Abstract
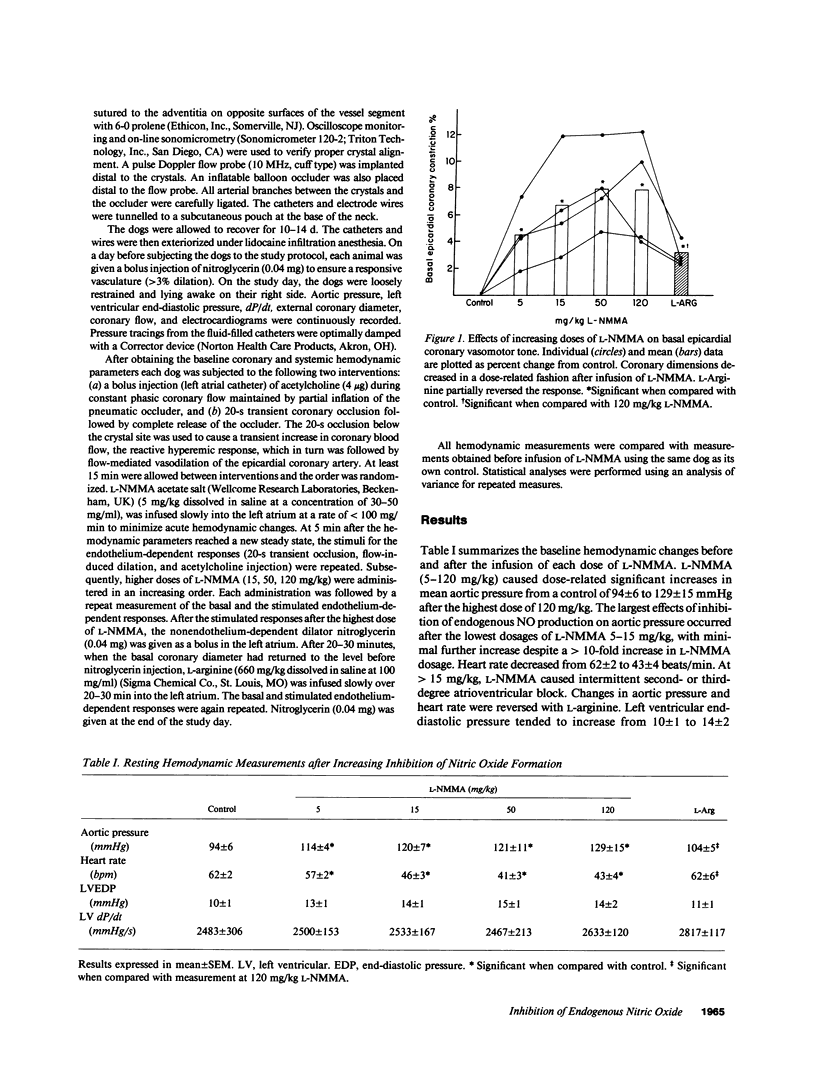
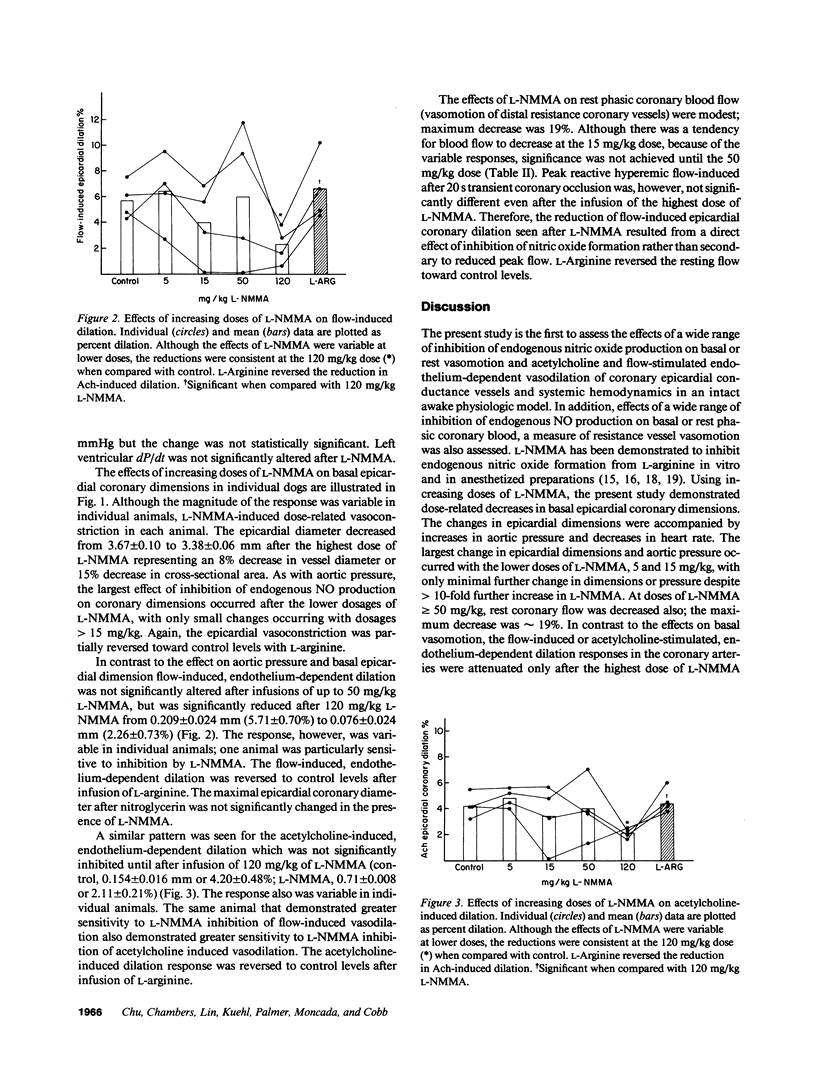
The role of nitric oxide in basal vasomotor tone and stimulated endothelium-dependent dilations in the coronary arteries in chronically instrumented awake dogs was studied by examining the consequences of inhibiting endogenous nitric oxide formation with the specific inhibitor of nitric oxide formation, NG-monomethyl-L-arginine (L-NMMA). In four awake dogs, coronary dimension crystals were chronically implanted on the circumflex artery for the measurement of epicardial coronary diameter, and Doppler flow probes were implanted for quantitation of phasic coronary blood flow (vasomotion of distal regulatory resistance vessels). Basal epicardial coronary diameter, acetylcholine-stimulated endothelium-dependent dilation, and flow-induced endothelium-dependent dilation of the epicardial arteries and phasic blood flow were recorded before, and after 5, 15, 50, and 120 mg/kg of L-NMMA. L-NMMA induced a dose-related increase in basal epicardial coronary vasomotor tone. There was an accompanying increase in aortic pressure and a decrease in heart rate. At doses greater than or equal to 50 mg/kg, rest phasic coronary blood flow was also decreased. Left ventricular end-diastolic pressure and contractility were not significantly changed. In contrast, the flow-induced or acetylcholine-stimulated endothelium-dependent responses were attenuated only after infusion of the highest does of L-NMMA (120 mg/kg). The changes in the basal vasomotor tone and acetylcholine-stimulated endothelium-dependent responses returned towards the control states in the presence of L-arginine (660 mg/kg). These data support the view that nitric oxide plays a significant role in modulating basal vasomotion and endothelial-dependent dilation stimulated by acetylcholine or increase in blood flow in epicardial coronary arteries and also influence the regulation of coronary blood flow during physiologic conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisaka K., Gross S. S., Griffith O. W., Levi R. NG-methylarginine, an inhibitor of endothelium-derived nitric oxide synthesis, is a potent pressor agent in the guinea pig: does nitric oxide regulate blood pressure in vivo? Biochem Biophys Res Commun. 1989 Apr 28;160(2):881–886. doi: 10.1016/0006-291x(89)92517-5. [DOI] [PubMed] [Google Scholar]
- Boulanger C., Hendrickson H., Lorenz R. R., Vanhoutte P. M. Release of different relaxing factors by cultured porcine endothelial cells. Circ Res. 1989 Jun;64(6):1070–1078. doi: 10.1161/01.res.64.6.1070. [DOI] [PubMed] [Google Scholar]
- Chu A., Chambers D. E., Lin C. C., Kuehl W. D., Cobb F. R. Nitric oxide modulates epicardial coronary basal vasomotor tone in awake dogs. Am J Physiol. 1990 Apr;258(4 Pt 2):H1250–H1254. doi: 10.1152/ajpheart.1990.258.4.H1250. [DOI] [PubMed] [Google Scholar]
- Chu A., Cobb F. R. Effects of atrial natriuretic peptide on proximal epicardial coronary arteries and coronary blood flow in conscious dogs. Circ Res. 1987 Oct;61(4):485–491. doi: 10.1161/01.res.61.4.485. [DOI] [PubMed] [Google Scholar]
- Chu A., Cobb F. R., Hagen P. O., Murray J. J. Effects of a stabilized endothelium-derived relaxing factor on the coronary vasculature in awake dogs. Am J Physiol. 1989 Dec;257(6 Pt 2):H1895–H1899. doi: 10.1152/ajpheart.1989.257.6.H1895. [DOI] [PubMed] [Google Scholar]
- Chu A., Morris K. G., Kuehl W. D., Cusma J., Navetta F., Cobb F. R. Effects of atrial natriuretic peptide on the coronary arterial vasculature in humans. Circulation. 1989 Dec;80(6):1627–1635. doi: 10.1161/01.cir.80.6.1627. [DOI] [PubMed] [Google Scholar]
- Chu A., Murray J. J., Lin C. C., Russell M., Hagen P. O., Cobb F. R. Preferential proximal coronary dilation by activators of guanylate cyclase in awake dogs. Am J Physiol. 1990 Aug;259(2 Pt 2):H340–H345. doi: 10.1152/ajpheart.1990.259.2.H340. [DOI] [PubMed] [Google Scholar]
- Damase-Michel C., Tran M. A., Montastruc J. L., Moatti J. P., Montastruc P. Effects of pinacidil on the sympatho-adrenal system in dogs. Br J Pharmacol. 1989 Aug;97(4):1019–1026. doi: 10.1111/j.1476-5381.1989.tb12557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res. 1989 Jul;65(1):1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Byrns R. E., Buga G. M., Wood K. S., Chaudhuri G. Pharmacological evidence that endothelium-derived relaxing factor is nitric oxide: use of pyrogallol and superoxide dismutase to study endothelium-dependent and nitric oxide-elicited vascular smooth muscle relaxation. J Pharmacol Exp Ther. 1988 Jan;244(1):181–189. [PubMed] [Google Scholar]
- Kontos H. A., Wei E. P., Povlishock J. T., Christman C. W. Oxygen radicals mediate the cerebral arteriolar dilation from arachidonate and bradykinin in cats. Circ Res. 1984 Sep;55(3):295–303. doi: 10.1161/01.res.55.3.295. [DOI] [PubMed] [Google Scholar]
- Moncada S., Radomski M. W., Palmer R. M. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem Pharmacol. 1988 Jul 1;37(13):2495–2501. doi: 10.1016/0006-2952(88)90236-5. [DOI] [PubMed] [Google Scholar]
- Myers P. R., Guerra R., Jr, Harrison D. G. Release of NO and EDRF from cultured bovine aortic endothelial cells. Am J Physiol. 1989 Apr;256(4 Pt 2):H1030–H1037. doi: 10.1152/ajpheart.1989.256.4.H1030. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio R., Berne R. M. Regulation of coronary blood flow. Prog Cardiovasc Dis. 1975 Sep-Oct;18(2):105–122. doi: 10.1016/0033-0620(75)90001-8. [DOI] [PubMed] [Google Scholar]
- Shikano K., Ohlstein E. H., Berkowitz B. A. Differential selectivity of endothelium-derived relaxing factor and nitric oxide in smooth muscle. Br J Pharmacol. 1987 Nov;92(3):483–485. doi: 10.1111/j.1476-5381.1987.tb11347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989 Oct 28;2(8670):997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]



