Abstract
In the title compound, C15H12N2O5S, the benzisothiazole group is approximately planar (r.m.s. deviation excluding H atoms and the two O atoms bonded to S = 0.023 Å). The dihedral angle between the benzisothiazole ring and the terminal phenol ring is 84.9 (1)°. In the crystal, molecules are joined by N—H⋯O and O—H⋯O hydrogen bonds, and π-stacking interactions are observed between alternating phenol and benzisothiazole rings [centroid–centroid distances = 3.929 (3) and 3.943 (3) Å].
Related literature
For background literature related to analgesics, see: Slattery et al. (1996 ▶); McGoldrick & Bailie (1997 ▶); Watkins et al. (2006 ▶). For the synthesis and biological activity of the title compound, see: Vaccarino et al. (2007 ▶); González-Martin et al. (1998 ▶); Bazan & Alvarez-Builla (1996a
▶,b
▶). For related structures, see: Arshad et al. (2009a ▶,b ▶,c
▶); Siddiqui et al. (2008a ▶,b
▶; 2007 ▶).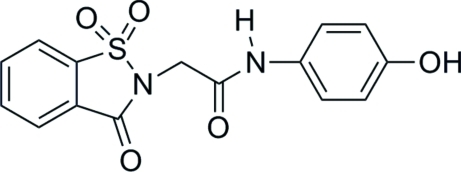
Experimental
Crystal data
C15H12N2O5S
M r = 332.33
Orthorhombic,

a = 16.3588 (10) Å
b = 9.6451 (6) Å
c = 9.9603 (6) Å
V = 1571.56 (17) Å3
Z = 4
Mo Kα radiation
μ = 0.23 mm−1
T = 230 K
0.60 × 0.20 × 0.20 mm
Data collection
Bruker SMART 1K CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2007 ▶) T min = 0.832, T max = 0.954
19429 measured reflections
3580 independent reflections
3283 reflections with I > 2σ(I)
R int = 0.033
Refinement
R[F 2 > 2σ(F 2)] = 0.039
wR(F 2) = 0.084
S = 1.07
3580 reflections
257 parameters
1 restraint
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.28 e Å−3
Δρmin = −0.38 e Å−3
Absolute structure: Flack (1983 ▶), 1681 Friedel pairs
Flack parameter: 0.02 (8)
Data collection: SMART (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809023022/bi2377sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809023022/bi2377Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Enhanced figure: interactive version of Fig. 1
Enhanced figure: interactive version of Fig. 2
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N10—H10⋯O14i | 0.87 (3) | 2.23 (3) | 3.078 (3) | 165 (3) |
| O14—H14⋯O9ii | 0.82 (3) | 1.91 (3) | 2.725 (2) | 173 (3) |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Acknowledgments
Helpful discussions with Professor M. L. Trudell, University of New Orleans, are gratefully acknowledged.
supplementary crystallographic information
Comment
Analgesics currently in use have high incidence of adverse reactions and can cause potentially lethal effects like hepatotoxicity and nephrotoxicity (Slattery et al., 1996; McGoldrick & Bailie, 1997; Watkins et al., 2006). A series of compounds bearing the acetaminophen (Tylenol) fragment linked to different lipophilic heterocyclic moieties were synthesized with a view to modulate its pharmacokinetic profile (Bazan & Alvarez-Builla, 1996a,b6; Vaccarino et al., 2007). Of these new derivatives, the title compound (commonly called SCP-1) has a similar profile to that of acetaminophen but with shorter elimination half-life and clearance.
Experimental
The title compound was synthesized following the procedure described by Vaccarino et al. (2007) and colourless needles suitable for X-ray analysis were obtained by recrystallization from an ethanol-water (8:1) mixture.
Refinement
All H atoms were located in a difference density map and their positional parameters and Uiso included in the full-matrix least-squares refinement. Observed C—H bond lengths are in the range 0.91 (3)–0.97 (3) Å.
Figures
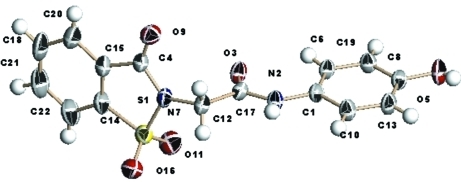
Fig. 1.
The asymmetric unit of the title compound. Displacement ellipsoids are drawn at the 50% probability level.
Fig. 2.
Perspective view of the contents of the unit cell showing the parallel stacking of the aromatic rings in layers perpendicular to the a axis.
Crystal data
| C15H12N2O5S | F(000) = 688 |
| Mr = 332.33 | Dx = 1.405 Mg m−3 |
| Orthorhombic, Pna21 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2c -2n | Cell parameters from 17706 reflections |
| a = 16.3588 (10) Å | θ = 2.5–30.5° |
| b = 9.6451 (6) Å | µ = 0.23 mm−1 |
| c = 9.9603 (6) Å | T = 230 K |
| V = 1571.56 (17) Å3 | Needle, colourless |
| Z = 4 | 0.60 × 0.20 × 0.20 mm |
Data collection
| Bruker SMART 1K CCD diffractometer | 3580 independent reflections |
| Radiation source: fine-focus sealed tube | 3283 reflections with I > 2σ(I) |
| graphite | Rint = 0.033 |
| φ and ω scans | θmax = 27.5°, θmin = 2.5° |
| Absorption correction: multi-scan (SADABS; Bruker, 2007) | h = −21→21 |
| Tmin = 0.832, Tmax = 0.954 | k = −12→12 |
| 19429 measured reflections | l = −12→12 |
Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H atoms treated by a mixture of independent and constrained refinement |
| R[F2 > 2σ(F2)] = 0.039 | w = 1/[σ2(Fo2) + (0.0183P)2 + 1.0724P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.084 | (Δ/σ)max < 0.001 |
| S = 1.07 | Δρmax = 0.28 e Å−3 |
| 3580 reflections | Δρmin = −0.38 e Å−3 |
| 257 parameters | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 1 restraint | Extinction coefficient: 0.0063 (8) |
| Primary atom site location: structure-invariant direct methods | Absolute structure: Flack (1983), 1681 Friedel pairs |
| Secondary atom site location: difference Fourier map | Flack parameter: 0.02 (8) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.11997 (4) | 0.20573 (6) | 0.93772 (6) | 0.03830 (15) | |
| O1 | 0.10058 (12) | 0.3352 (2) | 1.0007 (2) | 0.0534 (5) | |
| N2 | 0.09100 (11) | 0.0757 (2) | 1.0375 (2) | 0.0339 (4) | |
| O2 | 0.08966 (13) | 0.1841 (2) | 0.80485 (18) | 0.0554 (5) | |
| C3 | 0.15368 (14) | 0.0026 (2) | 1.0986 (2) | 0.0320 (5) | |
| O3 | 0.14167 (11) | −0.0882 (2) | 1.17895 (19) | 0.0451 (4) | |
| C4 | 0.31014 (15) | 0.0065 (3) | 1.0784 (3) | 0.0467 (6) | |
| H4 | 0.3137 (16) | −0.067 (3) | 1.135 (3) | 0.034 (7)* | |
| C4A | 0.23289 (14) | 0.0552 (3) | 1.0467 (2) | 0.0353 (5) | |
| C5 | 0.37621 (17) | 0.0677 (4) | 1.0128 (4) | 0.0608 (9) | |
| H5 | 0.430 (2) | 0.035 (4) | 1.032 (4) | 0.081 (11)* | |
| C6 | 0.36573 (17) | 0.1721 (4) | 0.9215 (4) | 0.0640 (9) | |
| H6 | 0.4123 (19) | 0.209 (3) | 0.873 (4) | 0.065 (9)* | |
| C7A | 0.22384 (13) | 0.1609 (3) | 0.9562 (3) | 0.0398 (5) | |
| C7 | 0.2887 (2) | 0.2225 (3) | 0.8913 (3) | 0.0561 (8) | |
| H7 | 0.283 (2) | 0.295 (3) | 0.830 (3) | 0.058 (9)* | |
| C8 | 0.00639 (14) | 0.0545 (3) | 1.0733 (2) | 0.0346 (5) | |
| H8B | −0.0203 (16) | 0.141 (3) | 1.072 (3) | 0.038 (7)* | |
| H8A | 0.0067 (16) | 0.018 (3) | 1.162 (3) | 0.035 (7)* | |
| C9 | −0.03588 (12) | −0.0469 (2) | 0.9791 (2) | 0.0298 (4) | |
| O9 | 0.00080 (9) | −0.11209 (18) | 0.89225 (16) | 0.0379 (4) | |
| N10 | −0.11625 (11) | −0.0573 (2) | 1.0033 (2) | 0.0331 (4) | |
| H10 | −0.1339 (17) | −0.008 (3) | 1.070 (3) | 0.045 (8)* | |
| C11 | −0.17479 (12) | −0.1418 (2) | 0.9368 (2) | 0.0295 (4) | |
| C12 | −0.25630 (14) | −0.1228 (3) | 0.9725 (3) | 0.0391 (6) | |
| H12 | −0.2690 (17) | −0.049 (3) | 1.033 (3) | 0.047 (8)* | |
| C13 | −0.31719 (13) | −0.2010 (3) | 0.9129 (3) | 0.0407 (6) | |
| H13 | −0.3736 (19) | −0.187 (3) | 0.933 (4) | 0.066 (9)* | |
| C14 | −0.29711 (13) | −0.2994 (2) | 0.8175 (2) | 0.0320 (5) | |
| O14 | −0.35473 (10) | −0.3808 (2) | 0.75619 (19) | 0.0442 (5) | |
| H14 | −0.397 (2) | −0.378 (3) | 0.802 (4) | 0.060 (10)* | |
| C15 | −0.21627 (14) | −0.3179 (3) | 0.7811 (2) | 0.0341 (5) | |
| H15 | −0.2007 (16) | −0.386 (3) | 0.716 (3) | 0.040 (7)* | |
| C16 | −0.15511 (13) | −0.2396 (3) | 0.8404 (2) | 0.0320 (5) | |
| H16 | −0.1000 (17) | −0.255 (3) | 0.814 (3) | 0.041 (7)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0371 (3) | 0.0407 (3) | 0.0371 (3) | −0.0060 (2) | −0.0064 (3) | 0.0063 (3) |
| O1 | 0.0547 (11) | 0.0422 (10) | 0.0633 (12) | 0.0011 (9) | −0.0100 (10) | 0.0011 (9) |
| N2 | 0.0253 (9) | 0.0422 (11) | 0.0343 (10) | −0.0052 (8) | −0.0035 (7) | 0.0048 (9) |
| O2 | 0.0640 (12) | 0.0651 (13) | 0.0371 (10) | −0.0036 (11) | −0.0141 (9) | 0.0105 (9) |
| C3 | 0.0288 (11) | 0.0366 (12) | 0.0306 (11) | −0.0023 (9) | −0.0040 (9) | −0.0026 (10) |
| O3 | 0.0430 (10) | 0.0481 (11) | 0.0443 (10) | 0.0011 (8) | −0.0010 (8) | 0.0133 (9) |
| C4 | 0.0312 (12) | 0.0594 (17) | 0.0496 (15) | 0.0012 (12) | −0.0040 (11) | −0.0117 (14) |
| C4A | 0.0278 (11) | 0.0438 (13) | 0.0343 (11) | −0.0037 (10) | −0.0027 (10) | −0.0065 (10) |
| C5 | 0.0260 (13) | 0.081 (2) | 0.075 (2) | −0.0045 (14) | −0.0007 (13) | −0.0239 (19) |
| C6 | 0.0398 (14) | 0.081 (2) | 0.072 (2) | −0.0244 (15) | 0.0125 (16) | −0.009 (2) |
| C7A | 0.0312 (11) | 0.0480 (13) | 0.0401 (14) | −0.0085 (10) | 0.0011 (10) | −0.0019 (11) |
| C7 | 0.0491 (16) | 0.065 (2) | 0.0547 (18) | −0.0222 (15) | 0.0078 (13) | 0.0049 (15) |
| C8 | 0.0256 (10) | 0.0460 (14) | 0.0323 (12) | −0.0015 (10) | −0.0004 (9) | −0.0030 (11) |
| C9 | 0.0246 (9) | 0.0372 (11) | 0.0275 (10) | −0.0002 (9) | −0.0012 (8) | 0.0020 (9) |
| O9 | 0.0238 (7) | 0.0520 (10) | 0.0379 (9) | −0.0013 (7) | 0.0043 (6) | −0.0089 (8) |
| N10 | 0.0249 (9) | 0.0425 (11) | 0.0319 (10) | −0.0037 (8) | 0.0045 (8) | −0.0087 (9) |
| C11 | 0.0240 (8) | 0.0352 (10) | 0.0293 (9) | −0.0040 (8) | 0.0016 (9) | −0.0002 (10) |
| C12 | 0.0276 (10) | 0.0465 (14) | 0.0432 (14) | −0.0007 (10) | 0.0078 (10) | −0.0145 (11) |
| C13 | 0.0219 (10) | 0.0511 (13) | 0.0492 (16) | −0.0017 (10) | 0.0059 (10) | −0.0093 (12) |
| C14 | 0.0254 (10) | 0.0402 (12) | 0.0304 (11) | −0.0067 (9) | 0.0012 (9) | 0.0024 (9) |
| O14 | 0.0284 (9) | 0.0613 (12) | 0.0427 (10) | −0.0135 (9) | 0.0038 (8) | −0.0150 (9) |
| C15 | 0.0296 (11) | 0.0408 (13) | 0.0319 (11) | −0.0023 (10) | 0.0053 (9) | −0.0044 (10) |
| C16 | 0.0216 (10) | 0.0401 (12) | 0.0343 (12) | −0.0010 (9) | 0.0055 (9) | −0.0010 (10) |
Geometric parameters (Å, °)
| S1—O2 | 1.4286 (19) | C8—H8B | 0.94 (3) |
| S1—O1 | 1.433 (2) | C8—H8A | 0.95 (3) |
| S1—N2 | 1.668 (2) | C9—O9 | 1.226 (3) |
| S1—C7A | 1.763 (2) | C9—N10 | 1.340 (3) |
| N2—C3 | 1.386 (3) | N10—C11 | 1.421 (3) |
| N2—C8 | 1.444 (3) | N10—H10 | 0.87 (3) |
| C3—O3 | 1.203 (3) | C11—C16 | 1.384 (3) |
| C3—C4A | 1.484 (3) | C11—C12 | 1.392 (3) |
| C4—C4A | 1.385 (3) | C12—C13 | 1.383 (3) |
| C4—C5 | 1.394 (4) | C12—H12 | 0.95 (3) |
| C4—H4 | 0.91 (3) | C13—C14 | 1.383 (3) |
| C4A—C7A | 1.369 (4) | C13—H13 | 0.95 (3) |
| C5—C6 | 1.367 (5) | C14—O14 | 1.370 (3) |
| C5—H5 | 0.96 (4) | C14—C15 | 1.383 (3) |
| C6—C7 | 1.384 (4) | O14—H14 | 0.82 (3) |
| C6—H6 | 0.97 (3) | C15—C16 | 1.385 (3) |
| C7A—C7 | 1.377 (4) | C15—H15 | 0.96 (3) |
| C7—H7 | 0.93 (3) | C16—H16 | 0.95 (3) |
| C8—C9 | 1.522 (3) | ||
| O2—S1—O1 | 117.12 (13) | N2—C8—H8B | 108.3 (16) |
| O2—S1—N2 | 110.07 (11) | C9—C8—H8B | 110.3 (16) |
| O1—S1—N2 | 109.35 (12) | N2—C8—H8A | 106.1 (16) |
| O2—S1—C7A | 113.30 (13) | C9—C8—H8A | 109.9 (16) |
| O1—S1—C7A | 112.41 (12) | H8B—C8—H8A | 110 (2) |
| N2—S1—C7A | 91.57 (11) | O9—C9—N10 | 124.6 (2) |
| C3—N2—C8 | 121.9 (2) | O9—C9—C8 | 122.84 (19) |
| C3—N2—S1 | 115.73 (16) | N10—C9—C8 | 112.52 (19) |
| C8—N2—S1 | 121.75 (17) | C9—N10—C11 | 128.3 (2) |
| O3—C3—N2 | 122.8 (2) | C9—N10—H10 | 115.0 (19) |
| O3—C3—C4A | 128.6 (2) | C11—N10—H10 | 116.6 (19) |
| N2—C3—C4A | 108.6 (2) | C16—C11—C12 | 119.3 (2) |
| C4A—C4—C5 | 117.2 (3) | C16—C11—N10 | 123.88 (19) |
| C4A—C4—H4 | 117.7 (17) | C12—C11—N10 | 116.8 (2) |
| C5—C4—H4 | 125.0 (17) | C13—C12—C11 | 120.6 (2) |
| C7A—C4A—C4 | 120.1 (2) | C13—C12—H12 | 121.3 (17) |
| C7A—C4A—C3 | 112.9 (2) | C11—C12—H12 | 117.9 (17) |
| C4—C4A—C3 | 127.0 (2) | C12—C13—C14 | 119.9 (2) |
| C6—C5—C4 | 121.8 (3) | C12—C13—H13 | 122 (2) |
| C6—C5—H5 | 120 (2) | C14—C13—H13 | 118 (2) |
| C4—C5—H5 | 119 (2) | O14—C14—C15 | 117.9 (2) |
| C5—C6—C7 | 121.2 (3) | O14—C14—C13 | 122.4 (2) |
| C5—C6—H6 | 120.3 (19) | C15—C14—C13 | 119.7 (2) |
| C7—C6—H6 | 118 (2) | C14—O14—H14 | 108 (2) |
| C4A—C7A—C7 | 123.2 (2) | C14—C15—C16 | 120.6 (2) |
| C4A—C7A—S1 | 110.83 (17) | C14—C15—H15 | 121.2 (16) |
| C7—C7A—S1 | 126.0 (2) | C16—C15—H15 | 118.1 (16) |
| C7A—C7—C6 | 116.6 (3) | C11—C16—C15 | 119.9 (2) |
| C7A—C7—H7 | 123 (2) | C11—C16—H16 | 121.3 (16) |
| C6—C7—H7 | 120 (2) | C15—C16—H16 | 118.8 (16) |
| N2—C8—C9 | 111.95 (19) | ||
| O2—S1—N2—C3 | 121.36 (18) | O2—S1—C7A—C7 | 63.0 (3) |
| O1—S1—N2—C3 | −108.64 (18) | O1—S1—C7A—C7 | −72.6 (3) |
| C7A—S1—N2—C3 | 5.82 (19) | N2—S1—C7A—C7 | 175.7 (3) |
| O2—S1—N2—C8 | −67.6 (2) | C4A—C7A—C7—C6 | 0.1 (5) |
| O1—S1—N2—C8 | 62.4 (2) | S1—C7A—C7—C6 | 179.4 (2) |
| C7A—S1—N2—C8 | 176.87 (19) | C5—C6—C7—C7A | −0.9 (5) |
| C8—N2—C3—O3 | 4.5 (4) | C3—N2—C8—C9 | −96.0 (3) |
| S1—N2—C3—O3 | 175.5 (2) | S1—N2—C8—C9 | 93.5 (2) |
| C8—N2—C3—C4A | −176.0 (2) | N2—C8—C9—O9 | 6.7 (3) |
| S1—N2—C3—C4A | −5.0 (2) | N2—C8—C9—N10 | −174.0 (2) |
| C5—C4—C4A—C7A | −1.1 (4) | O9—C9—N10—C11 | −0.2 (4) |
| C5—C4—C4A—C3 | 177.2 (3) | C8—C9—N10—C11 | −179.5 (2) |
| O3—C3—C4A—C7A | −179.5 (3) | C9—N10—C11—C16 | 6.3 (4) |
| N2—C3—C4A—C7A | 1.0 (3) | C9—N10—C11—C12 | −173.7 (2) |
| O3—C3—C4A—C4 | 2.1 (4) | C16—C11—C12—C13 | 0.3 (4) |
| N2—C3—C4A—C4 | −177.4 (2) | N10—C11—C12—C13 | −179.8 (2) |
| C4A—C4—C5—C6 | 0.3 (4) | C11—C12—C13—C14 | 0.3 (4) |
| C4—C5—C6—C7 | 0.7 (5) | C12—C13—C14—O14 | 179.3 (2) |
| C4—C4A—C7A—C7 | 0.9 (4) | C12—C13—C14—C15 | −0.7 (4) |
| C3—C4A—C7A—C7 | −177.6 (3) | O14—C14—C15—C16 | −179.4 (2) |
| C4—C4A—C7A—S1 | −178.4 (2) | C13—C14—C15—C16 | 0.6 (4) |
| C3—C4A—C7A—S1 | 3.1 (3) | C12—C11—C16—C15 | −0.4 (3) |
| O2—S1—C7A—C4A | −117.6 (2) | N10—C11—C16—C15 | 179.7 (2) |
| O1—S1—C7A—C4A | 106.8 (2) | C14—C15—C16—C11 | −0.1 (4) |
| N2—S1—C7A—C4A | −5.0 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N10—H10···O14i | 0.87 (3) | 2.23 (3) | 3.078 (3) | 165 (3) |
| O14—H14···O9ii | 0.82 (3) | 1.91 (3) | 2.725 (2) | 173 (3) |
Symmetry codes: (i) −x−1/2, y+1/2, z+1/2; (ii) x−1/2, −y−1/2, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BI2377).
References
- Arshad, M. N., Mubashar-ur-Rehman, H., Zia-ur-Rehman, M., Khan, I. U. & Shafiq, M. (2009a). Acta Cryst. E65, o1236. [DOI] [PMC free article] [PubMed]
- Arshad, M. N., Mubashar-ur-Rehman, H., Zia-ur-Rehman, M., Khan, I. U. & Shafique, M. (2009b). Acta Cryst. E65, o1011. [DOI] [PMC free article] [PubMed]
- Arshad, M. N., Tahir, M. N., Khan, I. U., Bilal, M. H. & Mubashar-ur-Rehman, H. (2009c). Acta Cryst. E65, o986. [DOI] [PMC free article] [PubMed]
- Bazan, N. G. & Alvarez-Builla, J. (1996a). U. S. Patent 5 554 636.
- Bazan, N. G. & Alvarez-Builla, J. (1996b). Chem. Abstr 125, 266037k.
- Bruker (2007). SMART, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- González-Martin, G., Lyndon, C. & Sunkel, C. (1998). Eur. J. Pharm. Biopharm.46, 293-297. [DOI] [PubMed]
- McGoldrick, M. D. & Bailie, G. R. (1997). Ann. Pharmacother 31, 221–227. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Siddiqui, W. A., Ahmad, S., Khan, I. U., Siddiqui, H. L. & Parvez, M. (2007). Acta Cryst. E63, o4116.
- Siddiqui, W. A., Ahmad, S., Siddiqui, H. L., Hussain, R. A. & Parvez, M. (2008a). Acta Cryst. E64, o1897. [DOI] [PMC free article] [PubMed]
- Siddiqui, W. A., Ahmad, S., Siddiqui, H. L. & Parvez, M. (2008b). Acta Cryst. E64, o724. [DOI] [PMC free article] [PubMed]
- Slattery, J. T., Nelson, S. D. & Thummel, K. E. (1996). Clin. Pharmacol. Ther 60, 241–246. [DOI] [PubMed]
- Vaccarino, A. L., Paul, D., Mukherjee, P. K., Rodriguez de Turco, E. B., Marcheselli, V. L., Xu, L., Trudell, M. L., Matia, M. P., Sunkel, C., Alvarez-Builla, J. & Bazan, N. G. (2007). Bioorg. Med. Chem.15, 2206–2215. [DOI] [PubMed]
- Watkins, P. B., Kaplowitz, N., Slattery, J. T., Colonese, C. R., Colucci, S. V., Stewart, P. W. & Harris, S. C. (2006). J. Am. Med. Assoc.296, 87–90. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809023022/bi2377sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809023022/bi2377Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Enhanced figure: interactive version of Fig. 1
Enhanced figure: interactive version of Fig. 2