17 | −94.6 (2) | C51—C61—C62—C67 | −98.4 (2) |
| C1—C11—C12—C13 | 87.5 (2) | C51—C61—C62—C63 | 81.8 (2) |
| C17—C12—C13—C14 | 0.4 (3) | C67—C62—C63—C64 | 1.5 (3) |
| C11—C12—C13—C14 | 178.31 (18) | C61—C62—C63—C64 | −178.71 (18) |
| C12—C13—C14—C15 | −0.1 (3) | C62—C63—C64—C65 | 0.1 (3) |
| C13—C14—C15—C16 | −0.2 (3) | C63—C64—C65—C66 | −1.2 (3) |
| C13—C14—C15—N1 | 176.24 (17) | C63—C64—C65—N51 | 176.95 (18) |
| C18—N1—C15—C16 | −37.7 (3) | C68—N51—C65—C64 | 160.18 (19) |
| C18—N1—C15—C14 | 146.1 (2) | C68—N51—C65—C66 | −21.7 (3) |
| C14—C15—C16—C17 | 0.1 (3) | C64—C65—C66—C67 | 0.8 (3) |
| N1—C15—C16—C17 | −176.15 (17) | N51—C65—C66—C67 | −177.28 (18) |
| C15—C16—C17—C12 | 0.2 (3) | C63—C62—C67—C66 | −2.0 (3) |
| C13—C12—C17—C16 | −0.4 (3) | C61—C62—C67—C66 | 178.24 (19) |
| C11—C12—C17—C16 | −178.37 (18) | C65—C66—C67—C62 | 0.9 (3) |
| C15—N1—C18—N2 | −5.6 (3) | C65—N51—C68—N52 | −6.8 (3) |
| C15—N1—C18—C21 | 174.88 (19) | C65—N51—C68—C71 | 173.91 (19) |
| C19—N2—C18—N1 | 179.85 (18) | C69—N52—C68—N51 | 178.00 (18) |
| C19—N2—C18—C21 | −0.6 (3) | C69—N52—C68—C71 | −2.7 (3) |
| C20—N3—C19—N2 | 2.2 (3) | C70—N53—C69—N52 | 5.2 (3) |
| C20—N3—C19—Cl1 | −177.30 (14) | C70—N53—C69—Cl51 | −175.13 (14) |
| C18—N2—C19—N3 | −2.6 (3) | C68—N52—C69—N53 | −3.6 (3) |
| C18—N2—C19—Cl1 | 177.00 (14) | C68—N52—C69—Cl51 | 176.80 (14) |
| C19—N3—C20—N5 | −178.37 (19) | C69—N53—C70—N55 | 177.51 (19) |
| C19—N3—C20—C21 | 1.2 (3) | C69—N53—C70—C71 | −0.9 (3) |
| C22—N5—C20—N3 | −179.8 (2) | C72—N55—C70—N53 | −177.88 (19) |
| C23—N5—C20—N3 | 1.1 (3) | C73—N55—C70—N53 | 4.3 (3) |
| C22—N5—C20—C21 | 0.5 (2) | C72—N55—C70—C71 | 0.8 (2) |
| C23—N5—C20—C21 | −178.52 (18) | C73—N55—C70—C71 | −176.98 (17) |
| N3—C20—C21—N4 | 179.46 (19) | C72—N54—C71—C70 | 0.5 (2) |
| N5—C20—C21—N4 | −0.9 (2) | C72—N54—C71—C68 | −176.9 (2) |
| N3—C20—C21—C18 | −3.8 (3) | N53—C70—C71—N54 | 177.86 (18) |
| N5—C20—C21—C18 | 175.82 (17) | N55—C70—C71—N54 | −0.8 (2) |
| C22—N4—C21—C20 | 0.9 (2) | N53—C70—C71—C68 | −4.3 (3) |
| C22—N4—C21—C18 | −175.1 (2) | N55—C70—C71—C68 | 176.99 (17) |
| N1—C18—C21—C20 | −177.17 (19) | N51—C68—C71—N54 | 2.5 (3) |
| N2—C18—C21—C20 | 3.3 (3) | N52—C68—C71—N54 | −176.80 (19) |
| N1—C18—C21—N4 | −1.4 (3) | N51—C68—C71—C70 | −174.68 (17) |
| N2—C18—C21—N4 | 179.1 (2) | N52—C68—C71—C70 | 6.0 (3) |
| C21—N4—C22—N5 | −0.6 (2) | C71—N54—C72—N55 | 0.1 (2) |
| C20—N5—C22—N4 | 0.1 (2) | C70—N55—C72—N54 | −0.6 (2) |
| C23—N5—C22—N4 | 179.07 (19) | C73—N55—C72—N54 | 177.10 (18) |
| C22—N5—C23—C25 | −59.4 (3) | C72—N55—C73—C74 | 26.2 (3) |
| C20—N5—C23—C25 | 119.4 (2) | C70—N55—C73—C74 | −156.61 (18) |
| C22—N5—C23—C24 | 64.7 (3) | C72—N55—C73—C75 | −98.3 (2) |
| C20—N5—C23—C24 | −116.5 (2) | C70—N55—C73—C75 | 78.9 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1A···N54 | 0.88 | 2.14 | 2.940 (2) | 152 |
| N51—H51A···N4 | 0.88 | 2.27 | 3.026 (2) | 144 |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: IM2124).
References
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Fiorini, M. T. & Abell, C. (1998). Tetrahedron Lett.39, 1827–1830.
- Hardcastle, I. R., Golding, B. T. & Griffin, R. J. (2002). Annu. Rev. Pharmacol. Toxicol.42, 325–348. [DOI] [PubMed]
- Oxford Diffraction (2006). CrysAlis CCD and CrysAlis RED Oxford Diffraction Ltd, Abingdon, England.
- Rouchal, M., Nečas, M., de Carvalho, F. P. & Vícha, R. (2009a). Acta Cryst. E65, o298–o299. [DOI] [PMC free article] [PubMed]
- Rouchal, M., Nečas, M. & Vícha, R. (2009b). Acta Cryst. E65, o1268. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Trávníček, Z. & Popa, I. (2007a). Acta Cryst. E63, o629–o631.
- Trávníček, Z. & Popa, I. (2007b). Acta Cryst. E63, o728–o730.
- Trávníček, Z. & Zatloukal, M. (2004). Acta Cryst. E60, o924–o926.
- Villhauer, E. B., Brinkman, J. A., Naderi, G. B., Burkey, B. F., Dunning, B. E., Prasad, K., Mangold, B. L., Russell, M. E. & Hughes, T. E. (2003). J. Med. Chem.46, 2774–2789. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809023629/im2124sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809023629/im2124Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



